Surface functionalization of Aspergillus versicolor mycelia: in situ fabrication of cadmium sulphide nanoparticles and removal of cadmium ions from aqueous solution
Sujoy K.
Das
*ab,
Ishita
Shome
a and
Arun K.
Guha
a
aDepartment of Biological Chemistry, Indian Association for the Cultivation of Science, Kolkata, 700 032, India
bEnvironmental Technology Division, Council of Scientific and Industrial Research (CSIR)-Central Leather Research Institute (CLRI), Chennai, 600 020, India. E-mail: sujoy@clri.res.in; Fax: +914424916351; Tel: +914424437132
First published on 18th January 2012
Abstract
Xanthate functionalization of Aspergillus versicolor mycelia (AVM) was carried out to synthesize cadmium sulphide (CdS) nanoparticles and for the removal of cadmium ions from aqueous solution. The synthesized nanoparticles were characterized by spectroscopic and microscopic techniques. Fourier transform infrared (FTIR) spectroscopy and elemental detection X-ray analysis (EDXA) results confirmed the binding of cadmium with sulphur groups of the functionalized mycelia. Scanning electron and atomic force microscopic studies revealed alteration of surface morphology following binding of cadmium, while high resolution transmission electron microscopy (HRTEM) and fluorescence micrographs demonstrated formation of CdS nanoparticles on AVM surface. Formation of 3.0 ± 0.2 nm size CdS nanoparticles was confirmed from HRTEM images. The maximum adsorption capacity of the functionalized mycelia for Cd+2 was enhanced to 141.5 mg g−1 from the corresponding value of 70.5 mg g−1 for pristine mycelia. An increase in adsorption capacity was attributed to cadmium binding affinity of sulfur atoms due to soft acid–base reaction and supported by a −ΔG value. The experimental results thus suggest that xanthate functionalization of AVM provides a feasible approach for CdS nanoparticle synthesis and also for efficient removal of heavy metal ions.
Introduction
Surface and interfaces play an important role in many areas of research ranging from nanoscience to environmental technology. In recent years, template directed synthesis of nanoscale materials has found potential applications in molecular electronics, photocatalysis, solar energy conversion, and active electronic devices.1,2 Moreover, a three dimensional dispersion of nanomaterials fabricated on template molecules increases the accessibility for catalytic reaction. Utilization of template molecules in the fabrication of nanomaterials are currently being explored in a number of systems like silica, metal oxide, aluminum hydroxide-coated phospholipid tubules, polymers, ceramics, cellulose, carbon nanotube, etc.3–7 Among these, biological materials have gained much interest to modulate the growth of a large variety of inorganic nanoparticles including metal, semiconductor and magnetic particles. These biological materials are useful because of their specific properties, such as precise molecular recognition and the spatial organization that they impart on the growth of nanoparticles through specific binding affinities, nucleation and assembly.8–11 Besides, biological fibers as scaffolds also allow the manipulation of size, shape and even packing density of nanoparticles.12 Chemical modification of a biomolecular scaffold with functional molecules has therefore emerged as an attractive and practicable way to rationally tailor the properties of the scaffolds in current years.13,14 It creates preferential binding sites to nucleate and organize nanoparticles on the surface. Meldrum and Seshadri reported 15 nm porous gold nanostructures synthesis on skeletal plates of echinoids (sea urchins) as templates.5 He et al.6 demonstrated the formation of porous and nonporous silver nanostructures using cellulose fibers as the template. The controlled interaction between surface functional groups and nanoparticles yield a complex form of higher order hybrid assemblies. Despite numerous reports on metal nanoparticles assembling on biomaterials, very few reports are available on the synthesis of semiconductor nanoparticles. Among various nanoparticles, the template directed synthesis of cadmium sulphide (CdS) nanoparticles has gained considerable attention in current research due to its size-dependent tunable spectroscopic properties.15–17 We therefore, have attempted to functionalize the surface of fibrilar fungal mycelia by covalently linking xanthate groups for synthesis of CdS nanoparticles and removal of these metal ions from water.In the context of environmental science the surface and interface also plays a crucial role. The most commonly used techniques for the removal of heavy metals like cadmium from water bodies are lime precipitation, ion exchange, ultrafiltration and reverse osmosis. But these techniques suffer from limitations like high operating cost, incomplete precipitation, and generation of a huge amount of metal-bearing toxic sludge. Adsorption is a recently developed technique for metal removal, but lack of affinity and inadequate uptake capacity of the adsorbent materials requires a long time to reach equilibrium.18,19 Surface functionalization of adsorbents with suitable functional group is believed to increase the uptake capacity and also increase the affinity of the adsorbents for the desired metal ions and hence improve their performance. Therefore, surface functionalization of adsorbent has practical significance in efficient removal of metal ions. Among different functional groups, xanthate functionalization is usually preferred due to their easy preparation procedures, low solubility products and high stability constant values of the metal complexes formed.20 In this manuscript we explored in situ synthesis of CdS nanoparticles on fibrilar Aspergillus versicolor mycelia (AVM) through surface functionalization by xanthate modification. Moreover, the functionalized mycelia exhibited high cadmium binding capacity compared to the pristine mycelia. We therefore, strongly believe that in situ synthesis of CdS nanoparticles and removal of cadmium ions from aqueous solution by surface functionalization has significant practical implication in terms of nanoparticles synthesis and bioremediation of environmental pollutants.
