Different types of phase separation in binary monolayers of long chain alkyltrichlorosilanes on silicon oxide†
Simon
Desbief‡
abc,
Lionel
Patrone
*abc,
Didier
Goguenheim
bc and
Dominique
Vuillaume
d
aAix-Marseille Université, IM2NP
bCNRS, IM2NP UMR 7334
cInstitut Supérieur de l'Electronique et du Numérique, IM2NP Maison des Technologies, Place G. Pompidou, F-83000, Toulon, France. E-mail: lionel.patrone@im2np.fr; Fax: +33 494 038 951; Tel: +33 494 038 950
dInstitute for Electronics, Microelectronics and Nanotechnology (IEMN), CNRS/University of Lille, Avenue Poincaré, BP 60069, F-59652 cedex, Villeneuve d'Ascq, France
First published on 21st December 2011
Abstract
In the present work, we studied the growth and morphology of binary monolayers made with triacontyltrichlorosilane (C30H61SiCl3) as the long chain and hexadecyltrichlorosilane (C16H33SiCl3), octadecyltrichlorosilane (C18H37SiCl3) or eicosyltrichlorosilane (C20H41SiCl3) as the short chain according to the deposition conditions on silicon oxide, using mainly atomic force microscopy, ellipsometry, and contact angle measurements. We distinguished three types of different phase separation. Besides the classical phase separation by islands of long chains in a surrounding phase of shorter molecules, we observed a separation involving dendritic “filaments” of long chains in a shorter chain phase, regardless of the couple of molecules studied. A third sort of phase separation also appears in the case of triacontyltrichlorosilane with octadecyltrichlorosilane or eicosyltrichlorosilane: formation of “holes”, i.e. islands of the shorter molecule in a surrounding phase of long chains. We obtained “hole” and “filament” type phase separation only by working below a critical temperature, which depends on the molecule length. Moreover, the level of ambient relative humidity was shown to have an impact on “hole” versus “filament” phase separation types. We then showed that for phase separation by islands or holes the composition of binary monolayers (RSAM) prepared at low humidity (18% RH) is almost the same as that of the silanisation solution (Rsol). Conversely, for SAMs prepared at high humidity (45% RH) RSAM is always lower than Rsol whatever the type of phase separation by islands or holes. Moreover, concerning phase separation with C30 filaments we observed that RSAM is independent from both the nature of the short molecule and the humidity conditions. Considering a diffusion limited aggregation growth model, we found that at high humidity the monolayer growth is mainly driven by the diffusion coefficient, while at low humidity the deposition rate from solution is the leading parameter of the growth. The efficiency of phase separation is also addressed.
Introduction
One of the most promising ways of tailoring surface properties is proposed by molecular self-assembly,1,2 which consists of the spontaneous adsorption and organization of molecules within a monolayer. Self-assembled monolayers (SAMs) can be used for various purposes like tribology, surface passivation, formation of specific interfaces, and so on. An important field of application is molecular electronics within which self-assembly is a very powerful approach to organize at large surface scale molecules showing particular electronic properties (like insulator, molecular wire, memory, diode, and so forth).3–6 For such applications, using a silicon substrate in a hybrid approach should allow compatibility with microelectronic technology.7,8 For this purpose, trichlorosilane grafting heads are convenient. Indeed, they enable the formation of cross-linked SAMs anchored with a high chemical stability to various hydroxyl terminated surfaces, such as surfaces pre-functionalized with an OH end-group9 or oxide surfaces including silicon oxide.10–15Besides the formation of dense and robust monolayers of a single molecular species, organosilane molecules are able to present a very interesting property: phase separation, as reported in the literature for aliphatic16–24 or aromatic–aliphatic25–28 organosilane binary mixtures. Focusing on binary alkylsiloxane SAMs, phase separation can be achieved from aliphatic molecules of different lengths,16–20 and/or bearing different terminal moieties.18,21–24 As a main result, that such SAMs are either phase separated within islands16,17,19 or homogeneously mixed,22 these studies highlight that SAM composition does not necessarily reproduce the solution composition, but is mainly determined by kinetics through the relative surface adsorption rates of molecules.18 Particularly, when two organosilanes composed of alkyl chains of different lengths are solubilised in the silanisation solution, the difference in the van der Waals interactions leads to the formation of nanometric or micrometric domains of one type of molecule in a continuous phase of the other one (see Fig. 1). It is thus possible to tailor the domain size by acting on several parameters such as the molecular length difference, the deposition conditions, the nature of the reactive head of one or all the compounds, and so forth.
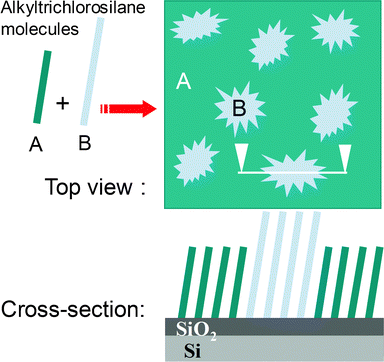 | ||
| Fig. 1 Scheme of the phase separation between two alkyltrichlorosilanes of different lengths. | ||
This phase separation can be used as a non lithographic method enabling it to go beyond the limit of conventional lithography for preparing active nanostructures if it is combined with a functionalization reaction.3–6 Indeed, one can give interesting properties (e.g. electronic properties, such as current rectification, resonant tunneling, and so on) to the type of molecules forming domains by grafting specific nanostructures or molecular species on the top. In order to be able to functionalize islands, the corresponding molecules have to bear a reactive moiety such as in the case of mercapto-terminated alkylsiloxane nanodomains decorated by gold nanoparticles.24 Alternate approaches have been developed based on various successive reaction steps, among which one can cite the grafting of gold nanoparticles on top of modified methyl-terminated alkylsiloxane islands,29 or of molecular species using surface vinyl reactive end-groups. For the latter case, when the self-assembly process is complete and the vinyl-terminated islands are formed, oxidation of the monolayer will transform vinyl moieties into carboxylic acid functions, which are necessary to achieve a subsequent esterification reaction. This gives the islands targeted electronic properties such as current rectification as demonstrated with phenyl cycles tethered on top of alkyltrichlorosilanes.5
Before controlling the phase separation, it is necessary to know the behaviour of the single component monolayers. First, the deposition temperature is a crucial parameter in determining the quality of the monolayer. Indeed, Brzoska et al.30,31 have highlighted the existence of a critical temperature, linearly dependent with the number of carbon atoms in the alkyl chain, above which the monolayer is disordered, and below which it is well ordered. Concerning the growth process of alkyltrichlorosilane SAMs, dendritic islands growing within a model based on diffusion limited aggregation32 are usually observed for long chains such as n-octadecyltrichlorosilane33,34 or longer alkyltrichlorosilanes.35 Nevertheless the existence of two critical temperatures defining three growth modes has been emphasized:36 the expanded liquid (EL) mode by formation of a homogeneous disordered monolayer at high temperatures (θ > θd), the condensed liquid (CL) mode by formation of dense and ordered islands at low temperatures (θ < θc), and the coexistence of these two growth modes (EL+CL) for intermediate temperatures (θc < θ < θd). From these studies, it appears that obtaining ordered alkyltrichlorosilane SAMs at ambient temperature should only be possible for long alkyl chains, with more than 16 carbon atoms.30,31
Another crucial parameter for obtaining dense and well ordered monolayers is the relative humidity in the deposition atmosphere.12,14,37–42 In fact, the water layer physisorbed on the bare substrate is essential for the growth of an alkyltrichlorosilane monolayer. On one hand, it is necessary for the hydrolyzation of the trichlorosilane heads. On the other hand, this layer acts as a lubricant, allowing the molecules to gather together on the surface in order to maximize van der Waals interactions between alkyl chains.
