NCP pincer palladacycle as a phosphine-free catalyst precursor for the Heck–Mizoroki coupling of aryl halides†
Gilber R.
Rosa
*a and
Diego S.
Rosa
b
aSchool of Chemistry and Food, FURG, R. Barão do Caí, 125, Santo Antônio da Patrulha, CEP 95500-000, RS, Brazil. E-mail: gilberrosa@furg.br; Fax: + 55 51 3662-6206
bLaboratory of Molecular Catalysis, Institute of Chemistry, UFRGS, Av. Bento Gonçalves, 9500 Porto Alegre, CEP 91501-970, RS, Brazil. E-mail: diegosrosa@yahoo.com.br; Fax: + 55 51 3308-6321
First published on 13th April 2012
Abstract
NCP pincer palladacycle 1 has been reported as an excellent catalyst precursor for the Suzuki–Miyaura reaction. Now, it’s properties have been evaluated in the Heck reaction. 1 is highly active for the coupling of ArI, ArBr and electron-poor ArCl with n-butyl acrylate showing limitations in the reaction of deactivated ArCl and sterically hindered olefins.
Palladium-promoted C–C cross-coupling reactions are one of the most important and investigated classes of organometallic reactions.1 These catalytic processes display several advantages when compared to the more classical organometallic methods such as Grignard reactions or other stoichiometric routes. As an example of the catalytic advantages, cross-coupling reactions have a larger functional group tolerance when aromatic rings and mild reaction conditions are employed.
The Heck–Mizoroki coupling is one of the most studied reactions in C–C bond formation between alkenes and aromatic rings.2,3 The industrial applications of this reaction can be observed in fine chemical fields, such as pharmaceuticals and herbicides.4 For example: (i) the sulfonyl urea herbicide Prosulforon™ is produced on a large scale with a process developed by Ciba-Geigy,1a,4b the key step is a Heck reaction, where a diazonium salt generates an arylpalladium intermediate, which couples with the olefin; (ii) a key step in the production of Singulair™ (antiasthma drug) is the use of the Heck reaction of methyl 2-iodo-benzoate on allylic alcohol.4c
The coupling of alkenes with aryl halides can be accomplished using a plethora of palladium catalyst precursors under various reaction conditions.5 Among these palladium sources, palladacycles have emerged as favourites, particularly due to their unique properties including their facile synthesis, their thermal stability and the possibility to readily modulate their steric and electronic properties.6 They have CP, CN, CS, PCP, SCS, NCN and NCP types7 in their molecular structures and they are able to have rings with 5 and 6 members.
Our group has developed several palladacycles (Fig. 1), including pincer NCP 1, a phosphinite palladacycle with an aliphatic backbone that is able to perform the cross-coupling of aryl chlorides with arylboronic acids via the Suzuki–Miyaura reaction.
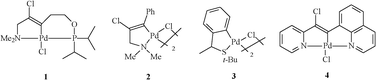 | ||
| Fig. 1 Palladacycles developed by our group. | ||
Other groups8–11 have worked with many phosphine-free palladium sources and have tested them in the Suzuki and Heck coupling reactions (Fig. 2).
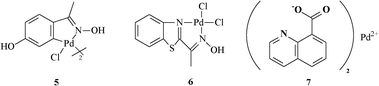 | ||
| Fig. 2 Other palladium sources tested in the Heck and Suzuki coupling reactions. | ||
The trend in working with phosphine-free catalytic systems lies in the high cost of producing tertiary (especially chiral) phosphines and their degradative tendency to converting to phosphine oxides, usually requiring high catalyst loadings.6e
With respect to the Suzuki coupling, 1 is certainly one of the most efficient catalysts, owing to its ability to promote the cross-coupling of both deactivated aryl chlorides and sterically hindered bromoarenes in high yields.7a,7b,7c However, the effectiveness of 1 in the Heck coupling reaction has not been tested.
