A new restriction effect of aging time on the shrinkage of ordered mesoporous carbon during carbonization
Bo
You
,
Zhidan
Zhang
,
Lili
Zhang
,
Jun
Yang
*,
Xiaolan
Zhu
and
Qingde
Su
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, China. E-mail: yjun8202@ustc.edu.cn; Fax: +86 551 3492065; Tel: +86 551 3492065
First published on 3rd May 2012
Abstract
A new effect of aging time (AT) on the shrinkage of ordered mesoporous carbon (OMC) prepared by an evaporation-induced triconstituent co-assembly method has been reported. Increasing AT from 0.5 to 2.0 h can gradually reduce framework shrinkage of the OMC during carbonization. This novel effect is effective and simple for preparing the OMC with large mesopore sizes, high pore volumes and surface area.
Ordered mesoporous carbons (OMCs) have received enormous attention owing to their high surface area, regular framework, large pore size with narrow distribution and excellent chemical/thermal stability, all of which lead to multiple potential applications in catalysis, separation and energy storage.1–5
The nanocasting,6 also called hard-template method, has been used extensively to prepare OMCs. Ryoo's group first used ordered mesoporous silica (MCM-48) as the hard template, the pores of which were impregnated with a carbon precursor, and then the composites were carbonized. Removal of the silica scaffold resulted in ordered mesoporous carbon replicating the silica mesochannels.6f This method is a powerful tool for the creation of nanostructured materials. Variable carbon precursors, e.g., phenol resin, sucrose, furfural and alcohol can be utilized. However, an extra step is needed to prepare ordered templates, which makes it costly and fussy.7 Also, the mesostructures and morphologies of the replicated carbon nanoparticles are limited to the parent silica template. More recently, an organic–organic self-assembly strategy has been successfully used to synthesize OMCs.8–10 The essence of such methods is the direct use of the surfactant as the templates (also called soft-template method) for the generation of porous carbon structures without the extra step of generating the silica templating structures. This method is simple and effective because most surfactants are commercially available and inexpensive. Nevertheless, serious framework shrinkage of the prepared OMCs during carbonization results in small mesopore sizes, low pore volumes and small specific surface areas.11 An effective solution for reducing framework shrinkage is the incorporation of a rigid constituent of silicates in OMCs.12 With an increase of Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios, the shrinkage decreases.13 While a change in the Si
C ratios, the shrinkage decreases.13 While a change in the Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio makes the amount of surfactant-template vary and it's not a simple function, giving rise to stringent reaction conditions.11 Therefore, it is imperative to facilely and effectively reduce the framework shrinkage of OMCs during high temperature carbonization.
C ratio makes the amount of surfactant-template vary and it's not a simple function, giving rise to stringent reaction conditions.11 Therefore, it is imperative to facilely and effectively reduce the framework shrinkage of OMCs during high temperature carbonization.
Herein, we report for the first time a new phenomenon whereby simply prolonging the AT of the triconstituent from 0.5 to 2.0 h can gradually reduce framework shrinkage of the OMCs prepared using an evaporation-induced triconstituent co-assembly method. This novel effect is effective and simple for preparing the OMCs with large mesopore sizes, high pore volumes and surface areas. The samples were prepared according to our previous reports with some modification (Scheme 1).5 In a typical preparation, 0.8 g of block copolymer F127 and 0.5 g of 0.2 M HCl were dissolved in 4.0 g ethanol and stirred for 1 h at 40 °C. Next, 1.04 g of TEOS and 2.5 g of 20 wt% resols’ ethanol solution were added in sequence. After stirring for 2.0 h (AT), the mixture was transferred into dishes. It took 8 h at room temperature to evaporate the ethanol and 24 h at 100 °C in an oven to thermopolymerize (named “as-made” samples). Then the products were scraped from the dishes. Calcination was carried out in a tubular furnace at 850 °C for 5 h under N2 flow to get ordered mesoporous carbon–silica nanocomposites, named as OMCS-2.0. “OMCS-x” denotes the ordered mesoporous carbon–silica nanocomposite samples, wherein x represents AT. After the carbon–silica nanocomposites were immersed in 10 wt% HF solutions for 24 h, the silica was removed and mesoporous carbon was left. We can get the various OMCs through simply varying AT, named as OMC-x.