Although temperature and ambient humidity are crucial parameters, their combined roles in binary alkylsiloxane SAM growth and nanostructuration still remain very poorly known. The present work addresses this point by studying three couples of alkyltrichlorosilane molecules mixed at various ratios, with two values of temperature and of humidity. Given the key issue of being able to develop good quality binary SAMs on silicon at ambient conditions, and according to our previous study of long alkylsiloxane SAM formation,35 we focused this study on binary SAMs on silicon oxide with triacontyltrichlorosilane as long chain, and hexadecyl-, octadecyl-, and eicosyltrichlorosilane as short chains.
Experimental
Molecules
n-Hexadecyltrichlorosilane (C16H33SiCl3, referred to as C16) was purchased from United Chemical Technologies Inc., n-octadecyltrichlorosilane (C18H37SiCl3, referred to as C18) and n-eicosyltrichlorosilane (C20H41SiCl3, referred to as C20) from ABCR GmbH & Co. KG and Gelest Inc., n-triacontyltrichlorosilane (C30H61SiCl3, referred to as C30) was obtained from Gelest Inc. and Fluorochem Ltd. C16 and C18 had a purity of 95%, C20 was distilled prior to use, and C30 purity was 80%, the other 20% being composed of 22 and 28 carbon atom alkyltrichlorosilanes. C30 was used as received, which was observed not to alter the formation of good quality n-triacontyltrichlorosilane single SAMs in a comparable manner to those of the shorter and purer alkyltrichlorosilanes.35 Note that shorter molecules blended with n-triacontyltrichlorosilane in the C30 product used (C22–C28) are distinct from short chains used in this work (C16–C20) since they are longer. Moreover the uncertainty of 20% on the real proportion of n-triacontyltrichlorosilanes may be hindered by that on the molecular proportions injected in solution which are controlled within ∼10% of uncertainty.SAM preparation protocol
The silanisation process is highly sensitive to various experimental parameters such as temperature, solvent type, residual water content, age of the solution, cleaning procedure of the substrates and humidity/ambient environment. Therefore, in order to obtain reproducible well ordered alkyltrichlorosilane SAMs on silicon oxide in normal laboratory conditions we developed the following deposition protocol adapted from that of Brzoska et al.30,31 and Breuil et al.17 Such a protocol allowed us to control key parameters of the silanisation process (substrate cleaning and preparation, temperature, humidity, solvent type, age of the solution, silanisation duration) and it was successfully applied to the preparation of reproducible, dense, and well-ordered SAMs of single alkyltrichlorosilane components varying from C16 to C30.35 First, the substrate (typically 5 × 5 mm²) cut from Si (100) wafers and covered with native oxide was degreased in a sonicated chloroform bath, and then dried under a nitrogen flux. Then the substrate was immersed for ∼30 min in a piranha mixture (H2SO4–H2O2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, caution, this mixture is exothermic and reacts violently with organics!) in order to remove any organic impurities from the surface and to increase the amount of hydroxyl moieties (OH) necessary for the grafting of the hydroxylated silane heads. Within ∼5 s after rinsing abundantly with pure de-ionised water (18 MΩ cm−1), in order to prevent contamination from the atmosphere, the substrate was quickly immersed into a beaker of deionised water, which was then transferred into a glovebox filled with nitrogen and where the relative humidity was kept constant. At this step, the silicon covered with an OH-rich clean native oxide surface was dried under a nitrogen flux and, still within ∼5 s, it was dipped into the silanisation solution composed of a mixture of hexadecane (99+%, Sigma-Aldrich), carbon tetrachloride (99% Sigma-Aldrich) and the alkyltrichlorosilanes (at 10−2 M). Beforehand, this solution was thermalized on a thermostatted plate for ∼20 min at the temperature of deposition at which it is maintained during all the silanisation time varying from several seconds to 1.5 h depending on the experiment. Then, the sample was rinsed in a sonicated chloroform bath, possibly further swept with a soft tissue soaked in chloroform in order to remove all the ungrafted material from the surface (this operation could sometimes form straight grooves in the monolayer visible in AFM images), and eventually dried with a nitrogen flux. Two silanisation temperatures were used for the SAM preparation: 11 °C and 20 °C named in the following LT (for low temperature) and HT (for high temperature), respectively. Concerning relative humidity (RH), SAMs were grown at either 18% RH or 45% RH referred to as LH (for low humidity) and HH (for high humidity), respectively.
3, caution, this mixture is exothermic and reacts violently with organics!) in order to remove any organic impurities from the surface and to increase the amount of hydroxyl moieties (OH) necessary for the grafting of the hydroxylated silane heads. Within ∼5 s after rinsing abundantly with pure de-ionised water (18 MΩ cm−1), in order to prevent contamination from the atmosphere, the substrate was quickly immersed into a beaker of deionised water, which was then transferred into a glovebox filled with nitrogen and where the relative humidity was kept constant. At this step, the silicon covered with an OH-rich clean native oxide surface was dried under a nitrogen flux and, still within ∼5 s, it was dipped into the silanisation solution composed of a mixture of hexadecane (99+%, Sigma-Aldrich), carbon tetrachloride (99% Sigma-Aldrich) and the alkyltrichlorosilanes (at 10−2 M). Beforehand, this solution was thermalized on a thermostatted plate for ∼20 min at the temperature of deposition at which it is maintained during all the silanisation time varying from several seconds to 1.5 h depending on the experiment. Then, the sample was rinsed in a sonicated chloroform bath, possibly further swept with a soft tissue soaked in chloroform in order to remove all the ungrafted material from the surface (this operation could sometimes form straight grooves in the monolayer visible in AFM images), and eventually dried with a nitrogen flux. Two silanisation temperatures were used for the SAM preparation: 11 °C and 20 °C named in the following LT (for low temperature) and HT (for high temperature), respectively. Concerning relative humidity (RH), SAMs were grown at either 18% RH or 45% RH referred to as LH (for low humidity) and HH (for high humidity), respectively.