Experimental section
Chemicals
Cd(NO3)2,4H2O, was purchased from Merck, Germany. Microbiological media were procured from Himedia, India. All other reagents were of analytical reagent grade and purchased from E-Merck, India.Metal solution and analysis
Aqueous solutions (1000 mg L−1) of cadmium were prepared by dissolving the required amount of Cd(NO3)2,4H2O in double distilled water and diluted to get the desired concentration. Concentration of the cadmium was measured by atomic adsorption spectrometer (Varian Spectra AA 55) using the respective standard solution.Synthesis of CdS nanoparticles
Functionalized 0.2 g of A. versicolor was treated with 25 mL Cd+2 solution (500 mg L−1) under shaking condition for 24 h at 30 °C. After 24 h, the mycelia were collected by centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min and dispersed in ultrapure water by sonication followed by filtration to remove large mycelium. UV-vis spectroscopic measurement of the dispersed solution was then recorded on a Varian Carry 50 Bio spectrophotometer. The control experiment was performed under identical condition excepting without addition of AVM.
000 rpm for 15 min and dispersed in ultrapure water by sonication followed by filtration to remove large mycelium. UV-vis spectroscopic measurement of the dispersed solution was then recorded on a Varian Carry 50 Bio spectrophotometer. The control experiment was performed under identical condition excepting without addition of AVM.
Characterization of as synthesized CdS
The synthesis of CdS on AVM was characterized by JEOL JSM 6700F field emission scanning electron microscope equipped with an energy dispersive X-ray spectrometer (FESEM-EDAX). Samples were coated with platinum before FESEM-EDAX analysis. The atomic force microscopy (AFM) images were recorded on a multimode AFM (Veeco Metrology, Autoprobe CP-II, Model No AP0100). The sample was prepared as described by Das et al.23 In brief, the functionalized AVM, before and after treatment with cadmium ions, were incubated the with an ultrasonically cleaned glass cover slip for 60 min, followed by repeated washing with ultrapure Millipore water (18.2 MΩ) to remove loosely attached AVM. The cover slip was then mounted for AFM study. Imaging in air at ambient conditions (20 ± 2 °C) was carried out using silicon probes (RTESPA-M, Veeco, Santa Barbara, CA) and in tapping mode for minimizing sample damage by the scanning tip. The cantilever used had long tips (aspect ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with spring constants ranging from 20 to 80 N m−1 and resonance frequencies of 245–285 kHz. The mycelium was scanned in both front and back directions several times before capturing an image to ensure minimal effects of non-linearity, such as hysteresis.
1) with spring constants ranging from 20 to 80 N m−1 and resonance frequencies of 245–285 kHz. The mycelium was scanned in both front and back directions several times before capturing an image to ensure minimal effects of non-linearity, such as hysteresis.
For HRTEM images, samples were prepared by drop-casting methodology. The dispersed solution of functionalized AVM after treatment with cadmium was drop casted on a carbon coated copper grid and then micrographs were recorded on a JEOL JEM 2010 high resolution transmission electron microscope operated at 200 kV. FTIR spectra of the samples were taken with Shimadzu FTIR Spectrometer under ambient condition. Pressed pellets were prepared by grinding the powder specimens with spectroscopic grade KBr with a sample/KBr ratio ∼1/100 in an agate mortar. The FTIR spectra were recorded with 500 scans at a resolution of 2 cm−1. The fluorescence microscopy images of cadmium treated functionalized AVM were recorded on a fluorescence microscope (Olympus BX-61) using an excitation filter of BP460–495 nm and a band absorbance filter covering wavelengths below 505 nm. The samples were excited with a 50 W mercury lamp. Fluorescent microscopy images of several randomly selected sites were captured with a digital camera connected to the microscope.
Adsorption experiment
Adsorption experiments were conducted in a batch process in 100 mL Erlenmeyer flasks to study the uptake capacity of functionalized mycelia. Effects of pH, kinetics and concentration were studied. The optimum pH for adsorption was determined by suspending 4 g L−1 pristine or functionalized A. versicolor mycelia in 100 mL Erlenmeyer flasks containing 50 mg L−1 cadmium, at different pH values (2.0–7.0). 50 mM citrate-phosphate buffer was used to prepare different pH solution containing 50 mg L−1 cadmium ions. The flasks were then incubated with shaking (120 rpm) at 30 °C (ambient temperature) for 24 h. At the end of incubation, adsorbent was separated by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min) and the concentration of cadmium in the supernatant was measured by atomic absorption spectrometry (AAS) as described above. The amount of cadmium adsorbed by the mycelia was calculated using the mass balance equation as described elsewhere.21 The equilibrium adsorption isotherm was carried out similarly in a batch process at pH 6.0 but with different cadmium concentrations (5–1000 mg L−1). Other experimental parameters were the same as described above. The kinetics of adsorption process was followed at regular intervals up to 6 h using 50 mg L−1 cadmium at pH value 6.0. As samples were collected from individual flasks, no correction was necessary regarding the withdrawal of the sampling volume. In all cases, the control experiments were conducted under identical conditions excepting without addition of any types of AVM.
000 rpm for 15 min) and the concentration of cadmium in the supernatant was measured by atomic absorption spectrometry (AAS) as described above. The amount of cadmium adsorbed by the mycelia was calculated using the mass balance equation as described elsewhere.21 The equilibrium adsorption isotherm was carried out similarly in a batch process at pH 6.0 but with different cadmium concentrations (5–1000 mg L−1). Other experimental parameters were the same as described above. The kinetics of adsorption process was followed at regular intervals up to 6 h using 50 mg L−1 cadmium at pH value 6.0. As samples were collected from individual flasks, no correction was necessary regarding the withdrawal of the sampling volume. In all cases, the control experiments were conducted under identical conditions excepting without addition of any types of AVM.