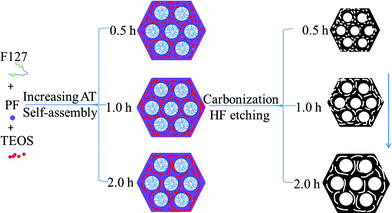 | ||
| Scheme 1 Illustration the restriction effect of aging time (AT) on the shrinkage of OMCs during carbonization. | ||
Calcination at 550 °C for 5 h in air was found to burn off carbons and generate mesoporous silica materials, named as OMS-x. These materials were characterized by XRD, TEM and N2 sorption techniques.
Fig. 1 shows the small-angle XRD patterns of the OMCs with different ATs. All the OMCs exhibit one intense and two weak diffraction peaks, associated with (10), (11), (20) reflections of two-dimensional hexagonal symmetry with the space group of p6mm.5b,11 It can be seen that the (10) peaks shift to low angle gradually along with increasing AT, implying that the cell parameter increases. The evolution of cell parameter with AT is shown in Table 1. This phenomenon clearly demonstrates that increasing aging time from 0.5 h to 2.0 h can efficiently reduce the framework shrinkage of the OMCs (further prolonging the AT, phase separation occurs). Moreover, the (10) diffraction peaks become narrow gradually with increasing AT, suggesting that the ordering of the three OMCs increases.14 While, even if the AT = 0.5 h, the cell parameter of OMC-0.5 is larger than C-FDU-15 with the same p6mm symmetry but without silicates inside the framework after heating treatment at 850 °C,5b This demonstrates that the presence of silica in the nanocomposite can efficiently reduce the framework shrinkage, which is in accordance with the previous reports.5
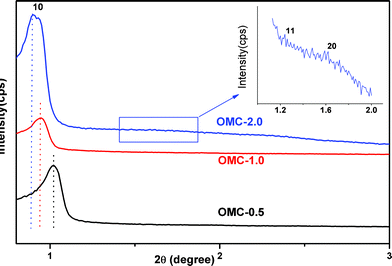 | ||
| Fig. 1 Small-angle XRD patterns of the OMCs with different AT, (black) OMC-0.5, (red) OMC-1.0 and (blue) OMC-2.0. | ||
| Sample | a0a | D b | W c | S BET d | V t e |
|---|---|---|---|---|---|
| a a0, the cell parameter (nm), was calculated using the formula a0 = 2d/√3. b D is the pore diameter (nm). c W the pore wall thickness (nm), was calculated using the formula W = a0 − D.15 d S BET is the BET surface area (m2 g−1). e V t is the total pore volume (cm3 g−1) . | |||||
| OMC-0.5 | 9.7 | 5.4 | 4.3 | 1123 | 1.55 |
| OMC-1.0 | 10.9 | 6.4 | 4.5 | 1349 | 1.82 |
| OMC-2.0 | 11.3 | 6.6 | 4.7 | 2280 | 1.93 |
TEM images of the three OMCs show large domains of ordered stripe-like arranged arrays (Fig. 2), indicating their highly ordered mesostructure.11 Their surfaces are uneven, indicative of many complementary pores that caused by many voids on the walls after etching of silica frameworks. The stripe-like arrange images (Fig. 2a) of the OM-C-0.5 are curved, which is ascribed to the asymmetric framework shrinkage during high temperature carbonization. By further increasing the AT, the stripe-like arranged images become more and more straight (Fig. 2b and 2c), suggesting the asymmetric shrinkage decreases and the ordering of the OMCs improves, which coincides with the small angle XRD patterns.