Ellipsometry
The monolayer thickness was first measured by ellipsometry in order to give the first proof of the deposition of a monolayer on the surface. Moreover, this measurement gives us the first indication on the global quality of the monolayer. Indeed, a monolayer thickness lower than the length of all-trans conformed molecules implies the SAM is either incomplete or disordered, i.e., the molecules are tilted with respect to the surface normal (the angle is usually ∼10°–15° in ordered all-trans alkyltrichlorosilane SAMs on SiO2) or the molecules have a gauche defect.According to Wasserman et al.,43 the expression of the expected monolayer thickness in a dense, well ordered phase, corresponding to the length of alkyltrichlorosilane in its all-trans conformation is:
| d(Å) = 1.26(n − 1) + 4.78 | (1) |
Thickness measurements were performed using a Sentech SE400 ellipsometer, with a 632.8 nm He–Ne laser at an incidence angle of 70°. In a dedicated study, we first determined the oxide thickness corresponding to our preparation procedure of the substrate prior to silanisation, namely ∼17 Å. From this reference value, we could measure the SAM thickness assuming an optical index of n = 1.45 for every molecule, considering the material is isotropic and homogeneous.44 Indeed, the alkyltrichlorosilanes used in this study are long enough to form well-ordered homogeneous and dense final SAMs within the temperature range used (11–20 °C),30,31 which supports such an assumption. Moreover, it is consistent with previous results that we obtained on single component alkyltrichlorosilane SAMs for which such ellipsometry measurements gave a thickness consistent with the molecule being perpendicular to the surface (with a possible tilt angle of up to 15°).35 All the results are an average of at least 5 measurements at different locations on the surface and from a minimum of 2 samples.
The thickness of binary SAMs measured by ellipsometry is directly related to the C30 rate ηL (0 ≤ ηL ≤ 1) in the SAM through the following relation:
| Thickness = dL.ηL + dS.(1 − ηL) | (2) |
The C30 proportion could then be estimated for each binary SAM allowing us to calculate the ratio RSAM of C30 on short chains in the SAM as follows:
| RSAM = ηL/(1 − ηL) | (3) |
Such a calculation gives an estimated value of RSAM but since experimental error should be similar for all measurements, it gives at least a general trend of the evolution of the SAM composition as a function of experimental preparation parameters. Taking into account the uncertainty on the tilt angle of the molecules (up to 15°), the error made on the composition of the binary SAM deduced from the measured thickness is within the range ±10%. Note that such an error should further mask that of the C30 content in solution caused by its low purity.
Contact angle measurements
Static deionised water contact angles were measured to determine the homogeneity of the monolayer. Measurements were carried out using a DSA 10 MK2 goniometer from Krüss GmbH. Contact angle values result from an average of at least 5 measurements performed at various locations on the surface and from a minimum of 2 samples.Fourier transform infrared spectroscopy (FTIR)
FTIR experiments were performed in transmission with a Perkin-Elmer Spectrum GX spectrometer equipped with a DTGS detector. Spectra were registered at a resolution of 2 cm−1, and 30 to 100 scans were accumulated.Atomic force microscopy (AFM)
AFM analyses were performed under ambient conditions, using a Multimode system equipped with a Nanoscope IIIa controller from Veeco Instruments Inc. All images were obtained in Tapping™ mode, using Si tips with a resonance frequency of 150–350 kHz. AFM images are presented with topography coded from dark to bright using WSxM software.45 The height of molecular structures present on the surface was measured through a sliced view of a given location in the image obtained from the profile function of WSxM. In addition, distribution of relative heights was plotted from roughness analysis in order to take into account height measurement dispersion.Results and discussion
We have studied monolayers with C30 as the long chain because, being the longest molecules in this study, they should own the highest critical temperature below which they could form ordered SAMs.30,31 Indeed, in a previous work,17 Breuil et al. showed that working with C18 and C12 at −3 °C (this temperature is below the critical temperature of those two molecules, according to Brzoska et al.30,31) was not cold enough for the two molecules to grow only within a condensed liquid phase of dense and homogeneous islands. In fact C12 was growing in a disordered way all the same, which was interfering with the diffusion of C18, leading to a poor phase separation, i.e., with isolated C18 scattered within the surrounding phase.As shown in the introduction, temperature is a crucial parameter for both the homogeneity and the density of the monolayer. In order to be able to work at room temperature (around 20 °C), we used longer molecules than Breuil et al.17 Thus we chose C30 as long chain in all our binary SAMs, and C20, C18 and C16 as short chains, regarding their respective critical temperatures estimated to be 70, 35, 28, and 21–24 °C,30,31 respectively. Water contact angle measurements performed on the final various binary SAMs prepared during 1.5 h of silanisation gave values ranging from ∼105° to ∼111°, consistent with a complete monolayer in an all-trans conformation.2 This is further supported by the spectral position of methylene stretching modes at ∼2850 cm−1 (symmetric) and ∼2919 cm−1 (asymmetric) probed by FTIR measurements (see Supplementary Information†).
We observed that these various binary SAMs could exhibit three different types of phase separation (Fig. 2), regardless of the molar ratio Rsol of C30 on short chain molecules injected in solution:
- Separation by protruding islands of the longer molecule, which is the more usual (Fig. 2a)
- Separation by “filaments” only at 11 °C (LT) for all the studied short chain molecules i.e., C16, C18, or C20 , which has never been reported in the literature to our knowledge (Fig. 2c)
- And, only in monolayers with C18 or C20 as short chains at both temperature values of 11 °C (LT) and 20 °C (HT), we obtained a separation by “holes” (Fig. 2b), that is to say islands of short chains in a continuous phase of long chains, which has never been reported in literature for alkyltrichlorosilanes to our knowledge. Modification of the molecular ratio in solution and/or of relative growth kinetics of the two molecules should determine whether the islands are composed of the longer (protruding) or shorter (holes) molecules.
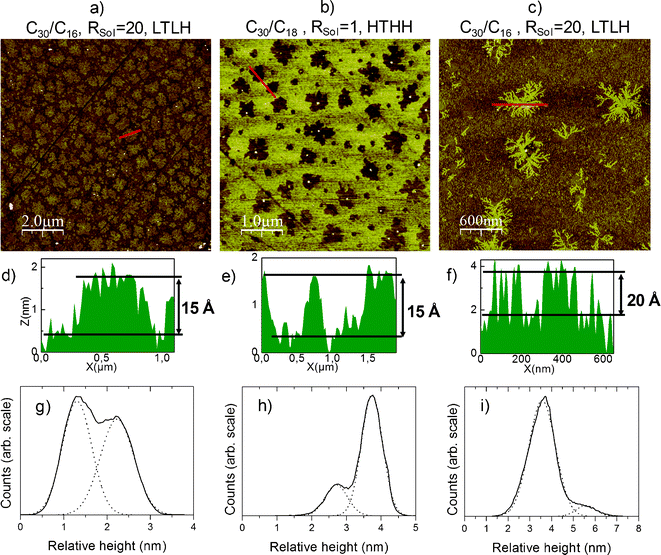 | ||
| Fig. 2 AFM images of the different types of phase separation observed: a) islands (10 × 10 μm2), b) holes (5 × 5 μm2), c) filaments (3 × 3 μm2), d), e), f) respective associated height profiles, and g), h), i) respective relative height bimodal distributions (solid) fitted by Gaussian functions (dot), the first peak corresponding to the short molecules, the second to the long. Rsol is the molar ratio of C30 on the short chain molecules injected in solution. | ||
We can see on the topographic profiles and relative height distributions associated with AFM images that the difference in height of these structures is consistent with the lengths of the molecules we used (41.3 Å, 28.7 Å, 26.2 Å and 23.7 Å for C30, C20, C18 and C16 respectively, from eqn (1) given by Wasserman et al.43). In relative height distributions, the global height difference calculated over the image is slightly reduced probably because long and short molecules are not perfectly phase separated.