The other palladium sources shown in Fig. 1 and 2 were tested in the Heck and the Suzuki coupling reactions. The results showed that 2 and 4 were excellent catalysts for the Heck reaction with acrylates and that 2 performed particularly well with activated aryl chlorides.7d No reaction was observed for the Suzuki coupling between 2-bromomesitylene and 2-tolylboronic acid. Good yields (60–80%) were achieved using 37e,7f and 710 for the Heck coupling of 4-bromoanisole and acrylates. Both 3 and 7 were also excellent for the Suzuki reaction between 4-bromoanisole and phenylboronic acid. Low yields (40%) were obtained with the use of 5 for the Heck reactions of 4-bromoanisole with acrylates8a and 5 performed slightly better in the Suzuki coupling, promoting the cross-coupling of 4-chloroanisole with phenylboronic acid in modest yield (57%).8b In the reactions with activated aryl chlorides, 6 was found to promote the Heck coupling with acrylates in good yields (82%), but was unable to promote the Suzuki reaction with phenylboronic acid.9 A Pd(OAc)2–1-phenylurea system has been described in the literature to provide high yields for the Heck coupling with activated aryl chlorides; however, the same has not been disclosed for the Suzuki reaction.11
As described previously, the discovery of a palladium source that is efficient for both the Heck and the Suzuki cross-coupling reactions with aryl chlorides is highly desired.
Herein, we describe the optimum reaction conditions for the phosphine-free Heck coupling of aryl halides with n-butyl acrylate and styrene using palladacycle 1 as the catalyst precursor.
The coupling reaction of the relatively unactivated 4-bromoanisole with n-butyl acrylate that is catalysed by palladacycle 1, was selected as a model system (Scheme 1).
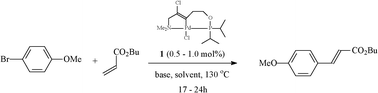 | ||
| Scheme 1 The model Heck coupling that was chosen for the optimisation of the catalytic system with palladacycle 1. | ||
DMF (N,N-dimethylformamide) and Na2CO3 were found to be the most effective solvent and base of those evaluated. NaOAc, K3PO4 and CsF could be used as bases; however, they were found to provide slightly lower yields. Reactions using NEt3 and DABCO (1,4-diazobicyclo[2.2.2]octane) provided very low conversions (Table 1). The bases and solvents were chosen from previous work that supported our investigation.7d,7e
| Entry | [Pd]/mol (%) | Base | Solvent | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a No NBu4Br. b 0.003 mmol of PPh3. The isolated yield is in parenthesis. | |||||
| 1 | 1/0.2 | NaOAc | DMA | 20 | 60 |
| 1/1.0 | 39 | ||||
| 2 | 1/0.2 | NaOAc | DMF | 20 | 57 |
| 1/0.5 | 34 | ||||
| 3 | 1/0.2 | K3PO4 | DMA | 20 | 40 |
| 4 | 1/0.2 | K3PO4 | DMF | 20 | 50 |
| 5 | 1/0.2 | CsF | DMF | 20 | 40 |
| 6 | 1/0.2 | NEt3 | DMF | 24 | 27 |
| 7 | 1/0.2 | DABCO | DMF | 20 | 12 |
| 8 | 1/0.2 | Na2CO3 | DMF | 17 | 50 |
| 1/0.5 | 98 (90) | ||||
| 9 | 1/0.2 | Na2CO3 | DMF | 17 | 40a |
| 10 | Pd(OAc)2/0.5 | Na2CO3 | DMF | 17 | 19b |
| 11 | Pd(OAc)2/0.5 | Na2CO3 | DMF | 17 | 26 |
Based on the results outlined in Table 1, we can see that the best result (entry 8) is obtained with the use of Na2CO3 as the base and DMF as the solvent. Repeating the same reaction in the absence of NBu4Br (entry 9), the reaction yield was lowered by 10%, when using 0.2 mol% of palladacycle 1. Changing the palladium source to 0.5 mol% of Pd(OAc)2 with and without PPh3 as a ligand (entries 10 and 11), the yield decreased by a factor of four. High amounts of palladacycle 1 are unfavourable for the Heck reaction when using NaOAc as the base and DMA (N,N-dimethylacetamide) or DMF as the solvent (entries 1 and 2).
