Biocentri-voltammetry for the enzyme assay: a model study
Serdar
Çevik
a,
Suna
Timur
b and
Ülkü
Anik
*a
aMugla University, Faculty of Science, Chemistry Department, Kotekli, Mugla, Turkey. E-mail: ulkuanik@yahoo.com; ulkuanik@gmail.com; Tel: +90 252 211 1503
bEge University, Faculty of Science, Biochemistry Department, 35100, Bornova, Izmir, Turkey.
First published on 2nd March 2012
Abstract
This work constitutes the first biocentri-voltammetric application for acetylcholinesterase (AChE) activity assay. After the optimization of working conditions like reaction time, centrifugation speed and time and enzyme amount, the analytical characteristics were investigated. As a result, the linear range of 1.0–350 μM was obtained with an equation of y = 0.0004x + 0.0163. The correlation coefficient is equal to (R2) 0.99 ± 0.001815 (n = 3) and the RSD value was calculated as 3.6% (n = 4). Then Donepezil-based drug analysis was also conducted by using the developed method and an inhibition of 80% was calculated.
1. Introduction
One of the products of hydrolysis of acetylthiocholine (ATCh) with acetylcholinesterase (AChE) is thiocholine. Detection of thiocholine can be used to assess the activity of AChE, a biomarker of the effect of pesticides (organophosphates (OPs) and carbamates) which inhibit cholinesterases.1 Analysis of ATCh is, therefore, of great importance, particularly in the development of sensors for the detection of environmental pollutants such as OPs and carbamates.2 Besides OPs and environmental pollutants, AChE based electrochemical sensors have been utilized for the examination of medicines based on Donepezil and tacrine.3 Donepezil is a benzylpiperidine and can be described as a second generation of cholinesterase inhibitor where it is used for Alzheimer disease (AD) treatment.3–5 AD has been reported to be consistent with cholinergic dysfunction in the brain.6 Thus restoration of cholinergic neurotransmission may help to ameliorate impaired memory in AD patients. For this purpose, many treatment strategies have focused on replacement therapy for deficits of central cholinergic neurotransmission. Promising results have been obtained with cholinesterase inhibitors aiming to amplify the physiological action of acetylcholine in AD patients.7,8Traditional methods available for determination of ATCh include methods like colorimetry and spectrophotometry.9–13 On the other hand, electrochemistry is a powerful tool for real-time detection compared with fluorescence and spectrophotometry. As a part of the electrochemical methods, amperometric sensors have been regarded as most suitable for biochemical analysis, because of their good selectivity, rapid response, miniature size, and reproducible results. These sensors have been applied in a variety of areas including food and environmental analysis.14,15 One of the important steps of biosensor construction is the enzyme immobilization. Enzymes can be directly mixed with the composite structure or suitable membranes can be used for this purpose.16,17 According to the literature, AChE can be immobilized on solid electrode surfaces by the use of a variety of matrices, for example cross-linked polymers,18 cross-linked bovine serum albumin,19,20 chitosan,21 cellulose22 and different support matrices, for example Nylon,23–25 controlled-pore glass,26 magnetic particles,27 or strong affinity linking with concanavalin A28 and metal ions.29
On the other hand, centri-voltammetry is a method that combines centrifugation and voltammetry. The first application of this method which includes the detection of lead(II) was carried out by us.30,31 In this method, generally, the analyte is pre-concentrated on the electrode surface with an appropriate carrier by means of centrifugation and then a voltammetric scan is directly applied in the same cell. By this way, the loss of the analyte is prevented which usually is the case for other pre-concentration techniques. As a result low detection limits with reproducible results are obtained.30,31
In another application, centrifugation was applied for the pre-concentration of mercury ions on a gold film electrode where they were reduced by using a suitable reducing agent. Then mercury(II) ions were detected by applying anodic stripping voltammetry to the mercury/gold amalgam formed on the electrode surface.32
Recently we have managed to develop biocentri-voltammetry by using centri-voltammetry as a part of biosensing systems. The technique was applied without using any carrier precipitate and the effective accumulation of the substrate onto the enzyme electrode is achieved via centrifugation force.33 A xanthine biosensor system was chosen for testing the performance of newly developed centri-voltammetric biosensor where xanthine oxidase enzyme was immobilized onto a planar Pt working electrode that was placed at the bottom of the centri-voltammetric cell. As a result, with the help of centrifugation, effective accumulation hence effective interaction of substrate–enzyme was accomplished that resulted with more sensitive current values.
