Multifunctional silver coated E-33/iron oxide water filters: Inhibition of biofilm growth and arsenic removal†
Mallikarjuna N.
Nadagouda
*,
Christina
Bennett-Stamper
,
Colin
White
and
Darren
Lytle
The U.S. Environmental Protection Agency, ORD, NRMRL, WSWRD, 26 W. Martin Luther King Dr, Cincinnati, Ohio 45268. E-mail: Nadagouda.mallikarjuna@epa.gov; Tel: 01-513-569-7232
First published on 21st February 2012
Abstract
Bayoxide® E33 (E-33) is a widely used commercial material for arsenic adsorption composed of a mixture of iron oxyhydroxide and oxides. Primarily used to remove arsenic from water, it is a non-magnetic, iron oxyhydroxide/oxide used in fixed bed pressure filters that generally lack multi-functionality. Like other adsorptive media, it is subject to surface fouling by precipitates, including iron and manganese, and biofilms that can create diffusion limitations. Modifying the surface of E-33 with silver nanoparticles to enhance the material properties could add multi-functionality and be beneficial, providing that the original arsenic adsorption properties are not compromised. Commercially available arsenic adsorption media (E-33) was combined to create core-shell composites, i.e. silver coated goethite (in magnetic and non magnetic forms), that is capable of removing arsenic, acting as an antibacterial, and has magnetic properties. Several green approaches were developed to prepare the silver coated goethite and it was tested for arsenic removal and antibacterial activity. Samples were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray florescence spectroscopy (XRF), and inductively coupled plasma mass spectroscopy (ICP-MS).
Introduction
An important step in exploring the potential application of materials to natural and engineered systems is the process of designing the surface composition or surface properties. The ability to create multifunctional materials that serve a range of simultaneous purposes is of increasing interest worldwide. For example, iron-based materials, including magnetic and non-magnetic Fe2O3 and iron particles have been synthesized1–5 and used for various applications6–8 such as arsenic removal, hepatocyte-targeted magnetic resonance (MR) contrast agents and MR contrast-enhancing agents,2 a substance for generating infrared (IR) ray-radiant heat, and the detection of an asialoglycoprotein receptor function in benign liver disease. Additionally, silver-coated magnetic materials have been examined in the development of advanced immunosensors based on chemically functionalized core-shell Fe3O4@Ag magnetic nanoparticles.6The antibacterial properties of silver are well known and the addition of silver to other matrices has been considered. These matrices include antibacterial textiles,9 zeolites,10 hyperbranched polyamine,11 3-(substituted-phenyl) sulfanylpropenoates,12 titania powder,13 sodium zirconium phosphate,14 carbon monolith,15 doped glasses and glass-ceramics,16 composites based on crosslinked resins,17 glass microspheres,18 carbon aerogels,19 activated carbon fibers,20 doped polyethyleneglycol-based polyamidoamine side chain dendritic polyurethane,21 polypyrrole/polyanion composite coatings, and polyacrylonitrile.22 There is also great interest in identifying and synthesizing multifunctional materials for environmental remediation; this includes the ability to remove arsenic and inhibit biofilm formation in drinking water treatment and distribution systems. Although there are a number of different approaches for arsenic control and biofilm reduction; most lack multifunctional capabilities and/or fail to meet desired quality.
Bayoxide® E33 (E-33) is a widely used commercial material for arsenic adsorption that is composed of a mixture of iron oxyhydroxide and oxides. Primarily used to remove arsenic from water, it is a non-magnetic, iron oxyhydroxide/oxide primarily used in fixed bed filters. As with other adsorptive media, E-33 is subject to surface fouling by precipitates, including iron and manganese, and generally lacks multi-functionality. The formation of biofilms can also create diffusion limitations and thus can potentially reduce treatment effectiveness. Through the modification of the surface of arsenic adsorption media, such as E-33, by the addition of silver nanoparticles, it is possible to enhance the materials' multi-functionality provided that the original materials' arsenic adsorption properties are not compromised.
