Up-conversion properties of lanthanide-organic frameworks and how to track ammunitions using these materials†
Ingrid T.
Weber
*ab,
Idelma A. A.
Terra
c,
Adenaule J. G. de
Melo
d,
Marcella A. de M.
Lucena
d,
Kaline A.
Wanderley
b,
Carlos de O.
Paiva-Santos
f,
Selma G.
Antônio
f,
Luiz A. O.
Nunes
c,
Filipe A. A.
Paz
e,
Gilberto F. de
Sá
b,
Severino A.
Júnior
b and
Marcelo O.
Rodrigues
*ab
aInstituto de Química, Universidade de Brasília, 70910-900, Brasília – DF, Brazil. E-mail: ingrid@ufpe.br; marcelozohio@unb.br; Fax: +55 61 3273-4149; Tel: +55 61 3107-3876
bDepartamento de Química Fundamental, UFPE, 50590-470, Recife - PE, Brazil. Fax: +55 81 2126-8442; Tel: +55 81 2126-7475
cInstituto de Física de São Carlos, Universidade de São Paulo, CP 369, 13560-970, São Carlos – SP, Brazil
dPrograma de Pós-Graduação em Ciências de Materiais, CCEN, UFPE, 50590-470, Recife - PE, Brazil
eDepartamento de Química, Universidade de Aveiro, CICECO, 3810-193, Aveiro, Portugal
fUniversidade Estadual Paulista, Departamento de Físico-Química, Instituto de Quıímica, Caixa Postal 355, CEP, 14801-970, Araraquara-SP, Brazil
First published on 21st February 2012
Abstract
This manuscript reports the first example of up-conversion properties involving Yb3+ and Tb3+ ions in five isostructural Lanthanide-Organic Frameworks (LnOFs), herein designated as UCMarker-1 to UCMarker-5, respectively, and their application as optical probes for the identification of gunshot residues (GSRs) and the ammunition encryption procedure. The excitation of the Yb3+ 2F7/2 ↔ 2F5/2 transition (980 nm) at room temperature leads to visible up-conversion (UC) emission of Tb3+ 5D4 → 7FJ. The GSR and lead-free primer residues are easily identified upon UV radiation (λ = 254 nm). These results prove that the exploration of LnOFs to identify GSR is attractive for the identification of ammunition origins or caliber recognition.
Introduction
Lanthanide-Organic Frameworks (LnOFs) have received special attention in recent years since they combine interesting structures, thermodynamic stability, well-known spectroscopic features of Ln3+ ions and promising applications as catalysts, markers and sensors.1–7 These LnOFs have been typically obtained by solvothermal synthesis, nevertheless, this process is restricted to laboratory scale since it is a time consuming methodology.8–10 Alternatively, microwave-assisted solvothermal synthesis allows the production of these materials in large scale since it provides a high yield synthesis in a few minutes.11–13 Thus, the use of microwave assisted synthesis opens a window to many new applications of LnOFs.14 Most of the work based on LnOFs has reported the conversion of UV into visible radiation (down-conversion), while the up-conversion process has been less explored.15,16 However, in the last decades the interest in up-conversion (UC) systems has grown significantly due to their remarkable applications in biomarkers,17,18 lasers,19 optical devices,20–23 and so on. It is well established that the Yb3+ ion is an excellent UC sensitizer due to the high oscillator strength of the 2F7/2 ↔ 2F5/2 transition (≈ 975 nm) and the lack of high excited states in the visible region.24 The lanthanides most commonly used in up-conversion materials are Er3+, Tm3+, Ho3+ and Pr3+, since all these ions present excited states almost resonant with Yb3+ 2F7/2 ↔ 2F5/2 transition.21,25 The use of Tb3+ and Eu3+ ions in up-conversion systems is less common due to a lack of possibility for one-step energy transfer owing to the large separation of relevant energy levels among these ions. Nevertheless, these ions are very attractive as emitters due to their high quantum efficiencies.24,26Even though UC properties of LnOF materials are available in the literature, investigation of UC luminescence in LnOFs containing simultaneously Yb3+ and Tb3+ ions is, to date, nonexistent.15,27 Hence, this work reports the first example of UC luminescent properties of the five isostructural 2D LnOFs containing Yb3+ and Tb3+, [(Tb1-xYbx)(DPA)(HDPA)] (where H2DPA is pyridine 2,6-dicarboxylic acid and x varies from 0.05 to 0.50 mol), synthesized via microwave solvothermal conditions, and herein designated as UCMarker-1 to UCMarker-5 respectively.