Controlling the size of the phase separated islands is a key issue for technological applications. However, one can see for instance in the AFM image of Fig. 2a that a broad distribution of island sizes is encountered within the same binary SAM. Nevertheless, if it appears difficult to obtain a narrow size distribution, the maximal size of C30 islands increases with the amount of C30 injected in solution, as shown in Fig. 3.
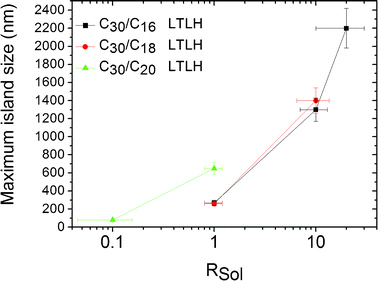 | ||
| Fig. 3 Maximal island size of C30 phase separated from C16, C18, and C20 at LTLH conditions, as a function of the ratio Rsol of C30 on the short molecule in the solution. | ||
Another key issue for potential applications of these binary SAMs stands in the control of the various morphologies and composition of the final SAM. For this purpose, we investigated each kind of phase separation according to the deposition parameters.
In all our experiments a given sample exhibited a homogeneous morphology being always exclusively composed of one type of phase separation: islands, holes, or filaments. Examples are provided in the Supplementary Information† (with Fig. S1, S2, and S3 displaying AFM images of different areas of the same samples as those of Fig. 2a, 2b, and 2c, respectively). Moreover, each experimental result characterized by a surface morphology and a type of phase separation was obtained several times with various samples (see Supplementary Information† Fig. S4, S5, and S6). In addition to topography AFM images shown in the paper, corresponding phase AFM images are presented in Fig. S7, S8 and S9 of the Supplementary Information† in order to show the contrast difference between the various structures in the SAM. This is usually more pronounced for the filaments (see Fig. S7c and S9†).
Phase separation by islands (protruding or holes)
Let us first analyse the formation of islands in a binary monolayer. Since it should highly depend on the SAM growth behaviour of each molecule separately, it is helpful to begin with the growth features of the various single component SAMs. As already underlined, at LT and HT C30 molecules are long enough to form islands during SAM growth.35 As for single SAMs of C18 and C20 they were observed to grow forming islands mixed with a disordered EL phase,35i.e., both 11 °C and 20 °C are located between the proposed two critical temperatures of these molecules36 (see Introduction), namely θc and θd. Nevertheless, C16 was shown to form islands mixed with an EL phase only at LT, whereas their SAMs grow exclusively in a complete disordered EL phase at HT,35i.e., the critical temperature value θd of C16 is strictly below 20 °C. Consequently, in binary SAMs of C30 mixed with C16, C18, or C20 there are three possible different scenarios whose scheme is displayed in Fig. 4, leading to the formation of islands of one type of molecule in a continuous phase of the other one. Each scenario should be determined by the temperature dependence of the growth mode of each molecule.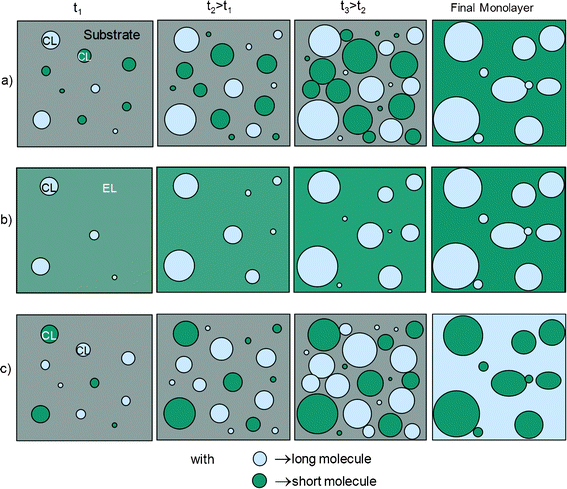 | ||
| Fig. 4 Scheme of the different scenarios for the growth of a binary monolayer leading to the formation of islands. a) Each compound forms dense islands, and b) the long molecule forms dense islands and the short one a disordered phase, in both cases with the final SAM eventually composed of islands of the long molecule. c) Each compound forms dense islands but with the final SAM eventually composed of islands of the short molecule (“holes”). | ||
Fig. 4a illustrates the case where the two molecules grow by dense and homogeneous islands, leading to the formation of protruding islands of the longer molecule (referred to as “islands” in the following) in a continuous phase of the shorter one. This case should not be possible only for C16 at HT. Fig. 4b illustrates the case where the longer molecule grows by dense islands, while the shorter one grows by a disordered phase. Such a case should only be encountered for the C16 short molecule at HT. In Fig. 4c, one can see the case where the two molecules grow by dense islands, which should be attainable in principle with short chains being C16 at LT and C18 or C20 at LT and HT, but resulting in the formation of islands of the shorter molecules (let us say “holes”) in a continuous phase of the longer molecules.
As explained above, cases a) and c) of Fig. 4 are possible only for deposition temperatures below the lowest critical temperature θd of the two compounds,36 leading to the formation of dense and homogeneous islands for the two molecules. However, even if C16 SAM growth does exhibit islands at LT, the latter should be mixed with a thicker and larger disordered EL phase than in the case of C18 or C20 longer molecules, which should hinder C16 island formation in binary SAMs. Therefore, it may explain why we only observed “hole” formation with C18 or C20 used as the short molecules, even at a deposition temperature of 11 °C (LT). For a given couple of molecules, switching between the scenario of Fig. 4a (protruding islands) to that of Fig. 4c (“holes”) should be induced by modification of the molecular ratio in solution and/or modification of relative growth kinetics of the two molecules. These two parameters should determine the relative surface coverage of the two molecules. In particular the relative growth kinetics may be modified through the diffusion coefficient of each molecule that should depend on the alkyl chain length, temperature and humidity conditions and that has a direct influence on the size and shape of islands (namely, a higher diffusion usually leads to larger and less dendritic islands in single molecule SAMs).35
As an example, Fig. 5 shows an experimental observation of the first scenario, i.e., formation of C30 islands obtained via island growth of the two compounds, with several AFM images of the growth of a C30/C18 monolayer at LTLH with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in hexane and CCl4, taken after different silanisation durations. As shown by the profiles and relative height distributions associated with the AFM images, at every step of the growth one can distinguish two different heights of islands consistent with the length difference of the molecules in an all-trans conformation. In the meantime, an expanded liquid (EL) molecular phase should be present in the images taken at 45 s and 5 min since the “island” heights are less than the length of the corresponding molecules in an all-trans conformation. At the end of the growth, the SAM exhibits islands with a mean height of ∼14 Å which corresponds to the theoretical length difference of the molecules (see the profile and the relative height distribution of the image showing the final monolayer in Fig. 5d).