In the present work, biocentri-voltammetry is applied for AChE activity monitoring. Scheme 1 demonstrates the steps of the procedure and Fig. 1 demonstrates the biocentri-voltammetric cell. As a working electrode, carbon paste electrode (CPE) was used where accumulation of the enzyme together with the substrate on this electrode was achieved by means of centrifugation force. After the optimization of centrifugation speed and time, optimum enzyme amount was investigated. Then analytical characteristics were done and the developed method was applied for Donepezil-based drug analysis.
 | ||
Fig. 1 A) Biocentri-voltammetric cell, B) Parts of the biocentri-voltammetric cell: body (a), cover for reference and counter electrode (b), protective cover for working electrode (c) and carbon paste working electrode (d), C) Eppendorf tube with the voltammetric cell, scale (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
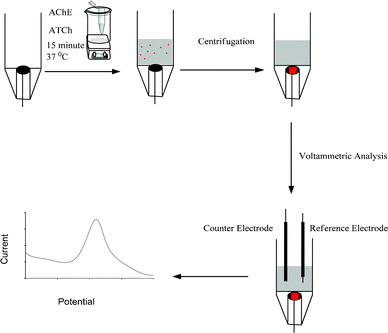 | ||
| Scheme 1 Schematic illustration of the experimental procedure. | ||
2. Experimental
2.1 Apparatus
Voltammetric studies were carried out with the AUTOLAB PGSTAT 12 electrochemical measurement system from ECO CHEMIE Instruments B.V. (The Netherlands) driven by GPES software. Sigma3-16PK centrifuge (refrigerated centrifuge from serial no: 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 321, max. speed (rpm): 15
321, max. speed (rpm): 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, max. capacity (mL): 1000: max. gravitational field (x g): 23
300, max. capacity (mL): 1000: max. gravitational field (x g): 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031, max. kin. energy (Nm): 9970, Rotor 19
031, max. kin. energy (Nm): 9970, Rotor 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 776-H; 1400 min-1, max. load 6 × 80 g, angle of the rotor 30° to the sample and 60° to the background) was used for centrifugation. The cell, made from a delrin tube, was constructed to be compatible with the centrifugation system (Fig. 1). Studies were made with a carbon paste electrode (7.06 mm2) and this electrode was placed at the bottom of the cell. Reference (Ag/AgCl) and counter (Pt rod) electrodes were immersed in the same cell (Scheme 1).
776-H; 1400 min-1, max. load 6 × 80 g, angle of the rotor 30° to the sample and 60° to the background) was used for centrifugation. The cell, made from a delrin tube, was constructed to be compatible with the centrifugation system (Fig. 1). Studies were made with a carbon paste electrode (7.06 mm2) and this electrode was placed at the bottom of the cell. Reference (Ag/AgCl) and counter (Pt rod) electrodes were immersed in the same cell (Scheme 1).
2.2 Reagents
Acetylcholinesterase from Electrophorus electricus (236 Units (U) mg-1 solid, EC number: 3.1.1.7, lot number: 041M7009V) and acetylthiocholine chloride were obtained from Sigma-Aldrich. Graphite powder was purchased from Merck and mineral oil was obtained from Sigma-Aldrich. Phosphate buffer (50 mM pH 7.0) served as supporting electrolyte. ATCh solutions were prepared by dilution of the proper amount of this reagent with phosphate buffer.2.3 Electrode preparation
CPE was prepared by mixing 70% graphite powder with 30% mineral oil.17 A portion of the resulting paste was then packed firmly into the electrode cavity. Electrical contact was established via a copper wire. The paste surface was smoothed on a weighing paper and rinsed carefully with double distilled water.2.4 Procedure
The desired amount of ATCh and AChE were put into an Eppendorf tube which includes 2 mL 50 mM PBS at 37 °C for 15 min. An Eppendorf tube is used for providing effective enzyme interaction in a small volume. After the enzymatic reaction, the solution was placed into the cell and was centrifuged for 5 min at 4000 rpm. Then the cell was carefully placed in the voltammetric stand and the reference and counter electrodes were immersed into the solution. DP voltammograms were recorded in the range 0 to 1.0 V with step potential of 0.005 V and amplitude of 0.025 V.2.5 Sample application
Neurem (Donepezil HCl, 5 mg) was smashed into a mortar. 1.0 mg Neurem was dissolved in phosphate buffer (pH 7.0). 350 μM ATCh, 49.56 × 10−4 U mL−1 AChE and 500 μL Neurem were then put into the Eppendorf and kept at 37 °C for 15 min. Afterwards this solution was placed into the cell and it was centrifuged for 5 min at 4000 rpm. After the centrifugation, the cell was placed in the voltammetric stand and the reference and counter electrodes were immersed into the solution. Then the potential was scanned from 0 to 1.0 V. The inhibition of Donepezil was calculated as follows: | (1) |
where Ip,control is the peak current of ATCh, Ip,exp is the peak current of ATCh with Donepezil inhibition.