One of the most promising applications of these luminescent LnOFs is as optical probes for identification of gunshot residues (GSRs) and the ammunition encryption procedure.28,29 Gunshot residues are a complex mixture of partially burned and unburnt propellant powder, solid particles from the ammunition primer and metals from the cartridge, projectile and the weapon itself, smoke, lubricants, grease, etc.30 These particles are produced when a firearm is discharged, and its detection provides very important evidence in forensic investigations.30–34 Currently, the identification of GSR particles is based on the morphological characteristics and elemental composition of the particles.31,35,36 GSR from conventional ammunitions should have lead, barium and antimony in composition and spherical morphology.35,37,38
A recent report performed by the Chicago Police has demonstrated difficulties in detection and recovering of GSR. Of the 201 samples analyzed, only 23 provided particles with Pb, Sb and Ba in the composition.39 It is noteworthy that these low instances of GSR particles identification arise from the large diversity of weapons and ammunition designs, complexity of the firing processes and physical conditions during the gun deflagration.40 Each shot is different from every other, even when the same firearm is used with the same ammunition type. Therefore, the development of new, simple, reliable and accurate methodology for GSR detection and ammunition traceability is a point of first interest in forensic science. The use of luminescent markers is an interesting alternative and LnOFs fit very well the requirements for use in the emerging GSR detection methodology.28
GSR identification could be done by visual or spectroscopic observation of luminescent markers. Besides the traditional luminescent properties of LnOF, these materials that contain more than one type of Ln3+ ion open a new window to design materials capable of modulating the luminescence over diverse regions of the visible and near-infrared spectrum.41 These properties allow encryption and tracing of ammunition origin, caliber, use (civil or law enforcement), etc.29 In addition, the unique chemical composition of the sample permits the use of traditional SEM-EDS methodology to confirm the presence of GSR particles.
Experimental
Synthesis of UCMarkers
A mixture of pyridine-2,6-dicarboxylic acid, H2DPA, (0.7 mmol), Ln(NO3)36·H2O, (0.35 mmol, Ln = Tb3+ and Yb3+ in appropriate ratios, 0.3325/0.0175, 0.315/0.035, 0.28/0.07, 0.245/0.105 and 0.175/0.175 mmol, herein designated as UCMarker-1 to 5 respectively, and H2O (ca. 5 mL) in a 10 mL IntelliVent reactor were placed inside a CEM Focused Microwave™ Synthesis System Discover S-Class equipment. Reactions took place at 160 °C during 10 min, under constant magnetic stirring. The final materials were obtained in a yield of ca. 90% after washing with water, acetone and being air-dried.General details
The powder synchrotron X-ray diffraction patterns were acquired at ambient temperature (300 K) in a 2θ range of 5–48° and using a Huber diffractometer in high resolution mode (low intensity, E = 10 keV) in multi-proposed powder station D10A-XRD2 beam line of Brazilian Synchrotron Light Laboratory (LNLS). Conventional X-ray powder diffraction analyses were performed at room temperature, using a Bruker D8 Advanced with DaVinci design equipped with a LynxEye Linear Position Sensitive Detector and a Copper (Cu) sealed tube (λkα1 = 1.5404 Å, λkα2 = 1.5444 Å, Iα2/Iα1 = 0.5). Intensity data were collected in step scanning mode, in the range from 5 to 50° (2θ), with a step size of 0.01°, Soller slit with 2.5° of divergence, 0.5° scattering slit and 0.6 mm receiving slit. The Rietveld refinement42 for UCMarkers were performed with the software GSAS/EXPGUI,43,44 using as starting premise the atomic coordinates of the structural model previously reported.45 The preferential orientation was corrected using the spherical harmonic model (sixth order) proposed by Jarvinen,46 the peak profile was adjusted by Thompson-Cox-Hastings function modified by Young and Desai (pV-TCHZ),47 surface roughness correction was refined by Pitschke function and background was fitted by an eighth-degree shifted Chebyshev polynomial function. In the final runs, the following parameters were refined: scale factor, background and absorption coefficients, spherical harmonic, unit-cell parameters and pV-TCHZ correction for asymmetric parameters.The emission spectra of Stokes and upconverted photoluminescence were achieved through standard optical setups. The visible and infrared signal went through a 0.30 m Thermo Jarrel Ash 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497 monochromator, and collected by photomultipler tube (R928) for visible and EG&G InGaAs photodetector for the infrared emission. Room temperature photoluminescence was acquired upon excitation at 325 nm with a He–Cd laser. The up-conversion spectra were obtained using a 980 nm InGaAs diode laser. The measurements of 5D4 emission decay curve were carried out with excitation at 355 nm from third harmonic of Nd:YAG laser (Continuun-Surelite SLII-10). The signal was dispersed by a monochromator and collected by a photomultiplier tube (R928) and registered by a digital oscilloscope. From these curves the average life time values were obtained as those for which the emission intensity drops by a factor 1/e.