1 ratio in hexane and CCl4, taken after different silanisation durations. As shown by the profiles and relative height distributions associated with the AFM images, at every step of the growth one can distinguish two different heights of islands consistent with the length difference of the molecules in an all-trans conformation. In the meantime, an expanded liquid (EL) molecular phase should be present in the images taken at 45 s and 5 min since the “island” heights are less than the length of the corresponding molecules in an all-trans conformation. At the end of the growth, the SAM exhibits islands with a mean height of ∼14 Å which corresponds to the theoretical length difference of the molecules (see the profile and the relative height distribution of the image showing the final monolayer in Fig. 5d).
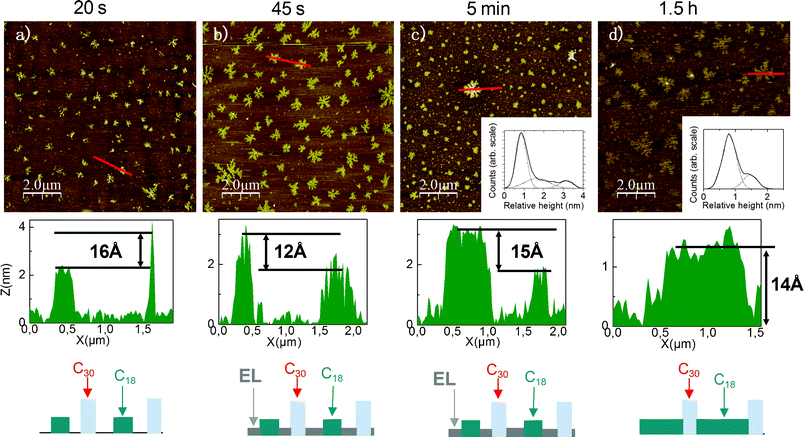 | ||
Fig. 5 AFM images (10 × 10 μm2) of the growth of a C30/C18 monolayer at LTLH with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio Rsol in hexane and CCl4, and associated representative height profiles. EL stands for the expanded liquid molecular phase. Inset shows relative height distributions (solid) fitted by Gaussian functions (dot). The results are consistent with the height profiles. In AFM image c) three peaks arise: the first one corresponds to the EL phase, the second to C18 islands, and the third to C30 islands. In AFM image d) the distribution is bimodal with C18 (1st peak) and C30 islands (2nd peak). 1 ratio Rsol in hexane and CCl4, and associated representative height profiles. EL stands for the expanded liquid molecular phase. Inset shows relative height distributions (solid) fitted by Gaussian functions (dot). The results are consistent with the height profiles. In AFM image c) three peaks arise: the first one corresponds to the EL phase, the second to C18 islands, and the third to C30 islands. In AFM image d) the distribution is bimodal with C18 (1st peak) and C30 islands (2nd peak). | ||
Phase separation by filaments
As shown in Fig. 2c, we observed only in SAMs prepared at 11 °C (LT) and with C16, C18, or C20 as short alkyl chain molecules a singular type of phase separation, which has never been reported in the literature to our knowledge for binary monolayers of alkyltrichlorosilanes: demixing by “filaments”.An example of filament growth studied at different steps is shown in Fig. 6. Height profiles associated with the AFM images of Fig. 6 and the relative height distribution at 5 min show that there are two different heights within a single “island”, at every step of the growth. It is also clear that the difference between those heights is consistent with the difference between respective theoretical lengths of C30 and C18. Thus, this should correspond to C30 filaments growing inside C18 islands. A clear contrast between those filaments and the surrounding islands is noticeable in the phase AFM images (see for example the image at 5 min in Fig. S9 of the Supplementary Information†). At the end of the growth, one can see on the AFM images corresponding to 1.5 h of silanisation, that the surface presents a lot of little filaments, which is clearer on the zoom below. The relative height distribution associated with this image shows that filaments are mainly ∼8 Å higher than the surrounding phase. This is consistent with C30 filaments surrounded by a phase mainly composed of C18, considering, moreover, that C30 molecules within filaments may be more tilted.
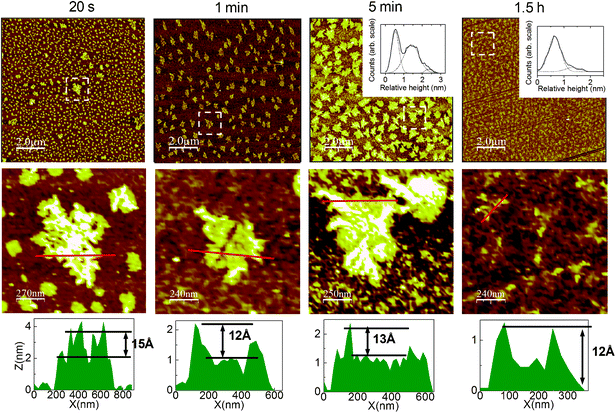 | ||
Fig. 6 Growth kinetics of a C30/C18 monolayer at LTLH with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio Rsol in hexadecane and CCl4. Upper AFM images are 10 × 10 μm2, and corresponding zooms are about 1.3 × 1.3 μm2. Insets show relative height distribution (solid) fitted by Gaussian functions (dot). At 5 min the first peak is associated to the EL phase, the second to C18 islands, and the third to C30 filaments. In the final SAM (1.5 h), the distribution is bimodal with C18 (1st peak) and C30 filaments (2nd peak). Typical height profiles are also shown. 1 ratio Rsol in hexadecane and CCl4. Upper AFM images are 10 × 10 μm2, and corresponding zooms are about 1.3 × 1.3 μm2. Insets show relative height distribution (solid) fitted by Gaussian functions (dot). At 5 min the first peak is associated to the EL phase, the second to C18 islands, and the third to C30 filaments. In the final SAM (1.5 h), the distribution is bimodal with C18 (1st peak) and C30 filaments (2nd peak). Typical height profiles are also shown. | ||
Fig. 7 shows another example of filament growth at LTLH within a C30/C18 SAM with a ratio in solution equal to 5. Fig. 7a represents the original AFM image, while in Fig. 7b we have highlighted the plateaus and the filaments for the sake of clarity. The height profile associated with the topographical AFM image shows indeed filaments 15 Å high embedded inside plateaus reaching a height of 8 Å from the surrounding C18 phase. Filaments are more clearly visible appearing darker in the corresponding phase AFM image displayed in Fig. 7c. Some of them are difficult to detect in the topographical image (see the bottom left-hand corner of Fig. 7c).
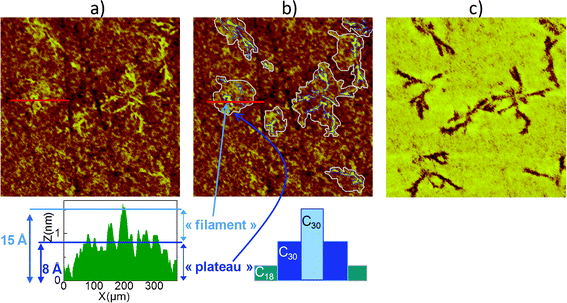 | ||
| Fig. 7 AFM images (1 × 1 μm²) of C30/C18 SAM prepared at LTLH with a ratio in solution Rsol = 5: a) topography and the associated height profile, b) topography with highlighted islands, c) phase (z scale = 15°). | ||
Since the formation of these filaments requires a low temperature (11 °C) it should involve the growth of islands for both long (C30) and all the short molecules of this study, including C16.35 However, compared to “island” or “hole” formation that also appears at 20 °C (HT), the filaments seem to require more ordered growth conditions corresponding to a temperature strictly below 20 °C. According to our observations, we can assume the existence of two different ways of obtaining “filament” type phase separation, which are illustrated in Fig. 8.