Results and discussion
Synthesis and characterization of CdS
Incubation of Cd(NO3)2 solution with functionalized AVM for 10 h caused a color change of the mycelia from pale yellow to orange yellow, indicating the formation of CdS nanoparticles on the AVM surface. The orange yellow colored mycelia (CdS fabricated mycelia) were collected by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min), dried by lyophilization and dispersed in ultrapure water. The UV-vis spectra of the dispersed solution exhibited an absorption maximum at about 380 nm (Fig. 1) due to the surface plasmon resonance (SPR) band of the CdS nanoparticles.24,25 However, the control functionalized AVM showed no such absorption band. This indicated formation of CdS nanoparticles on the surface of functionalized AVM. Fig. 2 showed the FESEM and AFM images of functionalized AVM before and after interaction with Cd(NO3)2. FESEM images (Fig. 2A–D) showed that the surface morphology of control functionalized AVM changed conspicuously following binding with cadmium. Compared to the control AVM (Fig. 2C), the surface of cadmium treated AVM became more rough and appearance of globular structures of CdS was observed in a high magnification image (Fig. 2D). AFM images demonstrated that functionalized AVM has domain like layer structures (Fig. 2E) on the surface. Following interaction with cadmium, disappearance of layer structures and subsequent appearance of globular structures of CdS nanoparticles (Fig. 2F) on AVM surface were witnessed. A similar structure was also reported by Liu et al.26 on the formation of CdS on a reduced graphene oxide surface. The TEM micrograph clearly shows the formation of CdS nanoparticles on the surface of functionalized mycelia (Fig. 3A). CdS nanoparticles were uniformly distributed throughout the surface. The HRTEM image as shown in Fig. 3B suggested formation of spherical particles with an average size (n = 100) of 3.0 ± 0.2 nm. The measured d-spacing of the lattice fringes in the HRTEM image was 3.3 Å, which corresponds to the (111) plane of cubic face CdS.25 The SAED pattern (Fig. 3B, inset) obtained from CdS nanoparticles showed Scherrer ring patterns characteristic of (111), (220), and (311) atomic planes of cubic CdS structure.
000 rpm for 10 min), dried by lyophilization and dispersed in ultrapure water. The UV-vis spectra of the dispersed solution exhibited an absorption maximum at about 380 nm (Fig. 1) due to the surface plasmon resonance (SPR) band of the CdS nanoparticles.24,25 However, the control functionalized AVM showed no such absorption band. This indicated formation of CdS nanoparticles on the surface of functionalized AVM. Fig. 2 showed the FESEM and AFM images of functionalized AVM before and after interaction with Cd(NO3)2. FESEM images (Fig. 2A–D) showed that the surface morphology of control functionalized AVM changed conspicuously following binding with cadmium. Compared to the control AVM (Fig. 2C), the surface of cadmium treated AVM became more rough and appearance of globular structures of CdS was observed in a high magnification image (Fig. 2D). AFM images demonstrated that functionalized AVM has domain like layer structures (Fig. 2E) on the surface. Following interaction with cadmium, disappearance of layer structures and subsequent appearance of globular structures of CdS nanoparticles (Fig. 2F) on AVM surface were witnessed. A similar structure was also reported by Liu et al.26 on the formation of CdS on a reduced graphene oxide surface. The TEM micrograph clearly shows the formation of CdS nanoparticles on the surface of functionalized mycelia (Fig. 3A). CdS nanoparticles were uniformly distributed throughout the surface. The HRTEM image as shown in Fig. 3B suggested formation of spherical particles with an average size (n = 100) of 3.0 ± 0.2 nm. The measured d-spacing of the lattice fringes in the HRTEM image was 3.3 Å, which corresponds to the (111) plane of cubic face CdS.25 The SAED pattern (Fig. 3B, inset) obtained from CdS nanoparticles showed Scherrer ring patterns characteristic of (111), (220), and (311) atomic planes of cubic CdS structure.