497 monochromator, and collected by photomultipler tube (R928) for visible and EG&G InGaAs photodetector for the infrared emission. Room temperature photoluminescence was acquired upon excitation at 325 nm with a He–Cd laser. The up-conversion spectra were obtained using a 980 nm InGaAs diode laser. The measurements of 5D4 emission decay curve were carried out with excitation at 355 nm from third harmonic of Nd:YAG laser (Continuun-Surelite SLII-10). The signal was dispersed by a monochromator and collected by a photomultiplier tube (R928) and registered by a digital oscilloscope. From these curves the average life time values were obtained as those for which the emission intensity drops by a factor 1/e.
Conventional .40 Smith and Wesson (S&W) bullets (calibre .40-inch) were disassembled to separate their components. UCMarkers were added to conventional gunpowder in a ratio of 10 wt% and the bullets were reassembled. For safety reasons, homogenization procedures were not performed. The bullets were then fired at distance of 40 cm from the target at an indoor shooting range. The UCMarkers were also added directly to the primer of lead-free ammunition in a ratio of 10.0 wt%. GSR particles from primers were obtained by manually discharging the primer by mechanical impact onto a sample holder (black metal support). All the marked GSR was analyzed by SEM/EDS and visual observation under UV irradiation.
Results and discussion
Fig. 1 displays the Tb3+ emission spectra associated with 5D4 → 7FJ transitions upon excitation (Laser diode) of UCMarkers at 980 nm at room temperature.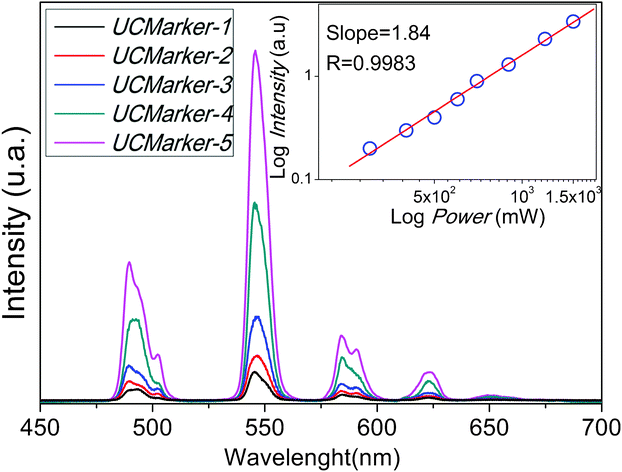 | ||
| Fig. 1 Room temperature UC photoluminescence of UCMarkers upon excitation at 980 nm. Insert plot represents the log-log dependence of the integrated intensity of Tb3+ 5D4 → 7FJ transition as function of incident excitation power after 980 nm excitation for UCMarker-5. | ||
The excitation of the Yb3+ 2F7/2 ↔ 2F5/2 transition (980 nm) at room temperature leads to visible UC emission of Tb3+ 5D4 → 7FJ transitions (Fig. 1). As the Tb3+ ion has no energy levels in this region (NIR) to absorb radiation, the Yb3+ ion does not have an energy level in green region either, therefore the UC processes cannot be explained by a direct energy transfer from excited Yb3+ to Tb3+ ion.24 The only way to access the Tb3+ 5D4 multiplet is via absorption of two photons. Insert plot in Fig. 1 exhibits, in log scale, the linear relation between the integrated intensity of the Tb3+ 5D4 → 7FJ transition and the incident pump power at 980 nm. A slope of 1.84 is in good agreement with the proposal that two photons are involved in the Tb3+ 5D4 → 7FJ emissions for UCMarker-5. Recent reports have demonstrated a temperature dependence of UC in these system, indicating that the processes must be mediated by phonons to bridge the energy gap between two times of Yb3+ 2F5/2 level and Tb3+ 5D4 emitter state. It is worth noting that GSA/ESA (Ground State Absorption/Exited State Absorption) and cooperative sensitization are the mechanisms (Fig. 2) proposed to justify the UC processes in materials where Yb3+ is used as a Tb3+ sensitizer.