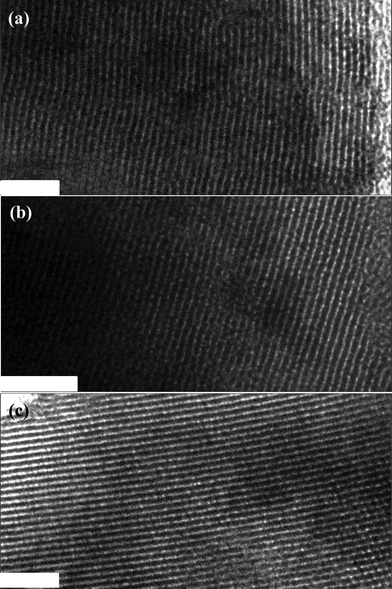 | ||
| Fig. 2 TEM images of the OMCs with different AT, (a) OMC-0.5, (b) OMC-1.0 and (c) OMC-2.0. Scale bar, 80 nm. | ||
Similar phenomenon can be observed from the N2 sorption isotherms (Fig. 3a) and the pore size distributions (Fig. 3b). The N2 sorption isotherms (Fig. 3a) of the three OMCs exhibit type-IV curves with sharp capillary condensation steps in the P/P0 range from 0.6 to 0.8, and obvious H1-type hysteresis loops which are typical of mesoporous materials with a cylindrical channel.14 With increasing the AT, the hysteresis loop shifts to high P/P0 slightly, implying that the primary pore increases gradually. Moreover, the increased adsorption at the relative pressure (P/P0) of 0.1–0.3 suggest that more and more small pores are formed,11 which is consistent with the TEM images. The increased BET surface areas (from 1123 m2 g−1 to 2280 m2 g−1) and pore volumes (from 1.55 cm3 g−1 to 1.93 cm3 g−1) also imply the increase of small pores (Table 1). Fig. 3b shows the BJH pore size distribution curves. The average mesopore sizes for the three OMCs are about 5.4, 6.4, and 6.6 nm, respectively, which indicate that the mesopore size of the OMCs slightly increases with increasing AT. This can be explained that prolonging the AT from 0.5 to 2.0 h increases the polymerization and cross-linking degree of the silicates. Then, large domain silica aggregations in the carbon well can be obtained, which can efficiently reduce the asymmetric framework shrinkage and hence the cell parameter (a0) and the wall thickness (W) of the resultant sample increase simultaneously (Table 1), so the difference between a0 and W (pore diameter, D = a0 − W15) is not so obvious.
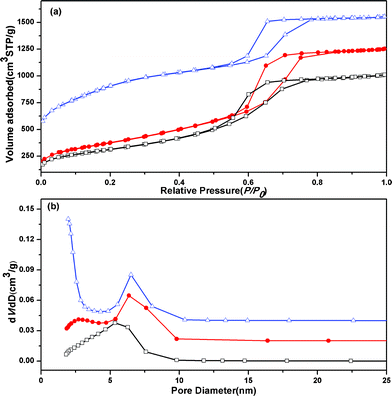 | ||
| Fig. 3 (a) N2 sorption isotherms and (b) pore size distribution curves of OMCs with different AT, (black) OMC-0.5, (red) OMC-1.0 and (blue) OMC-2.0. | ||
To further investigate the effect of AT on framework shrinkage of the resultant OMCs, we also synthesized the mother ordered mesoporous carbon–silica nanocomposites of the three OMCs. The small angle XRD patterns (Fig. 4a) of all the OMCs show three diffraction peaks at a 2θ range of 0.5 to 2° that can be indexed as (10), (11), (20) reflections,11 suggesting their highly ordered hexagonal mesosturcture. By increasing the AT from 0.5 h to 2.0 h, the (10) diffraction peak of the OMCs shifts to a lower angle and became sharp gradually, suggesting that the cell parameter is increased and the mesostructure regularity is improved gradually. This phenomenon is similar to that of the OMCs.