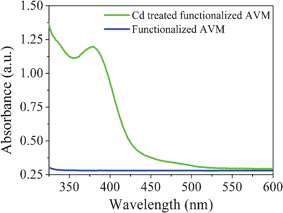 | ||
| Fig. 1 UV-vis spectra of dispersed solution of functionalized AVM before and after treatment with cadmium solution. | ||
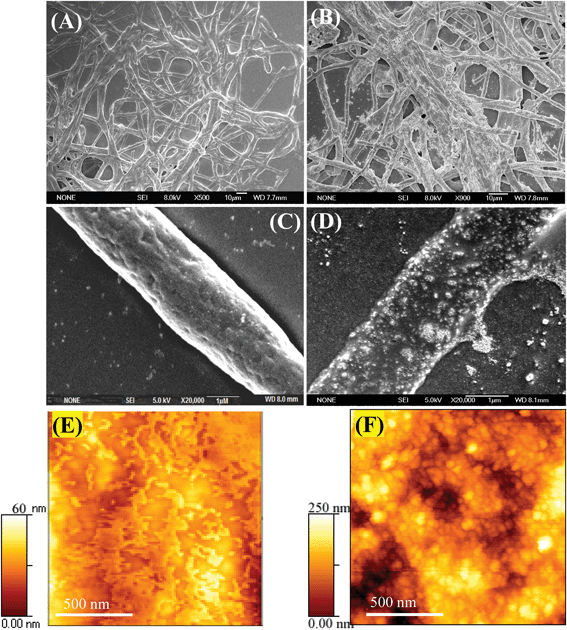 | ||
| Fig. 2 SEM images of functionalized AVM before (A, low magnification; C, high magnification) and after (B, low magnification; D, high magnification) binding with cadmium; AFM images of functionalized AVM before (E) and after (F) binding with cadmium. | ||
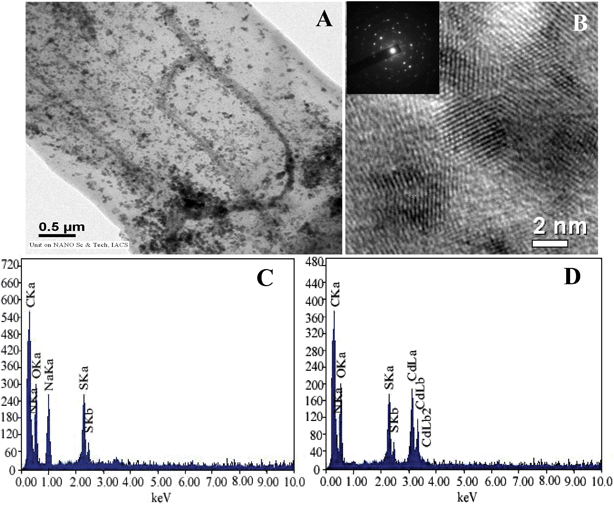 | ||
| Fig. 3 TEM image (A) of functionalized AVM after binding with cadmium; HRTEM image (B) of CdS nanocrystals formed on the functionalized AVM. SAED pattern (B, inset) of CdS nanocrystal; EDXA spectra of functionalized AVM before (C) and after (D) binding of cadmium. | ||
Energy dispersive X-ray analysis (EDXA) of the functionalized AVM (Fig. 3C) showed the presence of C, N, O, Na, Ca and S peaks. The C, N, O and Ca peaks appeared from carbohydrate and protein molecules present on AVM surface, whereas S and Na peaks demonstrated functionalization of AVM. The pristine AVM showed peaks of only C, N, O and Ca (data not shown). Following interaction with Cd+2 solution, functionalized AVM showed the presence of C, N, O, S and Cd peaks. It is interesting to note that in the post treated mycelia, the peaks of alkaline earth metal ions (Na and Ca) disappeared and concomitantly Cd peaks appeared (Fig. 3D) on the surface. This indicated that the formation of CdS nanoparticles on the surface occurred through ion exchange mechanism.
The FTIR spectra of the functionalized AVM showed perceptible changes after binding of cadmium ions. The xanthate functionalized AVM exhibited peaks at 655, 1040, 1052, 1081, 1103 and 1225 cm−1 (Fig. 4A) corresponding to γc-s, γc=s, γcss (a), γc-o-c and γcss (s) of xantahte groups.22,27,28 Downfield shifts of the wavenumber as well as reduction of intensity in the region 800–1200 cm−1, particularly 655, 1040 1052, 1081, 1103 and 1225 cm−1 were noted after binding of cadmium (Fig. 4B). The FTIR spectrum thus confirmed that the xanthate groups on the functionalized mycelia were the main binding sites for cadmium ions.20 Cadmium reacts with the sulphur atom of the xanthate group and forms CdS nanoparticles on the functionalized AVM. However, the absorption band for Cd–S was not detected on the current scale of the spectrum as it appeared at ∼250 cm−1.29
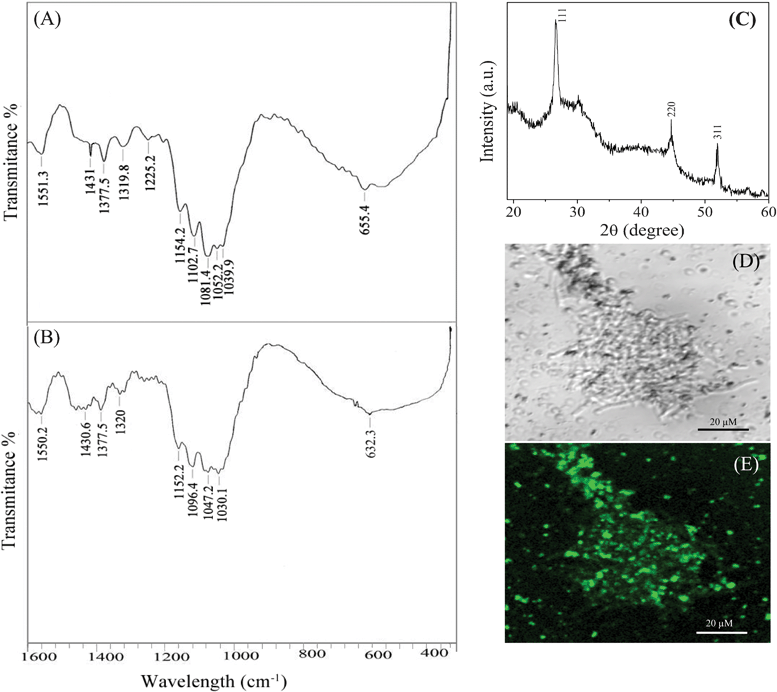 | ||
| Fig. 4 FTIR spectra of functionalized AVM before (A) and after (B) treatment with cadmium solution; XRD pattern (C) of the as-synthesized CdS nanoparticles; bright field (D) and fluorescence microscopy (E) image shows luminescence property of CdS nanocrystals formed on the functionalized AVM. Micrographs were recorded on a fluorescence microscope (Olympus BX-61) using an excitation filter of BP460-495 nm and a band absorbance filter covering wavelengths below 505 nm. | ||
XRD patterns also confirmed the formation of CdS on functionalized mycelia. The XRD pattern (Fig. 4C) exhibited diffraction peaks at 26.4°, 43.8° and 51.5° corresponding to (111), (220) and (311) planes of cubic phase CdS (JCPDS 10-454), respectively. The XRD data were in good agreement with TEM results and supported the successful synthesis of CdS nanoparticles on the surface of AVM. This result further showed that the main diffraction peaks of CdS-AVM composites are similar to pure CdS and demonstrated that in situ fabrication of CdS on AVM does not result in the development of new crystal orientations of CdS.