Following the optimisation of the catalytic system for the Heck reaction, we have performed the cross-coupling reactions using different aryl halides and n-butyl acrylate (Table 2).
| Entry | Ar–X | 1 (mol%) | Time (h) | Yield (%) |
|---|---|---|---|---|
| a GC yield. b Room temperature. | ||||
| 1 | 4-O2NC6H4I | 0.2 | 70 | 60a,b |
| 18 | 95 | |||
| 2 | 4-H2NC6H4I | 0.2 | 18 | 93 |
| 3 | 4-MeCOC6H4I | 0.2 | 18 | 96 |
| 4 | PhI | 0.2 | 20 | 97 |
| 5 | 4-MeOC6H4I | 0.2 | 23 | 95 |
| 6 | 4-MeOC6H4Br | 0.5 | 17 | 90 |
| 7 | PhBr | 0.5 | 17 | 99 |
| 8 | 4-O2NC6H4Br | 0.5 | 20 | 98 |
| 9 | 4-MeC6H4Br | 0.5 | 22 | 90 |
| 10 | 3-F3CC6H4Br | 0.5 | 18 | 95 |
| 11 | 2-MeCOC6H4Br | 0.5 | 25 | 90 |
| 12 | 4-NCC6H4Br | 0.5 | 16 | 91 |
| 13 | 2-MeC6H4Br | 0.5 | 23 | 93 |
| 14 | 2,5-MeC6H4Br | 0.5 | 30 | 70a |
| 15 | 4-NCC6H4Cl | 0.5 | 68 | 40a |
| 1.0 | 23 | 84 | ||
| 16 | 4-O2NC6H4Cl | 1.0 | 23 | 94 |
| 17 | 4-MeC6H4Cl | 1.0 | 68 | 0 |
| 18 | 4-MeOC6H4Cl | 1.0 | 68 | 0 |
Excellent yields are obtained for the coupling of the more reactive aryl iodides and a wide variety of functional groups were tolerated on the aromatic ring when using smaller quantities of 1 than that required with the chloro- and bromoarenes (Table 2). The coupling reaction of p-nitro-iodobenzene (entry 1) occurs at room temperature; however, a long reaction time is needed because the NCP pincer 1 requires high temperatures (100–130 °C) to reach full activity. It is believed that the catalytically active species is the product of the partial dechelation of NCP pincer 1, however, further investigations need to be carried out for such information.6e
Table 2 also shows that both electron-rich and electron-poor aryl bromides are efficiently coupled in the presence of palladacycle 1, furnishing the cross-coupled products with excellent yields. A wide variety of functional groups are tolerated in the coupling reactions with aryl bromides. As the main goal of our efforts with the Heck catalytic system is to work with aryl chlorides because they are the cheapest aryl halides, various reactions using different chloroarenes have been performed and very good yields have been obtained with electron-poor aryl chlorides (entries 15 and 16) using 1 mol% of 1. Electron-rich compounds, on the other hand, have been found to be unsuitable partners in this context, even with extended reaction times (entries 17 and 18).
In our previous work on the Suzuki cross-coupling reaction, we found that 1 was able to promote the coupling reaction of sterically hindered substrates with three methyl groups in the ortho position (Scheme 2). As a result, we decided to look at the ability of 1 to generate hindered Heck products with different olefin substrates (Table 3). Unfortunately, the catalytic system proved to be limited in scope as the more hindered olefins (entries 2 and 3) did not couple, even with the use of iodoarenes (entry 4). In both electron-rich aryl chlorides and sterically hindered substrates, 1 was inefficient for the Heck cross-coupling, being disabled with the generation of Pd(0) metallic. These results clearly indicate that the olefin coordination step is much more sensitive to steric effects than the oxidative addition step in the classical Heck coupling mechanism.