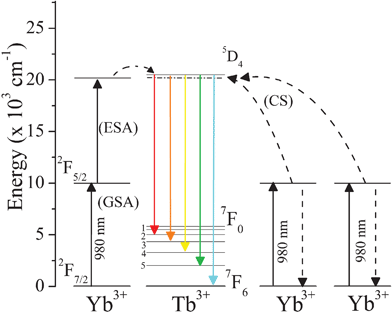 | ||
| Fig. 2 Schematic representation of UC mechanisms, exhibiting relevant levels of the Yb3+ and Tb3+ ions with the 5D4 → 7FJ Tb3+ transitions excited via Cooperative Sensitization (CS) and Ground State Absorption/Excited State Absorption (GSA/ESA). | ||
In accordance with Salley et al. the GSA/ESA mechanism at low temperature (10 K) is responsible for UC luminescence in materials whose ion-ion interaction has high dimer character (intermetallic separation of 3.9 Å), while at high temperature (T ≥ 100 K) the UC mechanism for Yb3+ → Tb3+ is phonon assisted cooperative sensitization of Tb3+ by two excited Yb3+ ions.26,48 Moreover, a temperature dependence of UC in these systems has been observed, indicating that the processes must be mediated by phonons to bridge the energy gap between two times of Yb3+ 2F5/2 level and Tb3+ 5D4 emitter state.
Assuming that in UCMarkers the effect of the lanthanide contraction is insignificant, the Ln3+–Ln3+ intermetallic distance in all materials are therefore close to those reported for ∞[Tb(DPA)(HDPA)], then, for each Ln3+ ion there are 14 nearest Ln3+ neighbors in the same layer, whose distances are 6.32, 6.75 and 9.93 Å (See Figure. S1 in Supporting Information†). In adjacent layers, the lanthanide ion is surrounded by 4 neighbors, the nearest metal-metal distance being 8.87 Å. Thus, the cooperative mechanism is more appropriate than GSA/ESA to justify the UC luminescence in UCMarkers due to both the temperature at which the experiments were conducted and the large intermetallic distances that corroborate to low Ln3+–Ln3+ coupling. Indeed, an unambiguous way to investigate an UC mechanism in a system is through the temporal evolution of luminescence under pulse excitation. Unfortunately, this experiment can not be performed, because the UC luminescence intensities exhibited by UCMarkers are extremely low, avoiding the acquisition of these data.
Fig. 3 shows the emission spectra of UCMarkers acquired at 300 K obtained by laser excitation at 325 nm. It also shows the visual effect, under UV lamp irradiation (λ = 254 nm), after firing a .40 pistol of unmarked ammunition (blank), ammunition and primers containing UCMarkers.