The objective of this work is to add multi-functionality to a commercially available arsenic adsorption media using a core-shell approach to create the multi-functional component that is absent from current approaches. Specifically, the work will create core-shell composites that are capable of removing arsenic, and have antibacterial and magnetic properties. In addition, this research will examine new methods to modify existing media, including their surface characteristics and utilize environmentally friendly techniques to meet objectives by building from environmentally-friendly green synthesis and coating approaches.23–27
Results and discussion
Self-propagating high temperature synthesis (SHS), also called combustion synthesis, is an extremely versatile, rapid, and energetically favorable process where the energy needed to sustain the process results from the chemical energy of the exothermic reaction mix. In this study, E-33 was mixed with varying PVA concentrations and ignited in a muffle furnace set at 500 °C for 30 min. This causes the PVA to self-ignite and drive the resulting chemical reaction. Through this self-propagating combustion reaction, E-33, which is non-magnetic at room temperature, can be converted to a magnetic form (see Fig. 1). | ||
| Fig. 1 A maghemite sample, placed on a magnet prepared through a self-propagating combustion reaction (Sample E composition). | ||
In order to synthesize magnetic E-33, different weight/weight ratios of E-33 and PVA were prepared and analyzed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). The XRD patterns of the E-33 control and above compositions are shown in Fig. 2 and 3 respectively. E-33 is comprised of crystalline goethite, FeOOH, based on XRD analysis (Fig. 2) although amorphous iron solids can be missed by XRD analysis.
5). The XRD patterns of the E-33 control and above compositions are shown in Fig. 2 and 3 respectively. E-33 is comprised of crystalline goethite, FeOOH, based on XRD analysis (Fig. 2) although amorphous iron solids can be missed by XRD analysis.
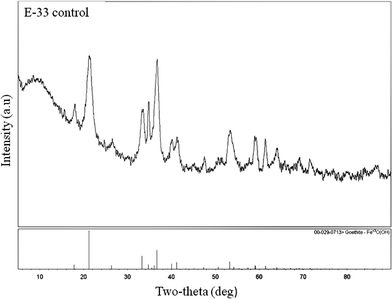 | ||
| Fig. 2 XRD pattern of control E-33 sample. | ||
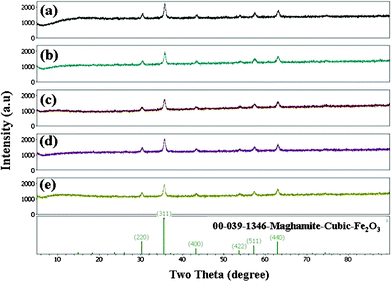 | ||
| Fig. 3 The XRD patterns of maghemite, obtained from E-33 using PVA. | ||
Independent of the ratio of E-33 to PVA, magnetic maghemite, Fe2O3, became the dominant iron mineral phase on the media surface (samples were not ground to expose internal surface prior to analysis). All of the maghemite XRD patterns were compared with the JCPDS pattern No. 00-039-1346. The composition ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was chosen for further analysis to make sure that most of the surface was converted to the magnetic phase. The magnetic and non-magnetic E-33 was coated with silver particles through several wet chemical approaches. Optical micrographs of silver-coated E-33 (magnetic and non-magnetic) are shown in Fig S1-S4, ESI.† Fig. S1 shows an optical image of (a) A, (b) B, (c) C, and (d) D samples and Fig. S2 shows sample E. After surface modification, the silver coating altered the surface color slightly from yellowish-orange to gray (See Fig. S3 and S4, ESI†). A thick coating of silver particles is observed on the E-33 control samples when compared to the magnetized E-33 samples. This is due to the adsorption of silver ions onto E-33 (goethite, Fe3+O (OH), iron oxyhydroxide) being relatively higher when compared with iron oxides (Fe2O3). SEM images of silver-coated E-33 (magnetic and non-magnetic) are shown in Fig. 4 through to 8.
5 was chosen for further analysis to make sure that most of the surface was converted to the magnetic phase. The magnetic and non-magnetic E-33 was coated with silver particles through several wet chemical approaches. Optical micrographs of silver-coated E-33 (magnetic and non-magnetic) are shown in Fig S1-S4, ESI.† Fig. S1 shows an optical image of (a) A, (b) B, (c) C, and (d) D samples and Fig. S2 shows sample E. After surface modification, the silver coating altered the surface color slightly from yellowish-orange to gray (See Fig. S3 and S4, ESI†). A thick coating of silver particles is observed on the E-33 control samples when compared to the magnetized E-33 samples. This is due to the adsorption of silver ions onto E-33 (goethite, Fe3+O (OH), iron oxyhydroxide) being relatively higher when compared with iron oxides (Fe2O3). SEM images of silver-coated E-33 (magnetic and non-magnetic) are shown in Fig. 4 through to 8.