It is well known that CdS nanoparticles have luminescence property under UV light. Therefore, the luminescence properties of the synthesized CdS were recorded by fluorescence microscopy. The as synthesized CdS has absorption maximum at about 380 nm, however cadmium treated functionalized AVM was excited at 460 nm to overcome the strong background fluorescence. The bright field and fluorescence images of cadmium treated functionalized AVM are illustrated in Fig. 4D and E, respectively. The bright green color under fluorescence microscopy further confirmed the formation of CdS nanoparticles on xanthate functionalized AVM following the binding of cadmium ions. Similar fluorescence properties of CdS were noted by Peretz, et al.30 by embedding CdS on polyvinyl pyrrolidone matrices. These results therefore clearly demonstrated CdS nanoparticle synthesis in a single step process employing a simple functionalization technique.
Batch adsorption experiment
Functionalized AVM was further tested for adsorptive removal of cadmium ions from water bodies. The surface functionalization of AVM through the xanthate group is believed to increase the adsorption capacity compared to the pristine mycelia as the sulphur atom of the xanthate group has a strong affinity for cadmium. The pH is an important factor and plays a crucial role in the adsorption of metal ions by changing the surface charge density on both the adsorbent and adsorbate. Moreover the metal speciation, sequestration, and/or mobility are strongly influenced by solution pH. Adsorption of cadmium by functionalized AVM was found to increase with increase in pH of the solution, with an optimum pH range 5.0–6.0 (Fig. 5A). This high adsorption at pH value 5.0–6.0 was associated with the formation of positively charged metal species having strong affinity for the surface functional groups. The experiment was restricted beyond the pH value of 6.0 due to precipitation of metal hydroxides such as [Cd(OH)3]− or [Cd(OH)4]−2, which have a lower binding affinity due to repulsive interaction with the negatively charged binding sites of the adsorbent.31,32 Speciation studies of cadmium salt demonstrated the formation of Cd(OH)(aq) and Cd(OH)2(aq), species at pH 6.0 and Cd(OH)3−(aq), and Cd(OH)4−2(aq) species beyond pH value 6.0.31,32 However, cadmium exists as Cd+2(aq) at low pH values. The reduced adsorption observed at low pH value (< 3.0) may be attributed to (i) higher hydrated [Cd+2(aq)] species having low mobility and (ii) protonation of the surface functional groups. Competition between Cd+2(aq) species and H+ or H3O+ ions present in the solution also hindered the approach of metal species due to coloumbic repulsion. Moreover, the xanthate group was found to be unstable at low pH values and dissociated from the mycelia with the elimination of carbon disulphide.22,33,34 At higher pH values (5.0–6.0), more functional groups are available for metal ion binding due to deprotonation, resulting in high adsorption. Therefore, maximum adsorption of cadmium within the pH values of 5.0–6.0 might be due to partial hydrolysis of species like Cd(OH)(aq), and Cd(OH)2(aq), having strong affinity for the negatively charged functional groups of the mycelia. EDXA data show (data not shown) that cadmium ions replace the Na and Ca peaks after cadmium adsorption on functionalized AVM, demonstrating that the cadmium adsorption process occurred through an ion-exchange mechanism.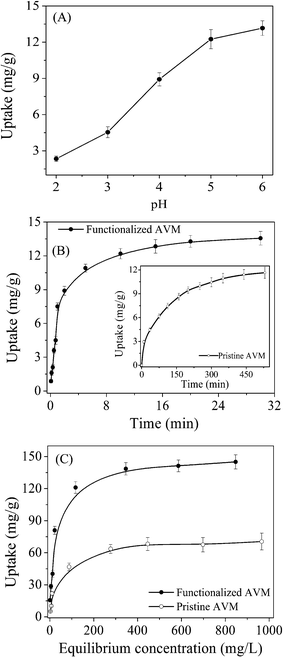 | ||
| Fig. 5 Effect of pH (A) on cadmium adsorption on functionalized AVM. Adsorption kinetics (B) of cadmium on the functionalized AVM; inset figure depicts adsorption kinetics on pristine AVM. Adsorption isotherm (C) of cadmium on the functionalized and pristine AVM. Data represent an average of four independent experiments ± S.D. shown by the error bar. | ||
The kinetic results (Fig. 5B) indicated that the cadmium adsorption process was very fast and reached equilibrium within 20 min in the functionalized AVM against 6 h for pristine mycelia (inset, Fig. 5B). The reasonably fast kinetics reflected good accessibility of the binding sites of the functionalized AVM to cadmium ions. The enhanced adsorption rates therefore have significant practical advantage in terms of time and space over the conventional techniques.