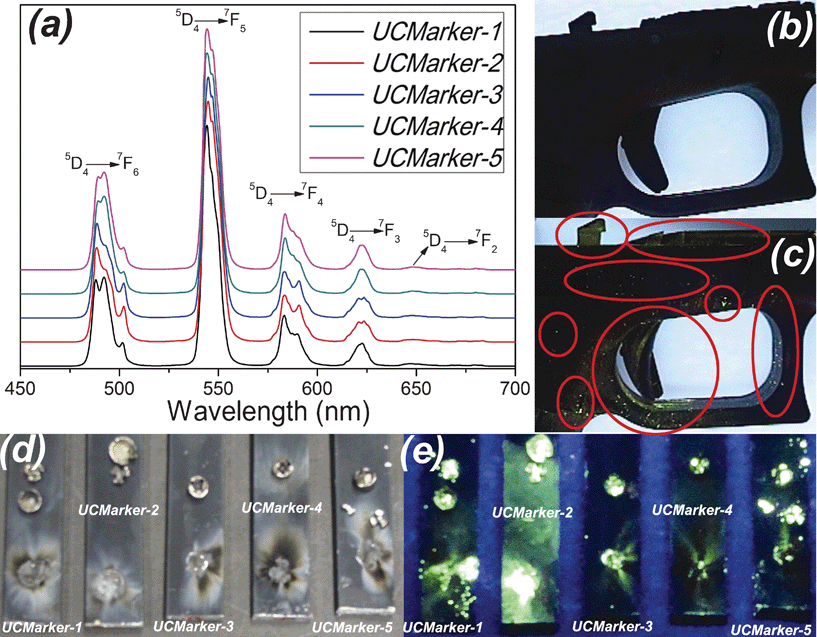 | ||
| Fig. 3 (a): Room temperature photoluminescence of UCMarkers upon excitation at 325 nm; (b) : Unmarked conventional ammunition (blank) on the .40 pistol after firing; (c) UCMarker-1 on the .40 gun pistol after firing; (d) and (e) Lead-free primers' residues containing UCMarkers. (d): no UV light irradiation; and (e): under UV irradiation. | ||
The UCMarkers shows an intense green emission associated with Tb3+ 5D4 → 7FJ transitions similarly to Tb3+ pure material, Fig. 3 (a). In order to prove the potential application of the materials as ammunition markers for GSR detection and ammunition encryption process, several assays were performed adding the LnOF samples to gun powder and primers. As observed in Fig. 3 (c) and (e), the GSR and primers residues are easily identified upon UV radiation (λ = 254 nm), while for unmarked ammunitions, Fig. 3 (b), the GSRs cannot be visually identified. These results have proven the efficacy of the methodology and that, although the UC emission presents low intensity for visual detection, the presence of Yb3+ ions does not compromise the visual detection of GSR by UV excitation, even in high concentration. By changing composition and optimizing the UC process one can identify GSR both by UV or NIR excitation, which is a very attractive tool for ammunition origin or caliber recognition.
In order to demonstrate the chemical traceability of the conventional ammunition and primers, SEM-EDS technique has been used to determine the composition of GSR (Fig. 4).
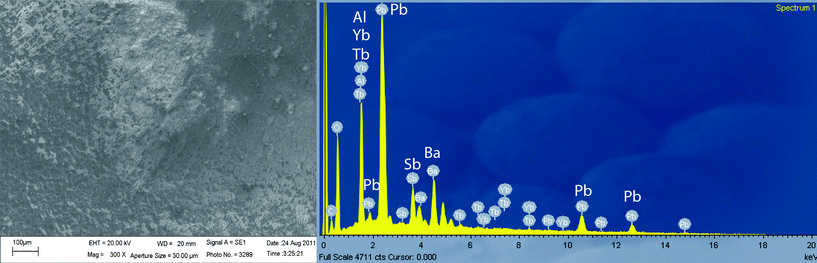 | ||
| Fig. 4 SEM image (x1000) of UCMarker-4 and EDS spectrum of its GSR particles acquired directly on aluminium plate. | ||
The EDS spectrum displays the presence of Yb3+ and Tb3+ ions and typical metals of GSR (Pb, Ba and Sb), demonstrating that the SEM-EDS methodology can be used to confirm their presence by visual and spectroscopic observations.