SEM images of magnetized A (a–b) and B (c–d) E-33 are shown in Fig. 4. After the combustion reaction, small spherical particles were formed on the surface of the E-33. These particles were densely packed with a size ranging from 100–500 nm. In the process of combustion, adsorbed poly (vinyl alcohol) on the E-33 surface reacts exothermically, resulting in surface reconstruction and a change in morphology.
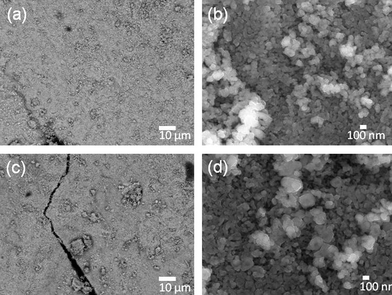 | ||
| Fig. 4 SEM images of A–B magnetized E-33 samples: A (a–b) and B (c–d). | ||
A similar trend is continued with other compositions (see Fig. 5 a–f). However, a lower PVA concentration had a slight influence on the formation of the surface morphology (see Fig. 5 (a–b)). In this case, the spherical particles are aggregated with larger particle sizes. One possible reason is that the exothermic reaction was not intense enough to combust and disperse the particles. Regardless, the lower and higher PVA concentration yielded densely packed spherical magnetic particles when compared to the middle compositions.
 | ||
| Fig. 5 SEM images of C (a–b), D (c–d) and E (e–f) magnetized E-33 samples. | ||
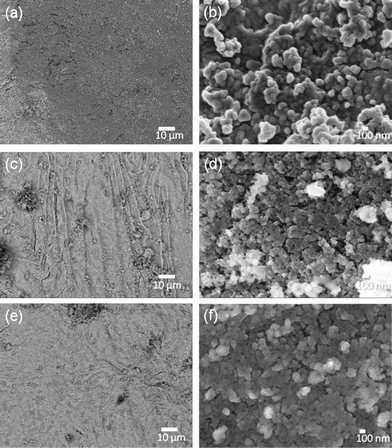
Using different reducing agents: tea extract, ascorbic acid, NaBH4, and sodium citrate, the magnetized E-33 samples were coated with silver particles and are shown in Fig. 6 (a–e), Fig. 7, and Fig. S5–S6, ESI.†Fig. 6 (a–b) shows SEM images of a silver-coated magnetized F sample with a coating of spherical silver particles that ranges in size from 50–200 nm. The ascorbic acid reduction produced relatively large silver particles (Fig. 6 (c–d)), but spherical particles along with few rare dendritic structures were also present (Fig. 6 (e–f)). These dendritic structures were on the micron scales (10–100) μm in length and a few hundred nanometers in thickness. Similar structures have been observed in the presence of copper metal.24 When sodium borohydride (NaBH4) was used as the reducing agent, silver particles with spherical and irregular shapes were observed (Fig. 7 (a–b)). These particles were aggregated and dense in nature. In the presence of sodium citrate, spherical to hexagonal silver crystals were formed with sizes ranging from 100 nm–1 μm with some irregular particle shapes also present (Fig. 7 (c–d)).
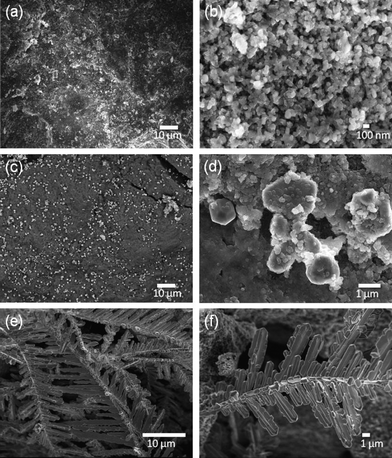 | ||
| Fig. 6 SEM images of magnetized E-33: F (a–b) and G (c–f) samples. | ||
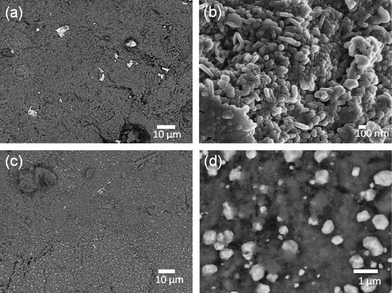 | ||
| Fig. 7 SEM images of silver-coated E-33: Samples H (a–b) and I (c–d). | ||
In order to achieve the desired product, it is important to have a uniform coating of silver particles throughout the surface of E-33. SEM images of non-magnetic E-33 coated silver images are shown in Fig. 8.