3. Result and discussions
In this study first of all, the reaction between ATCh and AChE was optimized following the obtained current values. In this manner, experimental parameters like reaction time, centrifugation speed, centrifugation time and enzyme amount were optimized. Then this optimum system was applied for Donepezil detection in Neurem-based medicine.3.1 Reaction time
In order to investigate the optimum reaction time, 0.25 mM ATCh reacted with 49.56 × 10−4 U mL−1 AChE at 37 °C for 0, 5, 15, 30, 60 min. Then the cell was centrifuged for 4 min, at 4000 rpm and DP voltammograms were recorded in order to obtain the current values. Results were expressed in terms of % electrode response. The current density that was obtained at the optimal working conditions was assumed as 100% and other measured values were calculated relative to this value.34 From the Fig. 2A it is obvious that the current increases up to 15 min and then a decrease is obtained. As a result, 15 min was chosen as the optimum reaction time and used for further studies. From the results it could be stated that the enzyme could lose the catalytic activity due to the longer incubation times under these working conditions. On the other hand, the formation of un-reactive species because of the reactions in which thiocholine might form a dimer or react with cysteine residues of the enzyme could be attributed to the decrease of the signal in longer reaction times than 15 min. On another point of view, 15 min can be defined as a short measuring period that is provided by the developed system.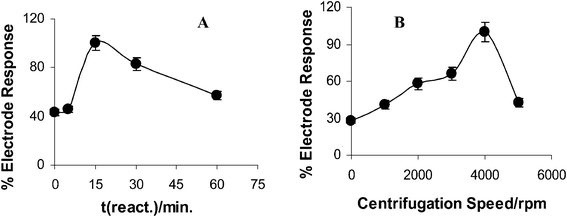 | ||
| Fig. 2 A) The effect of reaction time (0, 5, 15, 30 and 60 min) at 37 °C on the biocentri-voltammetric sensor current percentage for 0.25 mM ATCh, 50 mM phosphate (pH 7.0) as supporting electrolyte; Vcent; 4000 rpm, 4 min, 49.56 × 10−4 U mL−1 AChE. B). The obtained current values at different centrifugation speeds (0, 1000, 2000, 3000, 4000 and 5000 rpm). The reaction time is 15 min, all other conditions as in Fig. 2A. | ||
3.2 Optimum centrifugation speed and centrifugation time
The centrifugation parameters are very important in this method. From our previous studies30,31,33 it has been known that these parameters can sometimes have a profound effect on the current values. For observing the effect of centrifugation speed, 0, 1000, 2000, 3000, 4000 and 5000 rpm were applied to the biocentri-voltammetric cell in the presence of 0.25 mM ATCh, 50 mM PBS (pH 7.0) for 4 min with a reaction time of 15 min (Fig. 2B). Since the best current value was obtained at 4000 rpm, this speed was applied during centrifugation procedure when conducting the experiment.The increase up to 4000 rpm might be due to a convection procedure which results in an increase in current values since the increase in convection causes more analyte accumulation on the electrode surface and results in an increase in current values.31 On the other hand, the decrease that was observed after 4000 rpm, might be due to removal of some analyte on the electrode surface due to the high speed.
For the centrifugation time, 0, 1, 2, 3, 4, 5 and 6 min were applied under the same working conditions at 4000 rpm (Fig. 3). As can be seen from the figure, a significant increase has been obtained at 5 min. which makes this value the one to be used as the optimum centrifugation time for the system. A decrease of current values for longer periods of time can be attributed to the same cause, analyte loss, as explained above.