The functionalized AVM was further used to study the enhanced adsorption capacity of this mycelia compared to pristine mycelia. The maximum adsorption (Fig. 5C) capacity of the xanthate-functionalized AVM for cadmium was found to be 145.5 mg g−1 compared to 70.5 mg g−1 for pristine AVM. The isotherm profile in functionalized mycelia was much steeper than that of pristine mycelia and approached to an ideal type-1 isotherm according to IUPAC classification35 and best fitted with the Langmuir isotherm36 model with regression coefficient (r) 0.995. On the other hand, a regression coefficient of 0.875 for pristine mycelia plausibly indicated a lack of energy uniformity37 of the binding sites for metal ions compared to xanthate functionalized mycelia. The cadmium removal capacity of the adsorbent could also be expressed in terms of distribution coefficient (Kd).37
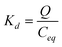
| (1) |
The uptake capacity (Qmax), distribution coefficient (Kd), and the Langmuir adsorption constant (KL),36 related to the adsorption energy for Cd(aq) species is summarized in Table 1. The Qmax, Kd, and KL values for cadmium on the functionalized mycelia were higher than those on the pristine AVM, indicting high affinity of the functionalized mycelia for cadmium. In addition the adsorption capacity of AVM through functionalization was increased significantly compared to other reported adsorbents.38–42 For examples, kaolinite clay after pre-treatment with tripolyphosphate adsorbed 113.64 mg g−1 of cadmium, whereas untreated kaolinite clay adsorbed only 13.23 mg g−1.38 Sodium tetraborate treated kaolinite clay adsorbed 44.05 mg g−1 of cadmium.39 Granular activated carbon and activated clay adsorbed 11.75 and 8.718 mg g−1 cadmium, respectively;40 whereas, other types of carbon adsorbed 40–97 mg g−1 of cadmium.41 Epichlorohydrin treated, NaOH treated and sodium bicarbonate treated rice husk adsorbed 11.12, 20.24 and 16.18 mg g−1 cadmium, respectively.42 The increased adsorption of cadmium in xanthate functionalized AVM is thus attributed to the metal-binding ability of sulphur groups with cadmium.43,44 Cadmium and sulphur groups are soft acid and soft base, respectively, hence high adsorption of cadmium by functionalized mycelia can be explained by soft acid–base interaction according to the Pearson rule.45
| Type of mycelia | Q max (mg g−1) | K d (L g−1) | K L (L g−1) | ΔG (kJ mol−1) |
|---|---|---|---|---|
| Functionalized AVM | 145.45 | 0.17 | 6.65 | −4.77 |
| Pristine AVM | 70.05 | 0.073 | 0.46 | −1.95 |
A. versicolor is easily grown in a cheap and simple growth medium. The handling of this fungus is very easy and growth rate is also high compared to other fungi. It secretes large amount of proteins and is widely used in the production of important enzymes including amylase, cellulase, xylanase, and pectinolytic enzymes, and also in biodiesel production.46,47 Large amount of waste mycelia are generated from these industries. Therefore, dissimilatory properties of this fungi could be exploited for low-cost and environmental friendly removal of metal ions. Xanthate functionalization of AVM not only increases the adsorption capacity, but also increases the affinity towards cadmium.42 Xanthate functionalized AVM took only 20 min to reach equilibrium, whereas other reported adsorbent took 5–10 h to attain the equilibrium.40,42,48,49 Most importantly, more than 85% of the adsorbed cadmium ions were eluated from the loaded AVM by low pH (< 2.0) solution. Functionalization of AVM thereby offers a low-cost green chemical approach toward reclamation of cadmium ions from water bodies.
The metal ions’ binding affinity of xanthate functionalized AVM can be explained by a model reaction X2S + M+2 → MS + 2X+ employing thermodynamics data as described by Brown et al.50 We therefore measured the ΔG (Gibbs free energy)35 values for the present adsorption process, considering above reaction with respect to cadmium and the result came out to be −4.77 kJ mol−1. The negative ΔG value indicates the high degree of spontaneity and energetically favorable adsorption process. In addition, formation of CdS nanoparticles following binding with the sulfur atom of the xanthate group is also responsible for higher adsorption of cadmium in the functionalized AVM. Thus increased cadmium binding efficiency of the functionalized mycelia demonstrated that the xanthate group has a strong binding affinity for cadmium and this has practical significance in process scale up for removal of heavy metal ions.
Conclusions
We developed a novel method for the fabrication of metal sulfide nanoparticles on the surface of fibrilar fungal mycelia and heavy metal removal through a xanthate functionalization process. The method includes in situ synthesis of CdS nanoparticles on the mycelia surface and removal of cadmium ions from water bodies. SEM and AFM images supported the appearance of globular structures of CdS nanoparticles on the surface of AVM following the binding with cadmium ions. TEM image showed that synthesized CdS nanoparticles have an average size of 3.0 ± 0.2 nm, while the EDXA result confirmed the involvement of an ion-exchange mechanism in the binding process. Fluorescence microscopy images showed the luminescence properties of the synthesized CdS nanocrystals. Functionalized mycelia also adsorbed 141.5 mg g−1 cadmium at pH value 6.0, while under identical conditions the pristine mycelia adsorbed 70.5 mg g−1 only. Kinetic results demonstrated very fast removal of cadmium by functionalized AVM and was completed within 20 min. This increased adsorption of cadmium by functionalized mycelia has significant practical application in process development.Acknowledgements
We thank Mr. R. N. Banik and Mr. S. Majhi of our Institute for their cooperation during AFM and FESEM experiments, respectively. Gratitudes are also due to Ms. Mousumi Basu (Institute of Environmental Studies and Wetland Management, Kolkata) for Atomic Absorption Spectroscopic analysis.References
- J. Du, L. Fu, Z. Liu, B. Han, Z. Li, Y. Liu, Z. Sun and D. Zhu, Facile route to synthesize multiwalled carbon nanotube/zinc sulfide heterostructures: Optical and electrical properties, J. Phys. Chem. B, 2005, 109, 12772–12776 CrossRef CAS.
- A. J. Hoffman, G. Mills, H. Yee and M. R. Hoffman, Q-sized cadmium sulfide: synthesis, characterization, and efficiency of photoinitiation of polymerization of several vinylic monomers, J. Phys. Chem., 1992, 96, 5546–5552 CrossRef CAS.