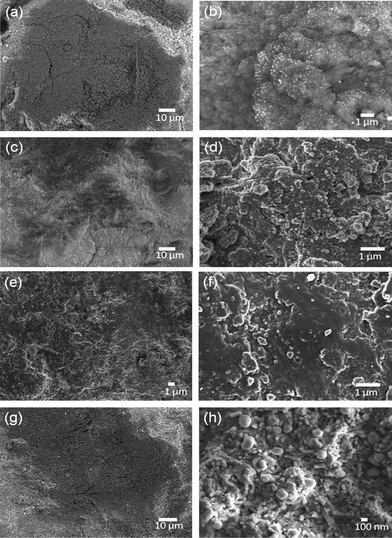 | ||
| Fig. 8 SEM image of J(a-b), K(c–d), L(e–f) L, and M (g–h) samples. | ||
Depending upon the reducing agent used, either a dense or sparse array of spherical silver particles coated the E-33. X-ray mapping was used to confirm the silver (color mapping) coating on the J, K, L, and M samples and are shown in Fig. 9. The yellow color indicates the presence of silver.
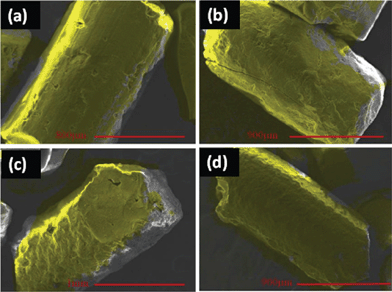 | ||
| Fig. 9 X-ray mapping images of Samples (a) J, (b) K, (c) L, and (d) M. | ||
Selected silver-modified, non-magnetic E-33 samples were ground and subjected to X-ray diffraction and X-ray fluorescence analysis (Fig. 10). Fig. 10 shows the XRD patterns of J, K, L, and M samples where no peaks corresponding to silver are observed other than the parent goethite phase. This is due to an insufficient quantity of silver, below the detection level of XRD. However, the presence of silver was confirmed using X-ray fluorescence and ranged from 0.54 to 0.84% depending upon the sample history as shown in Table 1 and are in agreement with energy dispersive X-ray analysis (EDX) data (Fig. S5–S9 and Table S1–S5, ESI†). The SEM image of control sample is shown in Fig. S10.
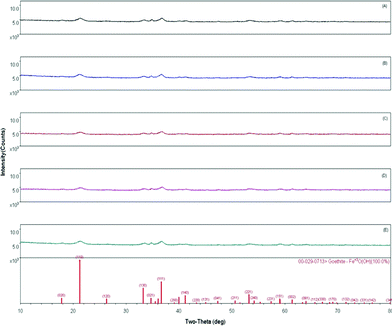 | ||
| Fig. 10 XRD patterns of silver-coated E-33 media. (a) J , (b) K, (c) L, (d) M, and (e) control E-33. | ||
| Element | O | Na | Mg | P | S | Ca | Cr | Mn | Fe | Ag |
|---|---|---|---|---|---|---|---|---|---|---|
| a All the data are in %. | ||||||||||
| J | 33 | 0.12 | 0.0060 | 0.014 | 0.11 | 0.09 | 0.022 | 0.21 | 75 | 0.72 |
| K | 33 | 0 | 0.0060 | 0.009 | 0.093 | 0.11 | 0 | 0.24 | 75 | 0.58 |
| L | 33 | 0.14 | 0.0060 | 0 | 0.025 | 0.18 | 0.030 | 0.21 | 75 | 0.54 |
| M | 33 | 0.27 | 0.0060 | 0.007 | 0.029 | 0.14 | 0.017 | 0.22 | 75 | 0.84 |
The arsenic removal data based on simple batch tests of the obtained materials is shown in Table 2. When compared to the E-33 control, the magnetized E-33 compositions (samples E–L) had a range of 68–74% arsenic removal capacity depending upon the preparation conditions. In comparison, non-magnetic E-33 modified silver samples (samples M–Q) tended to have a very high capacity (up to 94%) compared to the E-33 control. Additionally, the control, E-33 (goethite), can adsorb more arsenic than its oxide counterpart γ-Fe2O3 (magnetized iron oxide samples). the Arsenic removal capacity was not significantly impacted by the addition of silver. Recently, Aminabhavi et al. have reported28 nanofiltration membranes for arsenic removal. They have achieved very high arsenic rejection rates (99%) but the concentrations are very low compared with present study. Also, membrane based removal techniques are very expensive compared with conventional solid media based filtration and needs special apparatus.