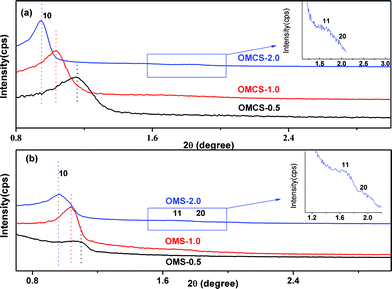 | ||
| Fig. 4 Small-angle XRD patterns of (a) the OMCs and (b) the OMCs with different AT, (black) 0.5 h, (red) 1.0 h and (blue) 2.0 h. | ||
We propose that prolonging the AT from 0.5 to 2.0 h increases the polymerization and cross-linking degree of silicates. Then, large domain silica aggregations in carbon walls can be obtained,11 which can efficiently reduce the asymmetric framework shrinkage, improve the mesostructural regularity of the carbon–silica nanocomposites and corresponding OMCs and make the resulting OMCs have larger mesopores (Scheme 1). Meanwhile, after etching silica, the voids interpenetrated with carbon walls become more and more large, which results in an uneven surface, many complimentary pores and finally an increase of the BET surface area of the OMCs.
To underpin our proposal, we also prepared the ordered mesoporous silica (OMS-x) by calcination of the carbon–silica nanocomposites at 550 °C for 5 h in air. The small angle XRD patterns of the three OMS-x samples (Fig. 4b) exhibit one intense and two weak diffraction peaks. Those can be indexed as (10), (11), (20) reflections, indicating that the three OMS-x samples have a high degree of hexagonal mesoscopic organization.11,14 With a prolonged AT, the (10) diffraction peak of the OMS-x shifts to a lower angle, implying the framework shrinkage is reduced gradually. This observation is ascribed to the increased polymerization and cross-linking degree of silicates with increasing aging time,16 which validates our proposal.
In summary, the effect of aging time on the shrinkage of ordered mesoporous carbon (OMC) prepared by an evaporation-induced triconstituent co-assembly method has been presented in detail. The results show that increasing the aging time of the triconstituent from 0.5 to 2.0 h can gradually reduce framework shrinkage of the OMCs during high temperature carbonization. This novel restriction effect provides a new approach for the synthesis of mesoporous carbons with large mesopore sizes, high pore volumes, and large mesopore surface areas. Furthermore, this method is simple and applicable to other mesoporous materials which are prone to shrink during high temperature carbonization.
This work was financially supported by the fundamental research fund for the central universities (WK2061020001).
References
- S. H. Joo, S. J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki and R. Ryoo, Nature, 2001, 412, 169 CrossRef CAS.
- (a) G. Gupta, D. A. Slanac, P. Kumar, J. D. Wiggins-Camacho, J. Kim, R. Ryoo, K. J. Stevenson and K. P. Johnston, J. Phys. Chem. C, 2010, 114, 10796 CrossRef CAS; (b) Y. Han, D. L. Zhang, L. L. Chng, J. L. Sun, L. Zhao, X. D. Zou and J. Y. Ying, Nat. Chem., 2009, 1, 123 CrossRef CAS; (c) H. Q. Qin, P. Gao, F. J. Wang, L. Zhao, J. Zhu, A. Q. Wang, T. Zhang, R. A. Wu and H. F. Zou, Angew. Chem., Int. Ed., 2011, 50, 1 CrossRef.
- (a) G. J. A. A. Soler-Illia and O. Azzaroni, Chem. Soc. Rev., 2011, 40, 1107 RSC; (b) M. Inagaki, H. Orikasa and T. Morishita, RSC Adv., 2011, 1, 1620 RSC; (c) C. C. Ting, Y. C. Pan, S. Vertrivel, D. Saikia and H. M. Kao, RSC Adv., 2012, 2, 2221 RSC.
- (a) D. Feng, Y. Y. Lv, Z. X. Wu, Y. Q. Dou, L. Han, Z. K. Sun, Y. Y. Xia, G. F. Zheng and D. Y. Zhao, J. Am. Chem. Soc., 2011, 133, 15148 CAS; (b) J. G. Li and S. W. Kuo, RSC Adv., 2011, 1, 1822 RSC.