- Y. Zhang, Y. Chen, H. Niu and M. Gao, Formation of CdS nanoparticle necklaces with functionalized dendronized polymers, Small, 2006, 2, 1314–1319 CrossRef CAS.
- M. L. Toebes, J. A. van Dillen and K. P. de Jong, Synthesis of supported palladium catalysts, J. Mol. Catal. A: Chem., 2001, 173, 75–98 CrossRef CAS.
- F. C. Meldrum and R. Seshadri, Porous gold structures through templating by echinoidskeletal plates, Chem. Commun., 2000, 29–30 RSC.
- J. He, T. Kunitake and T. Watanabe, Porous and nonporous Ag nanostructures fabricated using cellulose fiber as a template, Chem. Commun., 2005, 795–796 RSC.
- Z.-X. Cai and X.-P. Yan, In situ electrostatic assembly of CdS nanoparticles onto aligned multiwalled carbon nanotubes in aqueous solution, Nanotechnology, 2006, 17, 4212 CrossRef CAS.
- T. Shimizu, M. Masuda and H. Minamikawa, Supramolecular nanotube architectures based on amphiphilic molecules, Chem. Rev., 2005, 105, 1401–1443 CrossRef CAS.
- H. Cölfen and S. Mann, Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures, Angew. Chem., Int. Ed., 2003, 42, 2350–2365 CrossRef.
- S.-H. Yu, H. Cölfen, K. Tauer and M. Antonietti, Tectonic arrangement of BaCO3 nanocrystals into helices induced by a racemic block copolymer, Nat. Mater., 2005, 4, 51–55 CrossRef CAS.
- E. Braun, Y. Eichen, U. Sivan and G. Ben-Yoseph, DNA-templated assembly and electrode attachment of a conducting silver wire, Nature, 1998, 391, 775–778 CrossRef CAS.
- L. Yu, I. A. Banerjee and H. Matsui, Direct growth of shape-controlled nanocrystals on nanotubes via biological recognition, J. Am. Chem. Soc., 2003, 125, 14837–14840 CrossRef CAS.
- L.-S. Li and S. I. Stupp, One-dimensional assembly of lipophilic inorganic nanoparticles templated by peptide-based nanofibers with binding functionalities, Angew. Chem., Int. Ed., 2005, 44, 1833–1836 CrossRef CAS.
- C. Mao, D. J. Solis, B. D. Reiss, S. T. Kottmann, R. Y. Sweeney, A. H. Hayhurst, G. Georgiou, B. Iverson and A. M. Belcher, Virus-Based Toolkit for the Directed Synthesis of Magnetic and Semiconducting Nanowires, Science, 2004, 303, 213–217 CrossRef CAS.
- A. P. Alivisatos, Semiconductor clusters, nanocrystals, and quantum dots, Science, 1996, 271, 933–937 CrossRef CAS.
- L. Qu and X. Peng, Control of photoluminescence properties of CdSe nanocrystals in growth, J. Am. Chem. Soc., 2002, 124, 2049–2055 CrossRef CAS.
- R. Y. Sweeney, C. Mao, X. Gao, J. L. Burt, A. M. Belcher, G. Georgiou and B. L. Iverson, Bacterial biosynthesis of cadmium sulfide nanocrystals, Chem. Biol., 2004, 11, 1553–1559 CrossRef CAS.
- G. Bayramoglu, A. Deizli, S. Sektas and M. Y. Arica, Entrapment of Lentinus sajor-caju into Ca-alginate gel beads for removal of Cd(II) ions from aqueous solution: preparation and biosorption kinetics analysis, Microchem. J., 2002, 72, 63–76 CrossRef CAS.
- L. Hall-Stoodley, J. W. Costerton and P. Stoodley, Bacterial biofilms: from the natural environment to infectious diseases, Nat. Rev. Microbiol., 2004, 2, 95–108 CrossRef CAS.
- S. R. Rao, Xanthate and Related Compounds, Marcel Dekker, New York, 1971 Search PubMed.
- S. K. Das, A. R. Das and A. K. Guha, A study on the adsorption mechanism of mercury on Aspergillus versicolor biomass, Environ. Sci. Technol., 2007, 41, 8281–8187 CrossRef CAS.
- G. C. Panda, S. K. Das and A. K. Guha, Biosorption of cadmium and nickel by functionalized husk of Lathyrus sativus, Colloids Surf., B, 2008, 62, 173–179 Search PubMed.
- S. K. Das, A. R. Das and A. K. Guha, Structural and nanomechanical properties of Termitomyces clypeatus cell wall and its interaction with chromium(VI), J. Phys. Chem. B, 2009, 113, 1485–1492 Search PubMed.
- D. Diaz, M. Rivera, T. Ni, J.-C. Rodriguez, S.-E. Castillo-Blum, D. Nagesha, J. Robles, O.-J. Alvarez-Fregoso and N. A. Kotov, Conformation of ethylhexanoate stabilizer on the surface of CdS nanoparticles, J. Phys. Chem. B, 1999, 103, 9854–9858 CrossRef CAS.
- N. Pradhan and S. Efrima, Single-precursor, one-pot versatile synthesis under near ambient conditions of tunable, single and dual band fluorescing metal sulfide nanoparticles, J. Am. Chem. Soc., 2003, 125, 2050–2051 CrossRef.
- X. Liu, L. Pan, T. Lv, G. Zhu, Z. Suna and C. Sun, Microwave-assisted synthesis of CdS-reduced graphene oxide composites for photocatalytic reduction of Cr(VI), Chem. Commun., 2011, 47, 11984–11986 RSC.