| Sample | As in Sol'n (mg L−1) | Mass Media | Adsorption (mg g−1) | % As Removed |
|---|---|---|---|---|
| Control | 7.03000 | — | — | — |
| A | 2.12300 | 0.2481 | 19.78 | 69.80 |
| B | 1.92100 | 0.2525 | 20.23 | 72.67 |
| C | 1.90600 | 0.2512 | 20.40 | 72.89 |
| D | 1.85200 | 0.2486 | 20.83 | 73.66 |
| E | 2.18300 | 0.253 | 19.16 | 68.95 |
| F | 2.12800 | 0.2522 | 19.44 | 69.73 |
| G | 2.07600 | 0.25 | 19.82 | 70.47 |
| H | 1.93200 | 0.2502 | 20.38 | 72.52 |
| Control | 10.88000 | — | — | — |
| I | 3.75100 | 0.2492 | 28.61 | 65.52 |
| J | 1.05000 | 0.2493 | 39.43 | 90.35 |
| K | 0.64520 | 0.2504 | 40.87 | 94.07 |
| L | 0.97570 | 0.2509 | 39.48 | 91.03 |
| M | 1.03000 | 0.2488 | 39.59 | 90.53 |
| 397 Control | 0.29670 | 0.2494 | 42.44 | 97.27 |
| 399 Control | 0.35830 | 0.2506 | 41.99 | 96.71 |
In order to assess the potential of silver-coated E-33 on biofilm inhibition, all the prepared samples were subjected to a bacterial growth inhibition assay (Fig. 11). When plated on S. aureus or E. coli, the control sample, E-33, and the uncoated, magnetized E-33 compositions (samples A–E) did not show any bacterial inhibition. However, silver modified magnetic compositions had very high inhibition for S. aureus (6 to 16 mm) compared to E.coli (2 to 6 mm) depending upon the sample history. This preliminary data suggests that the silver-coated E-33 may serve as a bacterial growth inhibitor and current research is utilizing these materials in pilot column studies to assess their effects on biofilm development.
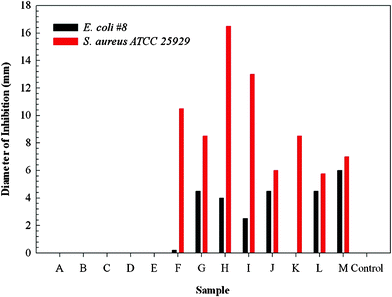 | ||
| Fig. 11 Growth inhibition of S. Aureus and E-Coli on various silver-coated media. | ||
Experimental
Synthesis of magnetic iron oxide from non-magnetic iron oxyhydroxide (E-33)
The materials were prepared by weighing out E-33 and poly(vinyl alcohol) (PVA) into five weight/weight ratios; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. For the material synthesis, each ratio of E-33 and PVA was added together in a crucible with milli-Q water (5 mL) and mixed using a spatula. Each crucible was put into a larger crucible to collect any spill over and placed in a muffle furnace (Fisher Price Isotemp Muffle Furnace, Fisher Scientific, USA) at 500 °C for 30 min. Once the materials were removed from the muffle furnace, they were allowed to cool at room temperature inside a hood prior to characterization. The samples were coded as A, B, C, D, and E for 1
5. For the material synthesis, each ratio of E-33 and PVA was added together in a crucible with milli-Q water (5 mL) and mixed using a spatula. Each crucible was put into a larger crucible to collect any spill over and placed in a muffle furnace (Fisher Price Isotemp Muffle Furnace, Fisher Scientific, USA) at 500 °C for 30 min. Once the materials were removed from the muffle furnace, they were allowed to cool at room temperature inside a hood prior to characterization. The samples were coded as A, B, C, D, and E for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively. (E-33: poly(vinyl alcohol)).
5, respectively. (E-33: poly(vinyl alcohol)).