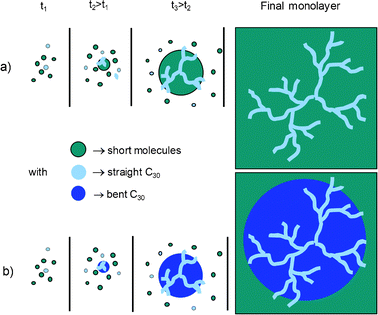 | ||
| Fig. 8 Scheme of two scenarios for the formation of C30 filaments surrounded in the final SAM (a) directly by a continuous phase of the shorter molecule, and (b) by an intermediate region of bent C30 that have collapsed. | ||
In the first scenario observed in Fig. 6, C30 filaments grow embedded into islands of short molecules (Fig. 8a). This behaviour involves a binary SAM growing below the lowest critical temperature θd of the couple of molecules allowing the two compounds to form dense and ordered islands in the film.36 Nevertheless, due to unclear reasons requiring further investigation, during this growth process C30 islands are trapped in between islands of the short molecule leading to the formation of these filaments.
Fig. 8b illustrates the second scenario for the formation of filaments reported in Fig. 7, where C30 and C18 grow by well separated dense and ordered islands. But, for some unclear reasons, C30 islands will collapse, except in the densest part of islands, leading to the formation of a plateau higher than the surrounding phase, and overhung by filaments.
Whatever the scenario, those filaments correspond to highly dendritic islands of the long C30 molecule with a slightly lower height than traditional larger islands which may indicate that molecules forming the filaments are more tilted. It is noticeable that filaments are observed only in binary SAMs of C30 at LT, but were never obtained during the growth of single C30 SAMs.35
Role of the experimental parameters on the type of phase separation
A selection of binary SAMs was prepared with various experimental conditions (couple of molecules, ratio in solution from 0.05 to 20, temperature, humidity). Each parameter was not systematically varied but a wide range of conditions were swept in order to extract a trend in the subsequent modification of the type of phase separation and of the SAM composition.Concerning all binary SAMs that have phase separated by islands or “holes”, Fig. 9 plots the ratio of long on short molecules (RSAM) estimated from the measured thickness (see eqn (2) and (3)) in the grafted monolayer versus the ratio of molecules injected in the silanisation solution (Rsol) at low (Fig. 9a) and high humidity (Fig. 9b). One can notice that at low humidity RSAM seems to follow Rsol, while at high humidity RSAM strays from Rsol. This difference of behaviour is enhanced for high Rsol values of 10 and 20 with RSAM staying well below Rsol at high humidity. Thus, this leads to the conclusion that at low humidity the growth is driven by thermodynamics, and by kinetics at high humidity.
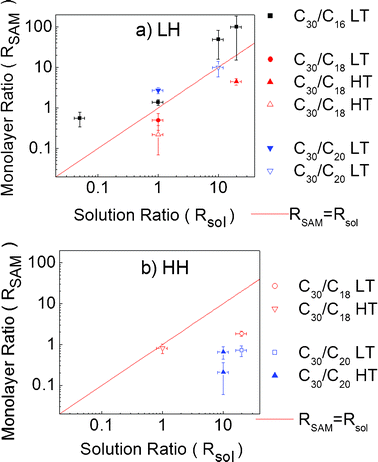 | ||
| Fig. 9 Plot of the ratio of molecules in the monolayer (RSAM) versus the ratio of molecules in the silanisation solution (Rsol) at a) low humidity (18% RH) and b) high humidity (45% RH). Filled symbols represent the separation by protruding islands, and empty ones represent the separation by “holes”. | ||
This can be understood in a simple way. Our experiments show that at low humidity we obtain nearly the same ratio of molecules on the substrate as in the solution. Indeed we know that at low humidity, the low amount of water on the substrate (∼one monolayer)46 hinders the diffusion of the molecules. Therefore the composition of the grafted monolayer is driven by the composition of the solution as the molecules that reach the surface cannot diffuse easily.
Conversely, in the high humidity case, there should be almost three monolayers of water on the SiO2 surface,17,37 which enables a very efficient surface diffusion of molecules. The composition of the monolayer is thus led by the SAM growth kinetics of each molecule. Considering that we have observed in a separate work that SAMs of C30 have lower growth kinetics than those of C16–20,35 it seems consistent that the proportion of molecules on the surface is not likely to be the same one as in the silanisation solution. More precisely, the more C30 are in the solution, the lower than Rsol RSAM should be. That indeed corresponds to what is observed in our experiments (see Fig. 9b).
From our observations summarized in Fig. 9, one can note that “hole” formation is more often observed at high humidity (Fig. 9b) than at low humidity (Fig. 9a). Therefore, favouring kinetic parameters may promote “hole” formation, which can be explained by the diffusion coefficient being higher for short molecules than for long molecules, thus allowing short molecules to diffuse more quickly to aggregate and form islands more easily.32,35
Fig. 10 shows the plot of the ratio RSAM of C30 on short molecules in monolayers that have phase separated by “filaments”, estimated from the thickness measured by ellipsometry (see eqn (2) and (3)), versus the corresponding ratio Rsol of molecules injected in the silanisation solution.
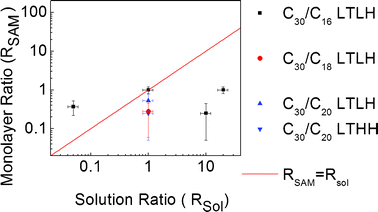 | ||
| Fig. 10 Plot of the ratio of molecules in the monolayer (RSAM) versus the ratio of molecules in the silanisation solution (Rsol) for monolayers that have phase separated by filaments. | ||
Whatever the couple of molecules, deposition condition, and solution ratio, one can notice that the plotted RSAM is around a value of 0.5. This observation leads to the conclusion that the estimated RSAM value is totally independent from the experimental conditions of deposition in the filament case, which may be favoured by C30 molecules being bent or tilted within filaments (Fig. 6 and 7). Moreover, as shown in Fig. 10, the high majority of “filament”-shaped binary SAMs were obtained at low humidity growth conditions. Since these “filaments” are highly dendritic, their formation is compatible with the weak molecular diffusion32,35 associated with the low amount of surface water adsorbed at low humidity.46
Analyses of Fig. 9 and 10 have shown that humidity plays a major role in determining the type of phase separation and the composition of the binary SAMs:
- for islands and “holes” the SAM composition tends to follow that of the solution at LH whereas kinetics effects dominate at HH,
- “holes” are favoured at HH whereas “filaments” are favoured at LH.