- P. Hellstrom, S. Oberg, A. Fredriksson and A. Holmgren, A theoretical and experimental study of vibrational properties of alkyl xanthates, Spectrochim. Acta, Part A, 2006, 65, 887–895 Search PubMed.
- G. Sundholm and P. Talonen, Adsorption of ethyl xanthate anions on a silver electrode. An in situ FTIR study, J. Electroanal. Chem., 1995, 380, 261–267 CrossRef CAS.
- A. G. Rolo, L. G. Vieira, M. J. M. Gomes, J. L. Ribeiro, M. S. Belsley and M. P. dos Santos, Growth and characterisation of cadmium sulphide nanocrystals embedded in silicon dioxide films, Thin Solid Films, 1998, 312, 348–353 Search PubMed.
- S. Peretz, B. Sava, M. Elisa and G. Stanciu, Cadmium sulphide nanoparticles embedded in polymeric matrices, J. Optoelectron. Adv. M.-, 2009, 11, 2108–2114 Search PubMed.
- L. Stoica, G. Dima, Biohydrometallurgy and the environment toward the mining of the 21st century, in: Amils R, Ballester A (ed.). Proceedings of the international biohydrometallurgy symposium, Spain, 20–23 June 1999, Elsevier, 1999, p. 409 (Part B Search PubMed.
- B. Cordero, P. Lodeiro, R. Herrero and M. E. S. de Vicente, Biosorption of cadmium by Fucus piralis, Environ. Chem., 2004, 1, 180–187 Search PubMed.
- Y. H. Kim, J. Y. Park, Y. J. Yoo and J. W. Kwak, Removal of lead using xanthated marine brown alga, Process Biochem., 1999, 34, 647–652 Search PubMed.
- S. K. Das, A. R. Das and A. K. Guha, Adsorption behavior of mercury on functionalized Aspergillus versicolor mycelia: Atomic force microscopic study., Langmuir, 2009, 25, 360–366 CrossRef CAS.
- S. Glastone, Textbook of physical chemistry, 2nd ed.; MacMillan Publishing Co: New York, 1962; p 1196 Search PubMed.
- I. Langmuir, The constitution and fundamental properties of solids and liquids. Part I. Solids, S., J. Am. Chem. Soc., 1916, 38, 2221–2295 CrossRef CAS.
- D. Pérez-Quintanilla, I. Hierro, M. Fajardo and I. Sierra, Adsorption of cadmium(II) from aqueous media onto a mesoporous silica chemically modified with 2-mercaptopyrimidine, J. Mater. Chem., 2006, 16, 1757–1764 RSC.
- E. I. Unuabonah, B. I. Olu-Owolabi, K. O. Adebowale and A. E. Ofomaja, Adsorption of lead and cadmium ions from aqueous solutions by tripolyphosphate-impregnated Kaolinite clay, Colloids Surf., A, 2007, 292, 202–211 CrossRef CAS.
- E. I. Unuabonah, K. O. Adebowale, B. I. Olu-Owolabi, L. Z. Yang and L. X. Kong, Adsorption of Pb(II) and Cd(II) from aqueous solutions onto sodium tetra borate -modified Kaolinite: Equilibrium and Thermodynamic studies, Hydrometallurgy, 2008, 93, 1–9 Search PubMed.
- K. L. Wasewar, P. Kumar, S. Chand, B. N. Padmini and T. T. Teng, Adsorption of cadmium ions from aqueous solution using granular activated carbon and activated clay, Clean-Soil Air Water, 2010, 38, 649–656 Search PubMed.
- C. Moreno-Castilla, M. A. Alvarez-Merino, M. V. López-Ramón and J. Rivera-Utrilla, Cadmium ion adsorption on different carbon adsorbents from aqueous solutions. Effect of surface chemistry, pore texture, ionic strength, and dissolved natural organic matter, Langmuir, 2004, 20, 8142–8148 Search PubMed.
- K. S. Rao1, M. Mohapatra, S. Anand and P. Venkateswarlu, Review on cadmium removal from aqueous solutions, Int. J. Eng. Sci. Technol., 2010, 2, 81–103 Search PubMed.
- L. Mercier and C. Detellier, Preparation, characterization, and applications as heavy metals sorbents of covalently grafted thiol functionalities on the interlamellar surface of montmorillonite, Environ. Sci. Technol., 1995, 29, 1318–1323 CAS.
- M. J. Winter, Complexes, d-block chemistry, Oxford University Press, New York, 1994 Search PubMed.
- R. G. Pearson, Absolute electronegativity and hardness: application to inorganic chemistry, Inorg. Chem., 1988, 27, 734–740 CrossRef CAS.
- G. Sathyaprabha, A. Panneerselvam and S. Muthukkumarasamy, Production of Cellulase and Amylase from wild and mutated fungal isolates, EJLS, 2011, 1, 39–45 Search PubMed.
- M. Jeya, S. Thiagarajan and P. Gunasekaran, Improvement of xylanase production in solid-state fermentation by alkali tolerant Aspergillus versicolor MKU3, Lett. Appl. Microbiol., 2005, 41, 175–8 Search PubMed.
- C. K. Ahn, Y. M. Kim, S. H. Woo and J. M. Park, Removal of cadmium using acid-treated activated carbon in the presence of non-ionic and/or anionic surfactants, Hydrometallurgy, 2009, 99, 209–213 Search PubMed.
- H. K. Boparai, M. Joseph and D. M. O'Carroll, Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles., J. Hazard. Mater., 2011, 186, 458–465 CrossRef CAS.
- J. Brown, L. Mercier and T. J. Pinnavaia, Selective adsorption of Hg+2 by thiol-functionalized nanoporous silica, Chem. Commun., 1999, 69–70 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