Synthesis of silver-coated magnetic E-33
To produce the initial mixture, 2 g of magnetic E-33 (obtained from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratios) is mixed with 20 mL of milli-Q water in a 100 mL conical flask. 10 mL of AgNO3 (0.1 N) is added to the flask and the flask is then shaken by hand for 2 min to ensure thorough mixing. To this initial mixture, the synthesis is completed by mixing with: 1) tea extract, 2) ascorbic acid, 3) NaBH4, or 4) sodium citrate.
5 ratios) is mixed with 20 mL of milli-Q water in a 100 mL conical flask. 10 mL of AgNO3 (0.1 N) is added to the flask and the flask is then shaken by hand for 2 min to ensure thorough mixing. To this initial mixture, the synthesis is completed by mixing with: 1) tea extract, 2) ascorbic acid, 3) NaBH4, or 4) sodium citrate.
Simplified versions of the above procedures are documented in Table 3.
| Sample Code | Composition |
|---|---|
| A | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven 4 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven |
| B | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven 3 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven |
| C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven 1 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven |
| D | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven 2 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven |
| E | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven 5 (E-33: poly(vinyl alcohol)), combusted at 500 °C in a preheated oven |
| F | 2 g magnetic E-33 + 20 mL H2O + 10 AgNO3(0.1N) + 20 mL tea extract |
| G | 2 g magnetic E-33 + 20 mL H2O + 10 AgNO3(0.1N) + 20 mL ascorbic acid(1N) |
| H | 2 g magnetic E-33 + 20 mL H2O + 10 AgNO3(0.1N) + 20 mL NaBH4 (1N) |
| I | 2 g magnetic E-33 + 20 mL H2O + 10 AgNO3(0.1N) + 20 mL sodium citrate(1N) |
| J | 10 g of E-33 + 35 mL of water + 2 mL AgNO3 + 10 mL of ascorbic acid (1N) |
| K | 10 g of E-33 + 35 mL of water + 2 mL AgNO3 + 10 mL of NaBH4 (1N) |
| L | 10 g of E-33 + 35 mL of water + 2 mL AgNO3 + 10 mL of tea extract (1N) |
| M | 10 g of E-33 + 35 mL of water + 2 mL AgNO3 + 10 mL of sodium citrate (1N) |
For the synthesis using tea extract, the tea extract is prepared by boiling 2 g of tea powder (Red Label, India) in 100 mL of milli-Q water and filtering the solution through a 25 μl filter. 20 mL of the tea extract is added to the initial mixture, shaken by hand for 2 min. and allowed to react overnight at room temperature. For ascorbic acid, 10 mL of 0.1 N ascorbic acid is added to the initial mixture, shaken by hand for 2 min., and allowed to react overnight at room temperature. By NaBH4, 10 mL of 0.1 N NaBH4 is added to the initial mixture, shaken by hand for 2 min. and allowed to react overnight at room temperature. Synthesis with sodium citrate occurs by adding 20 mL of 0.1 N sodium citrate and the mixture shaken by hand for 2 min. The solution is then boiled to accelerate the silver reduction and allowed to react overnight at room temperature. Following the overnight incubation, all samples are washed two times with milli-Q water and dried at room temperature prior to analysis and characterization.
Synthesis of silver-coated E-33 media
An initial mixture is prepared by mixing 10 g of E-33 and 35 mL of milli-Q water in a 100 ml conical flask. To the flask, 2 mL of AgNO3 (0.1 N) is added and the flask shaken by hand for 2 min. to ensure thorough mixing. Synthesis of the silver-coated E-33 media is completed as described above for: 1) tea extract, 2) ascorbic acid, 3) NaBH4, and 4) sodium citrate.Arsenic adsorption studies
The arsenic(V) adsorption capacity of the modified E-33 material was determined using a batch-scale capacity test. First, four liters of arsenic(V) solution were prepared using four liters of deionized water, 1.4 g of DIC, and 0.1267 g of Na2HAsO4·7H2O, for an initial As(V) concentration of 10 mg L−1. The solution was titrated with 0.6 M HCl and 0.5 N NaOH to obtain a pH of 7 and was added to eight 250 mL plastic sample bottles, each containing 0.25 g of the filter media (modified E-33), completely filling the bottles and leaving no head space. The sample bottles were placed in a tumbler and rotated at a rate of 50 rpm for 48 h. An initial 25 mL sample (at time zero) was taken with the excess arsenic solution to test the pH, alkalinity, total inorganic carbon (TIC), inductively coupled argon plasma atomic emission spectroscopy (ICAP), and As. After 48 h, the sample bottles were removed from the tumbler and tested for pH, As, and ICAP.Antibacterial activity
A pure culture of E. coli #8, Water main break, June 1997, Louisville, Ky and S. auerus (ATCC 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923) were grown on nutrient agar plates for 36 h at room temperature. To determine the zone of inhibition for each media preparation, two grains of media were placed on a plate containing a lawn of either E. coli or S. auerus with unmodified E-33 media used as a control. All inoculated plates were incubated for 36 h at room temperature and the zone of inhibition recorded by averaging the measurement of the diameter of duplicate trials.