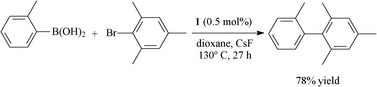 | ||
| Scheme 2 Suzuki cross-coupling reaction of sterically hindered substrates catalyzed by 1. | ||
The most accepted mechanism for this step (olefin coordination) suggests that the intermediate derived from the oxidative addition coordinates the olefin, having a rotation to this position in the plane of the Pd–Ar bond, so there is a concerted mechanism to the four centers where the Pd–C bond is broken and a C–C bond is formed. For the Suzuki coupling, 1 proved to be more efficient because the transmetallation step is less sensitive to the ligand.6e
Basic phosphines and other ancillary ligands capable of increasing the electron density on the palladium present in the active species facilitates the oxidative addition of substrates (the limiting step of Suzuki cross-coupling). However, the presence of these ligands may block the active sites, rendering a slower reaction (hampers olefin coordination) resulting in lower yields in Heck cross-coupling
To conduct a preliminary investigation on the catalytically active palladium species, we proceeded with the Hg poisoning test.7d The Hg poisoning test can confirm a homogeneous catalytic system but not a heterogeneous one. Thus, we conducted the reaction of 4-bromoanisole with n-butyl acrylate and observed that the addition of Hg0 (0.5 mmol) with the reagents did not inhibit the catalytic action of 1. Only at the end of 17 h was a decrease in yield (98–80%) seen when compared with the Hg0 free reaction. This negative response to the mercury poisoning test can be taken as confirmation of the presence of the singly ligated active Pd(0) species.
In summary, palladacycle pincer NCP 1 was found to be a highly efficient phosphine-free precatalyst for the Heck reaction of several aryl halides, including activated chloroarenes. Similar to other palladium sources, 1 appeared to have limitations for the Heck coupling of deactivated aryl chlorides with n-butyl acrylate. Sterically hindered olefins also proved to be difficult substrates for the Heck reaction. However, 1 deserves special attention because it is one of the few palladium sources that promotes high yields for both the Heck and the Suzuki cross-coupling reactions with activated aryl chlorides. Our group continues to work to elucidate the steps in the catalytic cycle for the Heck reaction of 1 and it’s applications in agrochemical fields.
Acknowledgements
This work was supported by grants from CNPq, FAPERGS and CAPES (Brazil). Professors Gunter Ebeling, Jairton Dupont and Adriano L. Monteiro (IQ/UFRGS) are thanked for their help in the preparation of the phosphinite ligand and for the use of their laboratories.References
- (a) E. Negishi, Handbook of Organopalladium Chemistry for Organic Synthesis, John Wiley & Sons, New York, USA, 1st edn, 2002 Search PubMed; (b) F. Diederich and A. Meijere, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH Verlag GmbH & Co, Weinheim, Germany, 2nd edn, 2004 Search PubMed; (c) F. Bellina, A. Carpita and R. Rossi, Synthesis, 2004, 15, 2419 Search PubMed; (d) A. Zapf and M. Beller, Chem. Commun., 2005, 431 RSC.
- (a) T. Mizoroki, K. Mori and A. Ozaki, Bull. Chem. Soc. Jpn., 1971, 44, 581 CrossRef CAS; (b) R. F. Heck and J. P. Nolley, J. Org. Chem., 1972, 37, 2320 CrossRef CAS; (c) M. Oestreich, The Mizoroki–Heck Reaction, John Wiley & Sons, Chichester, UK, 1st edn, 2009 Search PubMed.
- (a) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS; (b) V. Farina, Adv. Synth. Catal., 2004, 346, 1553 CrossRef CAS; (c) W. Cabri and I. Candiani, Acc. Chem. Res., 1995, 28, 2 CrossRef CAS; (d) B. Clique, C. H. Fabritius, C. Couturier, N. Monteiro and G. Balme, Chem. Commun., 2003, 272 RSC; (e) B. J. Burke and L. E. Overman, J. Am. Chem. Soc., 2004, 126, 16820 CrossRef CAS; (f) E. M. Beccalli, G. Broggini, M. Martinelli, N. Masciocchi and S. Sottocornola, Org. Lett., 2006, 8, 4521 CrossRef CAS.
- (a) J. G. de Vries, Can. J. Chem., 2001, 79, 1086 CrossRef CAS; (b) P. Baumeister, G. Seifert, H. Steiner (Ciba-Geigy AG), European Patent, EP584043, 1994 Search PubMed; (c) G. Higgs, Chem. Ind., 1997, 827 CAS.