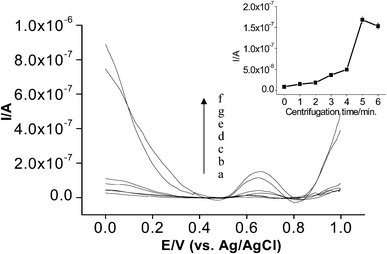 | ||
| Fig. 3 The centrifugation time effect on current values; a) 0, b) 1, c) 2, d) 3, e) 4, f) 5, g) 6 min; 0.25 mM ATCh, 50 mM phosphate buffer (pH 7.0) ; Vcent; 4000 rpm, reaction time 15 min, 49.56 × 10−4 U mL−1 AChE. DP parameters; scan rate 10 mV s−1, step potential 0.005 V, amplitude 0.025 V. Inset: obtained current values at different centrifugation times. | ||
3.3 Effect of enzyme amount
For investigation of the optimum AChE amount, 4.95 × 10−4, 9.91 × 10−4, 24.78 × 10−4, 49.56 × 10−4 and 74.34 × 10−4 U mL−1 of enzyme amounts were used and their effect on current values for 0.25 mM ATCh were examined under the centrifugation speed of 4000 rpm for 5 min (Fig. 4). As expected, the current values increase up to 49.56 × 10−4 U mL−1 and then a dramatic decrease is observed. This decrease might be attributed to the electrode passivation due to the presence of higher protein content that might also cause diffusion problems. Hence, further experiments were conducted by using this enzyme amount.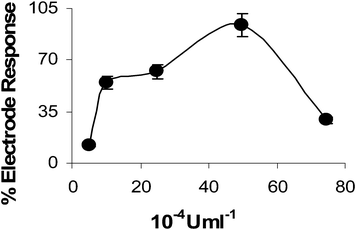 | ||
| Fig. 4 AChE amount (4.95 × 10−4, 9.91 × 10−4, 24.78 × 10−4, 49.56 × 10−4 and 74.34 × 10−4 U mL−1) effect on current values for 0.25 mM ATCl. Vcent; 4000 rpm, 5 min, reaction time 15 min, 50 mM phosphate buffer (pH 7.0). | ||
3.4 Analytical characteristics
After the optimization of experimental parameters, analytical characteristics were examined (Fig. 5). A wide linear range of 1.0–350 μM for ATCh was obtained with an equation of y = 0.0004x + 0.0163 and a correlation coefficient of R2 = 0.99 ± 0.001815 (n = 3). Moreover, a sharp decrease after 350 μM ATCh concentrations was observed because of the substrate inhibition (inset of the Fig. 6). The RSD value was calculated as 3.6% (n = 4).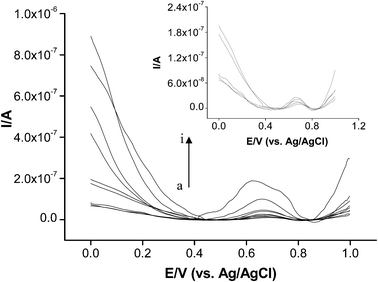 | ||
| Fig. 5 Voltammogram of the linear range 1, 2, 5, 10, 25, 50, 100, 250 and 350 μM ATCh (a–i). Inset; obtained DP voltammogram between 1.0–25 μM ATCh, Vcent; 4000 rpm, 5 min, 49.56 × 10−4 U mL−1 AChE, reaction time 15 min, 50 mM phosphate buffer (pH 7.0). DP parameters; step potential 0.005 V, amplitude 0.025 V. | ||
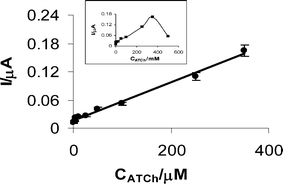 | ||
| Fig. 6 Calibration graph between 1.0–350 μM ATCh. All other conditions as in Fig. 5. | ||
The comparison of biocentri-voltammetric methods with other electrochemical biosensors was also done in terms of linear range and demonstrated in Table 1. As can be seen from the table, the widest linear range was obtained with biocentri-voltammetry for ATCh. This result confirms the sensitivity of the method once again.