- (a) B. You, J. Yang, Y. Q. Sun and Q. D. Su, Chem. Commun., 2011, 47, 12364 RSC; (b) B. You, J. Yang, G. P. Yong, S. M. Liu, W. Xie and Q. D. Su, Chin. J. Chem. Phys., 2011, 24, 365 CrossRef CAS; (c) N. Wickramaratne and M. Jaroniec, RSC Adv., 2012, 2, 1877 RSC.
- (a) Z. Sarshar, F. Kleitz and S. Kaliaguine, Energy Environ. Sci., 2011, 4, 4258 RSC; (b) P. N. Li, X. L. Hu, L. Zhang, H. X. Dai and L. Z. Zhang, Nanoscale, 2011, 3, 974 RSC; (c) L. C. Sang, A. Vinu and M. O. Coppens, J. Mater. Chem., 2011, 21, 7410 RSC; (d) C. Sanchez, P. Belleville, M. Popall and L. Nicole, Chem. Soc. Rev., 2011, 40, 696 RSC; (e) Y. F. Shi, Y. Wan and D. Y. Zhao, Chem. Soc. Rev., 2011, 40, 3854 RSC; (f) R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743 CrossRef CAS; (g) K. M. Choi and K. Kuroda, Chem. Commun., 2011, 47, 10933 RSC; (h) D. H. Choi and R. Ryoo, J. Mater. Chem., 2010, 20, 5544 RSC; (i) J. Changbum, K. Kyoungsoo and R. Ryoo, Microporous Mesoporous Mater., 2009, 124, 45 CrossRef.
- (a) A. H. Lu and F. Schüth, Adv. Mater., 2006, 18, 1793 CrossRef CAS; (b) Y. Fang, D. Gu, Y. Zou, Z. X. Wu, F. Y. Li, R. C. Che, Y. H. Deng, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2010, 49, 7987 CrossRef CAS; (c) Y. Wang, J. S. Zhang, X. C. Wang, M. Antonietti and H. R. Li, Angew. Chem., Int. Ed., 2010, 49, 3356 CrossRef CAS; (d) Z. W. Wu, J. B. Pang and Y. F. Lu, Nanoscale, 2009, 1, 245 RSC.
- (a) D. Gu, H. Bongard, Y. H. Deng, D. Feng, Z. X.Wu, Y. Fang, J. J. Mao, B. Tu, F. Schüth and D. Y. Zhao, Adv. Mater., 2010, 22 Search PubMed; (b) C. Z. Yuan, B. Gao, L.F. Shen, S. D. Yang, L. Hao, X. J. Lu, F. Zhang, L. J. Zhang and X. G. Zhang, Nanoscale, 2011, 3, 529 RSC.
- (a) J. Gorka, M. Jaroniec and W. L. Suchanek, Nanoscale, 2010, 2, 2868 RSC.
- B. D. Vogt, V. L. Chavez, M. Z. Dai, M. R. C. Arreola, L. Y. Song, D. Feng, D. Y. Zhao, G. M. Perera and G. E. Stein, Langmuir, 2011, 27, 5607 CrossRef CAS.
- R. L. Liu, Y. F. Shi, Y. Wan, Y. Meng, F.Q. Zhang, D. Gu, Z. X. Chen, B. Tu and D. Y. Zhao, J. Am. Chem. Soc., 2006, 128, 11652 CrossRef CAS.
- Y. Wei, D. L. Jin, C. C. Yang and G. Wei, J. Sol-Gel Sci. Technol., 1996, 7, 191 CrossRef CAS.
- C. F. Xue, B. Tu and D. Y. Zhao, Adv. Funct. Mater., 2008, 18, 3914 CrossRef CAS.
- D. H. Pan, P. Yuan, L. Z. Zhao, N. Liu, L. Zhou, G. F. Wei, J. Zhang, Y. C. Ling, Y. Fan, B. Y. Wei, H. Y. Liu, C. Z. Yu and X. J. Bao, Chem. Mater., 2009, 21, 5413 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chemlka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS.
- Q. Wei, Z. R. Nie, Y. L. Hao, L. Liu, Z. X. Chen and J. X. Zou, J. Sol-Gel Sci. Technol., 2006, 39, 103 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