923) were grown on nutrient agar plates for 36 h at room temperature. To determine the zone of inhibition for each media preparation, two grains of media were placed on a plate containing a lawn of either E. coli or S. auerus with unmodified E-33 media used as a control. All inoculated plates were incubated for 36 h at room temperature and the zone of inhibition recorded by averaging the measurement of the diameter of duplicate trials.
Materials analysis
For analysis by SEM, the samples were mounted on aluminum stubs using a carbon tape with the magnetic samples lightly coated with carbon using a Denton Desk 5 sputter coater to reduce the charging effects. Samples were analyzed using a JEOL JSM-6490LV and JEOL JSM-7600 F (JEOL USA, Inc., Peabody, MA) with an Oxford X-Act energy dispersive spectrum (EDS) system (Oxford Instruments, Concord, MA) for imaging and elemental analysis. Images and EDS were captured using an accelerating voltage of 1–3 kV and or 30 kV respectively and spectra collected for 50 live seconds on a process time of 5 at 30 to 50 percent dead-time.XRD analysis was performed on a Panalytical (Expert) 2-theta diffractometer (Panalytical, Almelo, The Netherlands) with copper Kα radiation used to identify crystalline phases. Scans were performed with the diffractometer, ranging from 10 to 80°. X-ray florescence spectra (Panalytical, Almelo, The Netherlands) were recorded on pressed pallets using a Panalytical Axios Instrument.
Conclusions
In our experiments, E-33 was modified with silver using green approaches with several different reducing agents such as NaBH4, ascorbic acid, sodium citrate, and tea extract to produce core-shell composites. These core-shell composites retained their ability to remove arsenic from water by adsorption while the silver coating exhibits the potential to inhibit bacterial growth. The samples prepared from NaBH4 and tea extract had 94 and 91 mg L−1 of arsenic removal capacity compared to the control E-33 sample (97 mg L−1). Also, E-33 coated with silver prepared from NaBH4 exhibited higher S. auerus (ATCC 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923) inhibition compared to other samples prepared and control sample. Current research focuses on further characterizing and analyzing silver-coated E-33 in pilot scale studies to examine arsenic adsorption and biofilm development in columns. This research highlights the potential of using green approaches to combine a core-shell approach to create multifunctional components that are absent from current approaches.
923) inhibition compared to other samples prepared and control sample. Current research focuses on further characterizing and analyzing silver-coated E-33 in pilot scale studies to examine arsenic adsorption and biofilm development in columns. This research highlights the potential of using green approaches to combine a core-shell approach to create multifunctional components that are absent from current approaches.
Notice
The U.S. Environmental Protection Agency, through its Office of Research and Development, funded and managed, or partially funded and collaborated in, the research described herein. It has been subjected to the Agency's administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the Agency, therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.Acknowledgements
The authors wish to thank Keith Kelty for ICP-MS analysis and Steve Harmon for XRF analysis. The authors would also like to thank Emily Nauman of Pegasus Technical Services, Inc. and John Olszewski for assistance with manuscript review and editing.References
- B. Baruwati, M. N. Nadagouda and R. S. Varma, J. Phys. Chem. C, 2008, 112(47), 18399–18404 CAS.
- V. Polshettiwar, M. N. Nadagouda and R. S. Varma, Chem. Commun., 2008, 6318–6320 RSC.
- M. N. Nadagouda and D. Lytle, Journal of Nanotechnology, 2011, 1–8, DOI:10.1155/2011/972486.
- M. N. Nadagouda and V. Abbaraju, Talanta, 2003, 60, 139–147 CrossRef.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS.