| Electrode | Range of linearity (μM) | Reference |
|---|---|---|
| MWCNT: Multiwalled carbon nanotubex. PANI: polyaniline. GnP: Oxidized exfoliated graphite nanoplatelet. TiO2–G: TiO2-decorated graphene. AuNPs: Gold nanoparticles. SiSG: silica sol–gel. PAN: Polyacrylonitrile. | ||
| GC/MWCNT/PANI/AChE | 10–89.2 | 35 |
| AChE/xGnP/Chitosan/GCE | 5–39 and 64–258 | 36 |
| AChE/TiO2–G/GCE | 300–1100 | 37 |
| AChE/AuNPs/SiSG/GCE | 10–1000 | 38 |
| AChE/PAN/AuNPs/Pt | 10–170 | 39 |
| Present work | 1–350 | |
The control experiments, where one set was prepared without AChE and the other one was prepared without ATChE, were also conducted and no signal was obtained.
For the inhibition study, as mentioned in the experimental, a Donepezil-based AD drug was analyzed. After the preparation of the sample that was taken from the drug, the measurement was conducted by introducing Donepezil together with ATCh and AChE in the reaction medium. Then the centrifugation was applied and the voltammogram was recorded (Fig. 7). As can be seen from the figure, a sharp decrease was obtained at the current value, demonstrating the inhibition of Donepezil. Based on the obtained current values and following the necessary formula, the inhibition was calculated as 80%.
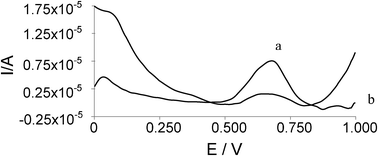 | ||
| Fig. 7 Obtained DP voltammograms with a) 350 μM ATCh, b) 350 μM ATCh and 500 μL Donepezil. All other conditions as in Fig. 5. | ||
4. Conclusion
A new method, biocentri-voltammetry was applied for monitoring the activity of the AChE enzyme. As can be seen from analytical characteristics, a wide linear range (1.0–350 μM) with a promising R2 value was obtained. Also this system was applied for inhibition studies by including the drug Donepezil and an inhibition of 80% was calculated. From these data, we can conclude that biocentri-voltammetry was suitable for monitoring these types of activity studies. Ongoing work in our lab continues on this subject. As future work, we are planning to perform drug analysis based on the inhibition process and also some OP detection will be made using the same procedure.Acknowledgements
The grant from The Technical and Scientific Council of Turkey (TUBİTAK) Project no: 109T885 is gratefully acknowledged.References
- H. Schulze, S. Vorlova, F. Villatte, T. T. Bachmann and R. D. Schmid, Biosens. Bioelectron., 2003, 18, 201 CrossRef CAS.
- D. Du, X. Huang, J. Cai, A. Zhang, J. Ding and S. Chen, Anal. Bioanal. Chem., 2007, 387, 1059 CrossRef CAS.
- M. F. Snape, A. Misra, T. K. Murray, R. J. De Souza, J. L. Williams, A. J. Cross and A. R. Gren, Neuropharmacology, 1999, 38, 181 CrossRef CAS.
- G. Ozturk, S. Alp and S. Timur, Dyes Pigm., 2008, 76, 792 CrossRef CAS.
- G. Ozturk, S. Alp and S. Timur, J. Mol. Catal. B: Enzym., 2007, 47, 111 CrossRef CAS.
- J. T. Coyle, D. L. Price and M. R. DeLong, Science, 1983, 219, 1184 CAS.
- G. L. Elman, K. D. Courtney, V. A. Jr. and R. M. Featherstone, Biochem. Pharmacol., 1961, 7, 88 CrossRef.
- Y. Q. Liang and X. C. Tang, Neurosci. Lett., 2004, 361, 56 CrossRef CAS.
- L. Pogacnik and M. Franko, Biosens. Bioelectron., 2003, 18, 1 CrossRef CAS.
- A. A. Dietz, H. M. Rubinstein and T. Lubrano, Clin. Chem., 1973, 19, 1309 CAS.
- A. A. Dietz and H. M. Rubinstein, Clin. Biochem., 1972, 5, 136 CrossRef CAS.
- G. L. Elman, Arch. Biochem. Biophys., 1958, 74, 443 CrossRef.