- (a) C. Vadapalli and N. R. Suriya, Tetrahedron Lett., 2011, 27, 3527 Search PubMed; (b) A. T. Hou, Y. J. Liu, X. Q. Hao, J. F. Gong and M. P. Song, J. Organomet. Chem., 2011, 696, 2857 CrossRef CAS; (c) J. D. Blakemore, M. J. Chalkley, J. H. Farnaby, L. M. Guard, N. Hazari, C. D. Incarvito, E. D. Luzik and H. W. Suh, Organometallics, 2011, 30, 1818 CrossRef CAS; (d) E. M. Beccalli, E. Borsini, S. Brenna, S. Galli, M. Rigamonti and G. Broggini, Chem.–Eur. J., 2010, 16, 1670 CrossRef CAS; (e) J. Vicente, I. Saura-Llamas, J. A. García-López and D. Bautista, Organometallics, 2010, 29, 4320 CrossRef CAS; (f) S. Yahiaoui, A. Fardost, A. Trejos and M. Larhed, J. Org. Chem., 2011, 76, 2433 CrossRef CAS; (g) W. Wang, Q. Yang, R. Zhou, H. Y. Fu, R. X. Li, H. Chen and X. J. Li, J. Organomet. Chem., 2012, 697, 1 CrossRef CAS.
- (a) W. A. Herrmann, V. P. W. Bohm and C. P. Reisinger, J. Organomet. Chem., 1999, 576, 23 CrossRef CAS; (b) J. Dupont, M. Pfeffer and J. Spencer, Eur. J. Inorg. Chem., 2001, 1917 CrossRef CAS; (c) R. B. Bedford, Chem. Commun., 2003, 1787 RSC; (d) I. P. Beletskaya and A. V. Cheprakov, J. Organomet. Chem., 2004, 689, 4055 CrossRef CAS; (e) J. Dupont, C. S. Consorti and J. Spencer, Chem. Rev., 2005, 105, 2527 CrossRef CAS; (f) J. Dupont and M. Pfeffer, Palladacycles—Synthesis, Characterization and Applications, Wiley-VCH Verlag GmbH & Co., Weinheim, Germany, 1st edn, 2008 Search PubMed.
- (a) G. R. Rosa, G. Ebeling, J. Dupont and A. L. Monteiro, Synthesis, 2003, 2894 CAS; (b) G. R. Rosa, C. H. Rosa, F. Rominger, A. L. Monteiro and J. Dupont, Inorg. Chim. Acta, 2006, 359, 1947 CrossRef CAS; (c) G. R. Rosa, Quim. Nova, in press Search PubMed; (d) C. S. Consorti, F. R. Flores and J. Dupont, J. Am. Chem. Soc., 2005, 127, 12054 CrossRef CAS; (e) A. S. Gruber, D. Zim, G. Ebeling, A. L. Monteiro and J. Dupont, Org. Lett., 2000, 2, 1287 CrossRef CAS; (f) D. Zim, A. S. Gruber, G. Ebeling, J. Dupont and A. L. Monteiro, Org. Lett., 2000, 2, 2881 CrossRef CAS.
- (a) L. Botella and C. Nájera, J. Org. Chem., 2005, 70, 4360 CrossRef CAS; (b) D. A. Alonso, J. F. Cívicos and C. Nájera, Synlett, 2009, 3011 CrossRef CAS; (c) D. A. Alonso and C. Nájera, Chem. Soc. Rev., 2010, 39, 2891 RSC.
- K. M. Dawood, Tetrahedron, 2007, 63, 9642 CrossRef CAS.
- X. Cui, J. Li, Z. P. Zhang, Y. Fu, L. Liu and Q. X. Guo, J. Org. Chem., 2007, 72, 9342 CrossRef CAS.
- X. Cui, Y. Zhou, N. Wang, Y. Fu, L. Liu and Q. X. Guo, Tetrahedron Lett., 2007, 48, 163 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: General procedures for the Heck reactions of aryl halides with olefins catalyzed by palladacycle 1 and characterization data of products. See DOI: 10.1039/c2ra01261h/ |
| This journal is © The Royal Society of Chemistry 2012 |