- D. Tang, R. Yuan and Y. Chai, J. Phys. Chem. B, 2006, 110, 11640–11646 CrossRef CAS.
- A. Tanimoto and S. Kuribayashi, Magn. Reson. Med. Sci., 2005, 4, 53–60 CrossRef CAS.
- E. R. Wisner, E. G. Amparo, D. R. Vera, J. M. Brock, T. W. Barlow, S. M. Griffey, C. Drake and R. W. Katzberg, J. Comput.-Assisted Tomogr., 1995, 192, 211–5 Search PubMed.
- E. Matyjas-Zgondek, A. Bacciarelli and E. Rybicki, Proceedings of the Aachen-Dresden International Textile Conference 2008, 2nd, Dresden, Germany, Dec. 4–5 Search PubMed.
- Y. Zhang, S. Zhong, M. Zhang and L. Maisheng, J. Mater. Sci., 2009, 44, 457–462 CrossRef CAS.
- S. Mahapatra and N. Karak, Mater. Chem. Phys., 2008, 112, 1114–1119 CrossRef CAS.
- E. Barreiro, J. Casas, M. Couce, A. Sanchez, R. Seoane, J. Sordo, J. Varela and E. Vazquez-Lopez, Eur. J. Med. Chem., 2008, 43, 2489–2497 CrossRef CAS.
- J. Thiel, L. Pakstis, S. Buzby, M. Raffi, C. Ni, D. J. Pochan and S. Ismat Shah, Small, 2007, 3(5), 799–803 CrossRef CAS.
- S. Tan, L. Zhang, Y. Liu, Q. Shi, Y. Ouyang and Y. Chen, J. Ceram. Soc. Jpn., 2008, 116, 767–770 CrossRef CAS.
- M. Vukcevic, A. Kaijadis, S. Dimitrijevic-Brankovic, Z. Lausevic nad and M. Lausevic, Sci. Technol. Adv. Mater., 2008, 9, 015006 CrossRef.
- E. Verne, M. Miola, S. Ferraris and S. Key, Eng. Mat., 2008, 361–363 (Pt. 2, Bioceramics), 1195–1198 Search PubMed.
- L. Maria, J. Souza, M. Aguiar, S. Wang, J. Mazzei, I. Felzenszwalb and S. J. Amico, J. Appl. Polym. Sci., 2008, 107, 1879–1886 CrossRef CAS.
- N. Masuda, M. Kawashita and T. Kokubo, J. Biomed. Mater. Res. Part B, 2007, 83, 114–120 CrossRef.
- S. Zhang, D. Wu, L. Wan, H. Tan and R. Fu, J. Appl. Polym. Sci., 2006, 102, 1030–1037 CrossRef CAS.
- S. Chen, J. Liu and H. Zeng, J. Mater. Sci., 2005, 40, 6223–6231 CrossRef CAS.
- S. J. Ghosh, J. Macromol. Sci., Part A: Pure Appl. Chem., 2005, A42, 765–770 CAS.
- M. Ignatova, D. Labaye, S. Lenoir, D. Strivay, R. Jerome and C. Jerome, Langmuir, 2003, 19, 8971–8979 CrossRef CAS.
- M. N. Nadagouda and R. S. Varma, Green Chem., 2008, 10, 859–862 RSC.
- M. N. Nadagouda and R. S. Varma, Aust. J. Chem., 2009, 62, 260–264 CrossRef CAS.
- H. J. Allen, C. A. Impellitteri, D. A. Macke, J. L. Heckman, H. C. Poynton, J. M. Lazorchak, S. Govindaswamy, D. L. Roose and M. N. Nadagouda, Environ. Toxicol. Chem., 2010, 29(12), 2742–2750 CrossRef.
- M. N. Nadagouda and R. S. Varma, Macromol. Rapid Commun., 2008, 29, 155–159 CrossRef CAS.
- M. N. Nadagouda, T. F. Speth and R. S. Varma, Acc. Chem. Res., 2011, 44(7), 469–478 CrossRef CAS.
- R. S. Harisha, K. M. Hosamani, R. S. Keri, S. K. Nataraj and T. M. Aminabhavi, Desalination, 2011, 252, 75–80 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: Additional SEM images, EDX data and steromicrographs. See DOI: 10.1039/c2ra01306a/ |
| This journal is © The Royal Society of Chemistry 2012 |