- G. L. Elman, Arch. Biochem. Biophys., 1959, 82, 70 CrossRef.
- E. Suprun, G. Evtugyn, H. Budnikov, F. Ricci, D. Moscone and G. Palleschi, Anal. Bioanal. Chem., 2005, 383, 597 CrossRef CAS.
- O. I. Covaci, B. Bucur, M. P. Bucur and G. L. Radu, Microchim. Acta, 2010, 169, 335 CrossRef CAS.
- M. Çubukçu, S. Timur and Ü. Anik, Talanta, 2007, 74, 434 CrossRef.
- Ü. Anik and S. Çevik, Microchim. Acta, 2009, 166, 209 CrossRef.
- K. Stein and G. Schwedt, Anal. Chim. Acta, 1993, 272, 73 CrossRef CAS.
- G. Palleschi, M. Bernabei, C. Cremisini and M. Macsini, Sens. Actuators, B, 1992, 7, 513 CrossRef.
- A. M. N. Hendji, N. Jaffrezic-Renault, C. Martelet, P. Clechet, A. A. Shlu'ga, V. I. Strikha, L. I. Netchiporuk, A. P. Soldatkin and W. B. Wlodarski, Anal. Chim. Acta, 1993, 281, 3 CrossRef CAS.
- V. B. Kandimalla and H. X. Ju, Chem.–Eur. J., 2006, 12, 1074 CrossRef CAS.
- T. T. Bachmann and R. D. Schmid, Anal. Chim. Acta, 1999, 401, 95 CrossRef CAS.
- M. Bernabei, C. Cremisini, M. Mascini and G. Palleschi, Anal. Lett., 1991, 24, 1317 CrossRef CAS.
- A. N. Ivanov, G. A. Evtugyn, R. E. Gyurcsányi, K. Tóth and H. C. Budnikov, Anal. Chim. Acta, 2000, 404, 55 CrossRef CAS.
- F. Pariente, C. La Rosa, F. Galan, L. Hernández and E. Lorenzo, Biosens. Bioelectron., 1996, 11, 1115 CrossRef CAS.
- H. S. Lee, Y. A. Kim, Y. A. Cho and Y. T. Lee, Chemosphere, 2002, 46, 571 CrossRef CAS.
- R. Kindervater, W. Künnecke and R. D. Schmid, Anal. Chim. Acta, 1990, 234, 113 CrossRef CAS.
- B. Bucur, A. F. Danet and J. L. Marty, Biosens. Bioelectron., 2004, 20, 217 CrossRef CAS.
- S. Andreescu, L. Barthelmebs and J.-L. Marty, Anal. Chim. Acta, 2002, 464, 171 CrossRef CAS.
- Ü. A. Kirgöz, H. Tural and F. N. Ertas, Talanta, 2005, 65, 48 Search PubMed.
- Ü. A. Kirgöz, H. Tural and F. N. Ertas, Electroanalysis, 2004, 16, 765 CrossRef.
- I. Urkmez, H. I. Gökçel, F. N. Ertas and H. Tural, Microchim. Acta, 2009, 167, 225 CrossRef CAS.
- U. Anik and S. Çevik, Microchim. Acta, 2011, 174, 207 CrossRef CAS.
- Ü. A. Kirgöz, S. Timur, D. Odaci, B. Perez, S. Alegret and A. Merkoçi, Electroanalysis, 2007, 19, 893 CrossRef.
- I. Cesarino, F. C. Moraes and S. A. S. Machado, Electroanalysis, 2011, 23, 2586 CrossRef CAS.
- A. C. Ion, I. Ion, A. Culetu, D. Gherase, C. A. Moldovan, R. Iosub and A. Dinescu, Mater. Sci. Eng., C, 2010, 30, 817 CrossRef CAS.
- K. Wang, H. Li, J. Wu, C. Ju, J. Yan, Q. Liu and B. Qiu, Analyst, 2011, 136, 3349 RSC.
- D. Du, S. Chen, J. Cai and A. Zhang, Biosens. Bioelectron., 2007, 23, 130 CrossRef CAS.
- I. Marinov, Y. Ivanov, K. Gabrovska and T. Godjevargova, J. Mol. Catal. B: Enzym., 2010, 62, 67 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