Conclusions
The excitation of the Yb3+ 2F7/2 ↔ 2F5/2 transition (980 nm) at room temperature leads to visible UC emission of Tb3+ 5D4 → 7FJ transitions. A slope of 1.84 is in good agreement with the proposal that two photons are involved in Tb3+ 5D4 → 7FJ emissions. The inclusion of these photoluminescent markers opens a new perspective to GSR analysis, since they permit the visual identification of GSR particles. Additionally, the encryption of ammunition may be performed via changes in the composition of these markers, being a versatile alternative for identifying the ammunition origin. This evidence is important in the reconstruction of crime scenes, especially, when firearms or ammunition from distinct origins are involved in shooting incidents.Acknowledgements
The authors gratefully acknowledge CNPq (INCT/INAMI, RH-INCT/INAMI and CNPq-550388/2007-9), PRONEX-FACEPE-CNPq (APQ-0859-1.06/08) and FCT (PTDC/QUI-QUI/098098/2008—FCOMP-01-0124-FEDER-010785) for its financial support and CETENE and LNLS (proposal D10A-XRD2—10164) for providing the facilities. We are also indebted to Patrícia Silva.References
- M. O. Rodrigues, F. A. A. Paz, R. O. Freire, G. F. de Sá, A. Galembeck, M. C. Montenegro, A. N. Araujo and S. Alves, J. Phys. Chem. B, 2009, 113, 12181–12188 CrossRef CAS.
- M. O. Rodrigues, N. B. da Costa, C. A. de Simone, A. A. S. Araújo, A. M. Brito-Silva, F. A. A. Paz, M. E. de Mesquita, S. A. Júnior and R. O. Freire, J. Phys. Chem. B, 2008, 112, 4204–4212 CrossRef CAS.
- H. L. Guo, Y. Z. Zhu, S. L. Qiu, J. A. Lercher and H. J. Zhang, Adv. Mater., 2010, 22, 4190–4192 CrossRef CAS.
- L. Cunha-Silva, S. Lima, D. Ananias, P. Silva, L. Mafra, L. D. Carlos, M. Pillinger, A. A. Valente, F. A. A. Paz and J. Rocha, J. Mater. Chem., 2009, 19, 2618–2632 RSC.
- B. L. Chen, Y. Yang, F. Zapata, G. N. Lin, G. D. Qian and E. B. Lobkovsky, Adv. Mater., 2007, 19, 1693–1696 CrossRef CAS.
- B. L. Chen, L. B. Wang, Y. Q. Xiao, F. R. Fronczek, M. Xue, Y. J. Cui and G. D. Qian, Angew. Chem., Int. Ed., 2009, 48, 500–503 CrossRef CAS.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. Houk, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC.
- Y.-Q. Sun, J. Zhang, Y.-M. Chen and G.-Y. Yang, Angew. Chem., Int. Ed., 2005, 44, 5814–5817 CrossRef CAS.
- J. Rocha, F. A. A. Paz, F. N. Shi, D. Ananias, N. J. O. Silva, L. D. Carlos and T. Trindade, Eur. J. Inorg. Chem., 2011, 13, 2035–2044 CrossRef.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS.
- S. T. Meek, J. A. Greathouse and M. D. Allendorf, Adv. Mater., 2011, 23, 249–267 CrossRef CAS.
- K. A. Wanderley, S. Alves and C. O. Paiva-Santos, Quim. Nova, 2011, 34, 434–438 CAS.
- Y. Yoo and H. K. Jeong, Chem. Commun., 2008, 2441–2443 RSC.
- P. Silva, A. A. Valente, J. Rocha and F. A. A. Paz, Cryst. Growth Des., 2010, 10, 2025–2028 CAS.
- J. Yang, Q. Yue, G.-D. Li, J.-J. Cao, G.-H. Li and J.-S. Chen, Inorg. Chem., 2006, 45, 2857–2865 CrossRef CAS.
- K. L. Wong, W. M. Kwok, W. T. Wong, D. L. Phillips and K. W. Cheah, Angew. Chem., Int. Ed., 2004, 43, 4659–4662 CrossRef CAS.
- S. A. Hilderbrand, F. W. Shao, C. Salthouse, U. Mahmood and R. Weissleder, Chem. Commun., 2009, 4188–4190 RSC.
- H. Hu, M. Yu, F. Li, Z. Chen, X. Gao, L. Xiong and C. Huang, Chem. Mater., 2008, 20, 7003–7009 CrossRef CAS.
- S. Sanders, R. G. Waarts, D. G. Mehuys and D. F. Welch, Appl. Phys. Lett., 1995, 67, 1815–1817 CrossRef CAS.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185–1189 CAS.
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS.
- F. C. Guinhos, P. C. Nóbrega and P. A. Santa-Cruz, J. Alloys Compd., 2001, 323-324, 358–361 CrossRef CAS.