In order to have a global view on this effect of humidity, and to make it clearer how the temperature and the molecular ratio in solution influence the morphology of the SAM, the different types of phase separation observed, i.e., islands (I), “holes” (H), or “filaments” (F), have been summarized in Table 1 for every couple of molecules, deposition condition, and solution ratio.
If, for a given couple of molecules, the same conditions of preparation (i.e., ratio Rsol, temperature, humidity) could sometimes lead to different types of phase separation, a careful analysis of the results reported in Table 1 shows that it occurs only at “low temperature” (LT) conditions. Indeed, at “high temperature” (HT) preparation the parameters determined an exclusive type of phase separation: namely “holes” (see the case of C30/C18 at Rsol = 1 whatever the humidity), or islands (at LH for C30/C18 at Rsol = 20 and at HH for C30/C20 at Rsol = 1 or 10). Therefore, at HT the type of phase separation was controlled for all the samples we prepared. On the contrary, at LT a given couple of molecules and fixed preparation parameters could provide either “islands” or “filaments” all over the sample surface. This was only observed at LH, particularly for C30/C16 whatever the Rsol ratio used, but also for C30/C18 and C30/C20 at Rsol = 1.
| Rsol | ||||||
|---|---|---|---|---|---|---|
| 0.05 | 0.1 | 1 | 10 | 20 | ||
| a Data non reported in Fig. 9 and 10. | ||||||
| C30/C16 | LTLH | I/F | I/F | I/F | I/F | |
| C30/C18 | LTLH | I/F | ||||
| LTHH | H | |||||
| HTHH | H | |||||
| HTLH | H | I | ||||
| C30/C20 | LTLH | Fa | I/F | H | ||
| LTHH | F | H | ||||
| HTHH | Ia | I | ||||
Regarding the influence of temperature on the type of phase separation, as already mentioned, results gathered in Table 1 clearly show that “filaments” are only observed at LT. Concerning “hole” formation, C16 never forms “holes” in the various C30/C16 binary SAMs, not even at LT as in the scenario of Fig. 4c. We have already shown in a previous paper that C16 SAM grows in a disordered expanded liquid phase at HT and that LT is not low enough for promoting their growth with pure condensed liquid islands.35 Moreover, it appears here that C16 is not long enough to form “holes” when mixed with C30 in any conditions. On the contrary, although C18 SAMs have been shown to grow within mixed islands and expanded liquid,35 it appears long enough to form “holes” even at HT. “Holes” are usually observed at ratios Rsol greater or equal to 1. For predominant long chains at Rsol greater than 1, the possibility to obtain “holes” depends on temperature: it is possible at LT (see C30/C18 at LTHH), but never at HT. Therefore if temperature definitely controls the possible formation of “filaments”, it appears to also be a crucial parameter determining the presence or not of “holes”.
Finally, as a major concern, the quality of phase separation should be addressed. In most of the cases, the SAM thickness measured by ellipsometry is in agreement with that estimated from the island, “hole” or “filament” coverage in AFM images assuming molecules are perfectly phase separated and in an all-trans conformation (see Supplementary Information†). This means that phase separation is rather efficient and it further supports the use of ellipsometry in measuring the thickness of binary SAMs with two different alkyl chain lengths as already reported in the literature.44 However, one can note a discrepancy in some cases that mainly corresponds to high temperature (HT) for the shortest molecule tested at HT, i.e.C18, but interestingly not for C20. It is consistent with a subsequent more disordered phase of the short molecule preventing molecules to diffuse and to gather together. Whereas the role of humidity in phase separation does not show up, that of temperature appears again to be of prime importance in determining the efficiency of phase separation in those binary SAMs.
Conclusions
Binary monolayers of triacontyltrichlorosilane (C30) mixed with shorter alkyltrichlorosilanes (C16–20) exhibit different types of phase separation regardless of the ratio of molecules injected in solution. We observed standard protruding islands of the longest molecule, and other two original morphologies that have never been reported for such molecules in the literature to our knowledge: “holes” corresponding to islands of the short molecule, and C30 “filaments” that could be surrounded either directly by the phase of the short molecule or by an intermediate phase of C30 that has collapsed. These “holes” or “filaments” were observed only for depositions below the critical temperature of the two molecules, but exclusively at a lower temperature strictly below 20 °C for “filaments”. We thus proposed a possible explanation of their growth involving condensed liquid islands of both molecules in the SAM. As a general trend, we noticed that “hole” formation may be favoured by high humidity at which the diffusion of short molecules is promoted. On the contrary, “filaments” are more often observed at low humidity, consistent with their highly dendritic shape which is usually a signature of a low diffusion within a diffusion limited aggregation model. Regardless of the ratio of molecules injected in solution and the relative humidity, “holes” could not be achieved in two conditions: using C16 as short molecules and working at HT. This can be explained considering LT (respectively HT) has been shown as not being low enough for promoting a pure condensed liquid “island” growth mode of C16 (resp. C16, C18, C20) SAM.35 Therefore, working at a low enough temperature to allow a condensed liquid growth mode of short chain SAMs is necessary to enable the formation of “holes” in the binary SAMs. Moreover, from our experiments, we showed that for protruding islands and “holes” ambient relative humidity is a key parameter for controlling final SAM composition. Indeed, the growth is driven by thermodynamics at low humidity (18% RH), and by kinetics at high humidity (45% RH). Nevertheless for C30 “filaments” the final binary SAM composition seems to be independent of both relative humidity and the shorter molecule. As for the important issue of the efficiency of phase separation, it is usually better at low temperature enabling an ordered growth type for the short chains. However, in order to use those structures to build nanometric devices, some complementary studies should be addressed. We must understand more clearly which mechanisms lead to the formation of islands, “filaments” and “holes”, in order to better control the type and the morphology of phase separation. For the latter, it should be important to improve the control of the width of the size distribution of the molecular domains with a view to possible applications in molecular-based nanotechnology such as molecular electronics.Acknowledgements
Equipment was mainly funded by the “Objectif 2” EEC program, the FEDER, the “Conseil Général du Var” Council, the PACA Regional Council, Toulon Provence Méditerranée, and ISEN-Toulon which are acknowledged. Authors thank Hamidou Haidara and Roberto Lazzaroni for valuable and helpful discussions. D. V. thanks Francis Rondelez for stimulating discussions at the early stage of this work and for suggesting some ideas.References
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418 CrossRef CAS
.
-
A. Ulman, An introduction to ultrathin organic films, Academic Press, Boston, 1991 Search PubMed
.
- J. Collet, M. Bonnier, O. Bouloussa, F. Rondelez and D. Vuillaume, Microelectron. Eng., 1997, 36, 119 CrossRef CAS
.
- J. Collet and D. Vuillaume, Appl. Phys. Lett., 1998, 73, 2681 CrossRef CAS
.
- S. Lenfant, C. Krzeminski, C. Delerue, G. Allan and D. Vuillaume, Nano Lett., 2003, 3, 741 CrossRef CAS
.
- D. Guérin, S. Lenfant, S. Godey and D. Vuillaume, J. Mater. Chem., 2010, 20, 2680 RSC
.
- D. K. Aswal, S. Lenfant, D. Guérin, J. V. Yakhmi and D. Vuillaume, Anal. Chim. Acta, 2006, 568, 84 CrossRef CAS
.
- D. Vuillaume, C. R. Phys., 2008, 9, 74 CrossRef
; ibid, Proceedings of the IEEE, 2010, 98, 2111; ibid, in The Oxford handbook of nanoscience and nanotechnology, ed. A.V. Narlikar & Y.Y. Fu, Oxford university press, 2010, vol. III, ch. 9, pp. 312–342.
- F. Berger, J. Delhalle and Z. Mekhalif, Appl. Surf. Sci., 2010, 256, 7131 CrossRef CAS
.
- J. Gun and J. Sagiv, J. Colloid Interface Sci., 1986, 112, 457 CrossRef CAS
.
- J. Sagiv, J. Am. Chem. Soc., 1980, 102, 92 CrossRef CAS
.
- P. Silberzan, L. Léger, D. Ausserré and J. J. Benattar, Langmuir, 1991, 7, 1647 CrossRef CAS
.
- S. R. Wasserman, Y. T. Tao and G. M. Whitesides, Langmuir, 1989, 5, 1074 CrossRef CAS
.
- J. D. Le Grange, J. L. Markham and C. R. Kurjian, Langmuir, 1993, 9, 1749 CrossRef CAS
.
- R. Maoz and J. Sagiv, J. Colloid Interface Sci., 1984, 100, 465 CrossRef CAS
.
-
J. H. Miernick, K. K. Chittur, J. Weimer, Society of automotive engineers technical paper series 951684 from the Proceedings of the 25th International Conference on Environmental Systems, San Diego, CA, 1995 Search PubMed
.
-
L. Breuil, PhD Thesis, University of Lille I, 2002 Search PubMed
.
- D. A. Offord and J. H. Griffin, Langmuir, 1993, 9, 3015 CrossRef CAS
.
- B. L. Kropman, D. H. A. Blank and H. Rogalla, Langmuir, 2000, 16, 1469 CrossRef CAS
.
- M. J. Wirth, R. W. P. Fairbank and H. O. Fatunmbi, Science, 1997, 275, 44 CrossRef CAS
.
- A. Heise, M. Stamm, M. Rauscher, H. Duschner and H. Menzel, Thin Solid Films, 1998, 327–329, 199 CrossRef CAS
.
- P. Martin, S. Marsaudon, L. Thomas, B. Desbat, J.-P. Aimé and B. Benneteau, Langmuir, 2005, 21, 6934 CrossRef CAS
.
- S. H. Lee, T. Ishizaki, N. Saito and Q. Takai, Appl. Surf. Sci., 2008, 254, 7453 CrossRef CAS
.
- I. Choi, Y. Kim, S. K. Kang, J. Lee and J. Yi, Langmuir, 2006, 22, 4885 CrossRef CAS
.
- L. Patrone, V. Gadenne and S. Desbief, Langmuir, 2010, 26, 17111 CrossRef CAS
.
- M. B. Smith, K. Efimenko, D. A. Fischer, S. E. Lappi, P. K. Kilpatrick and J. Genzer, Langmuir, 2007, 23, 673 CrossRef CAS
.
- S. Turgman-Cohen, M. B. Smith, D.
A. Fischer, P. K. Kilpatrick and J. Genzer, Langmuir, 2009, 25, 6260 CrossRef CAS
.
- F. Fan, C. Maldarelli and A. Couzis, Langmuir, 2003, 19, 3254 CrossRef CAS
.
- N. Hartmann, D. Dahlhaus and S. Franzka, Surf. Sci., 2007, 601, 3916 CrossRef CAS
.
- J. B. Brzoska, I. Ben Azouz and F. Rondelez, Langmuir, 1994, 10, 4367 CrossRef CAS
.
- J. B. Brzoska, N. Shahidzadeh and F. Rondelez, Nature, 1992, 360, 719 CrossRef CAS
.
- T.A. Witten and L.M. Sander, Phys. Rev. Lett., 1981, 47, 1400 CrossRef CAS
.
- T. Balgar, R. Bautista, N. Hartmann and E. Hasselbrink, Surf. Sci., 2003, 532–535, 963 CrossRef CAS
.
- S. R. Yang and B. Kolbesen, Appl. Surf. Sci., 2008, 255, 1726 CrossRef CAS
.
-
(a) S. Desbief, L. Patrone, D. Goguenheim, D. Guérin and D. Vuillaume, Phys. Chem. Chem. Phys., 2011, 13, 2870 RSC
; (b) S. Desbief, PhD Thesis, University of Provence (Aix-Marseille), 2006 Search PubMed
.
- C. Carraro, O. W. Yauw, M. M. Sung and R. Maboudian, J. Phys. Chem. B, 1998, 102, 4441 CrossRef CAS
.
- D. L. Angst and G. W. Simmons, Langmuir, 1991, 7, 2236 CrossRef CAS
.
- A. N. Parikh, D. L. Allara, I. Ben Azouz and F. Rondelez, J. Phys. Chem., 1994, 98, 7577 CrossRef CAS
.
- T. Bailey, B. J. Choi, M. Colburn, M. Meissi, S. Shaya, J. G. Ekerdt and S. V. Sreenivasan, J. Vac. Sci. Technol., B, 2000, 18, 3572 CAS
.
- D. W. Britt and V. Hlady, J. Colloid Interface Sci., 1996, 178, 775 CrossRef CAS
.
- K. Iimura, Y. Nakajima and T. Kato, Thin Solid Films, 2000, 379, 230 CrossRef CAS
.
- T. Vallant, H. Brunner, U. Mayer, H. Hoffmann, T. Leitner, R. Resch and G. Friedbacher, J. Phys. Chem. B, 1998, 102, 7190 CrossRef CAS
.
- S. R. Wasserman, Y. T. Tao and G. M. Whitesides, Langmuir, 1989, 5, 1074 CrossRef CAS
.
- C. D. Bain and G. M. Whitesides, J. Am. Chem. Soc., 1989, 111, 7164 CrossRef CAS
.
- I. Horca, R. Fernández, J. M. Gómez-Rodríguez, J. Colchero, J. Gómez-Herrero and A. M. Baró, Rev. Sci. Instrum., 2007, 78, 013705 CrossRef
.
- J. L. Mrotek, M. J. Matthewson and C. R. Kurkjian, J. Non Crystalline Solids, 2002, 297, 91 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: AFM images of various binary SAMs in order to give information on reproducibility (Fig. S1–6), phase AFM images corresponding to topographical images of Fig. 2, 5 and 6, (Fig. S7–9), FTIR spectrum of binary C30/C18 SAM (Fig. S10), and Table SI summarizing thickness values measured by ellipsometry and estimated from AFM images for all the studied conditions. See DOI: 10.1039/c2ra01327d |
| ‡ Current address: Université de Mons, Service de Chimie des Matériaux Nouveaux, 22 Place du Parc, B-7000 Mons (Belgium); E-mail: simon@desbief.com |
| This journal is © The Royal Society of Chemistry 2012 |