- J. E. C. da Silva, G. F. de Sá and P. A. Santa-Cruz, J. Alloys Compd., 2002, 344, 260–263 CrossRef CAS.
- R. Martin-Rodriguez, R. Valiente, S. Polizzi, M. Bettinelli, A. Speghini and F. Piccinelli, J. Phys. Chem. C, 2009, 113, 12195–12200 CAS.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- G. M. Salley, R. Valiente and H. U. Guedel, J. Lumin., 2001, 94-95, 305–309 CrossRef.
- D. Weng, X. Zheng and L. Jin, Eur. J. Inorg. Chem., 2006, 2006, 4184–4190 CrossRef.
- I. T. Weber, A. J. G. de Melo, M. A. M. Lucena, M. O. Rodrigues and S. Alves Júnior, Anal. Chem., 2011, 83, 4720–4723 CrossRef CAS.
- I. T. Weber, A. J. G. Melo, S. Alves Júnior, M. A. M. Lucena, M. O. Rodrigues, BR Patent WO/2010/105326, 2010 Search PubMed.
- O. Dalby, D. Butler and J. W. Birkett, J. Forensic Sci., 2010, 55, 924–943 CrossRef.
- S. Kage, K. Kudo, A. Kaizoji, J. Ryumoto, H. Ikeda and N. Ikeda, J Forensic Sci, 2001, 46, 830–834 CAS.
- H. E. Berryman, A. K. Kutyla and J. R. Davis, J. Forensic Sci., 2010, 55, 488–491 CrossRef CAS.
- H. H. Meng and B. Caddy, J Forensic Sci, 1997, 42, 553–570 CAS.
- L. Garofano, M. Capra, F. Ferrari, G. P. Bizzaro, D. Di Tullio, M. Dell'Olio and A. Ghitti, Forensic Sci. Int., 1999, 103, 1–21 CrossRef CAS.
- V. J. M. Di Maio, in Gunshot Wounds: Practical Aspects of Firearms, Ballistics, and Forensic Techniques, CRC Press LLC, New York, Second Edition edn 1999 Search PubMed.
- D. M. Wright and M. A. Trimpe, Forensic Sci. Commun., 2006, 8, 3 Search PubMed . Available at: http://www.fbi.gov/about-us/lab/forensic-science-communications/fsc/july2006/research/2006_07_research01.htm.
- T. A. Brettell, N. Rudin and R. Saferstein, Anal. Chem., 2003, 75, 2877–2890 CrossRef CAS.
- ASTM Standart E1588-10e1, Standard Guide for Gunshot Residue Analysis by Scanning Electron Microscopy/Energy Dispersive X-ray Spectrometry, 2008, DOI:10.1520/E1588-10E01. www.astm.org.
- R. E. Berk, S. A. Rochowicz, M. Wong and M. A. Kopina, J. Forensic Sci., 2007, 52, 838–841 CrossRef.
- M. R. Rijnders, A. Stamouli and A. Bolck, J. Forensic Sci., 2010, 55, 616–623 CrossRef.
- S. Biju, D. B. A. Raj, M. L. P. Reddy, C. K. Jayasankar, A. H. Cowley and M. Findlater, J. Mater. Chem., 2009, 19, 1425–1432 RSC.
- H. M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65–71 CrossRef CAS.
- A. C. Larson, R. B. Von Dreele, General Structure Analysis System (GSAS), Los Alamos ational Laboratory, Los Alamos, 2004 Search PubMed.
- B. H. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef CAS.
- A. Fernandes, J. Jaud, J. Dexpert-Ghys and C. Brouca-Cabarrecq, Polyhedron, 2001, 20, 2385–2391 CrossRef CAS.
- M. Jarvinen, J. Appl. Crystallogr., 1993, 26, 525–531 CrossRef CAS.
- R. A. Young and P. Desai, Archiwun Nauki o Materialach, 1989, 42, 71–90 Search PubMed.
- G. M. Salley, R. Valiente and H. U. Gudel, Phys. Rev. B: Condens. Matter, 2003, 67, 134111–1-134111-9 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, crystallographic structures, X-ray powder patterns with final Rietveld fits, primers and target images under UV irradiation are included in the supporting information. See DOI: 10.1039/c2ra01214f |
| This journal is © The Royal Society of Chemistry 2012 |
