One-pot synthesis of phenylboronic acid-functionalized core-shell magnetic nanoparticles for selective enrichment of glycoproteins†
Zian
Lin
*,
Jiangnan
Zheng
,
Zhiwei
Xia
,
Huanghao
Yang
,
Lan
Zhang
* and
Guonan
Chen
Ministry of Education Key Laboratory of Analysis and Detection for Food Safety, Fujian Provincial Key Laboratory of Analysis and Detection Technology for Food Safety, Department of Chemistry, Fuzhou University, Fuzhou, Fujian 350002, China. E-mail: zianlin@fzu.edu.cn (L. A. Lin); zlan@fzu.edu.cn (L. Zhang); Fax: (+86)591-22866135
First published on 30th March 2012
Abstract
A facile one-pot approach was developed for synthesis of phenylboronic acid-functionalized core-shell magnetic nanoparticles. The resulting magnetic nanocomposites demonstrated great potential in selective enrichment of glycoproteins.
Glycosylation is one of the most common post-translational modifications and is estimated to occur in more than half of the proteins encoded in eukaryotic genomes. It is involved in many biological processes.1 Numerous studies have revealed that many clinical biomarkers and therapeutic targets are glycoproteins. Although mass spectrometry has been proved as a powerful tool in glycoproteomic analysis, it is almost impossible to directly determine the glycoproteins and identify their glycosylation sites without any enrichment process due to their low-abundance in organisms. Therefore it is necessary to develop an efficient method for the enrichment of glycoproteins. Several methods such as hydrophilic interaction chromatography,2 hydrazine chemistry,3 and lectin-based affinity chromatography4 have been widely developed for isolation and enrichment of glycoproteins in complex biological samples. Besides, boronate affinity chromatography, as a effective means for recognition of cis-diol-containing small molecules,5 has gained increasing attention for glycoproteins and glycopeptides enrichment.6 The recognition principle relies on reversible covalent complex formation/dissociation between boronic acids and cis diols in basic/acidic media.7 The unique properties of boronic acid makes it an ideal probe for the capture of glycoproteins.
With the rapid development of nanostructured materials and nanotechnology in the fields of biotechnology and biomedicine, magnetic nanomaterials have received considerable attention for their strong magnetic properties and low toxicity.8 Recently, lectin- and boronic acid-functionalized magnetic nanoparticles (MNPs) have already been successfully introduced to enrich glycosylated peptides and proteins.9 Using multistep surface modifications is the predominant strategy for preparation of the functionalized MNPs. Although this strategy allows the selective introduction of functional groups and core-shell structures of MNPs can be well controlled, these procedures are rather tedious and time-consuming.9a,10 Unlike the multistep modification strategies, the significant feature of an one-pot procedure is the simultaneous use of organic monomers and alkoxysilanes. In this process, polycondensation of the hydrolyzed alkoxysilanes was carried out at a relatively low temperature (∼40 °C) by a sol–gel process and then the temperature was elevated to above 60 °C to accomplish the copolymerization of vinyl-containing organic monomer and vinyl-silica matrix. The unique “one-pot” approach greatly simplifies the synthetic procedure, which opens up a new route for the preparation of organic-inorganic hybrid magnetite nanocomposites with desirable organic functionalities.
Herein, we describe a facile one-pot approach for the preparation of phenylboronic acid-functionalized monodisperse core-shell MNPs using the hydrolyzed tetramethyloxysilane (TMOS) and 3-methacryloxypropyltrimethoxysilane (γ-MAPS) as co-precursors, 4-vinylphenylboronic acid (VPBA) as functionalized monomer, and ethylene glycol dimethacrylate (EDMA) as crosslinker. The general route for one-pot synthesis of phenylboronic acid-functionalized MNPs is illustrated in Scheme 1, which includes as follows: (a) solvothermal synthesis of amine-magnetite nanoparticles; (b) preparation of VPBA-functionalized MNPs via a successive injection of precursors and monomer in one pot. As depicted in Scheme 1, the resultant VPBA-functionalized MNPs were applied to selectively isolate and enrich glycoproteins with the help of magnet.
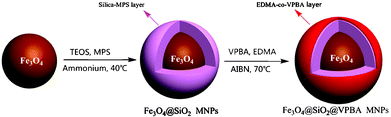 | ||
| Scheme 1 One-pot synthesis of VPBA-functionalized MNPs. | ||
By using FeCl3·6H2O as a single iron source and controlling the amount of 1,6-hexanediamine and reaction temperature, the high-quality amine-functionalized Fe3O4 nanoparticles were prepared via a solvothermal method. The as-synthesized MNPs functionalized with amino groups have good water-solubility and dispersibility, which is favorable for further one-pot reaction in hydrophilic media. A homogeneous silica coating was formed by hydrolysis and polycondensation of TMOS and γ-MAPS on the surface of amine-functionalized MNPs at 40 °C for 7 h. Subsequently, successive injection of EDMA and VPBA into the above suspension and in situ copolymerization was triggered at 70 °C with AIBN as an initiator. After mechanical stirring for about 11 h, the Fe3O4@SiO2@VPBA nanoparticles could be obtained.
Fig. 1 shows the typical TEM images of amine-functionalized MNPs and VPBA-functionalized MNPs, respectively. It was observed from Fig. 1(a) that dark Fe3O4 particles had good dispersibility and corresponding particle size ranged from 30 nm to 50 nm. The nanoscale MNPs made it possible to provide a high adsorption capacity for target proteins. Fig. 1(b) revealed the core-shell structures of VPBA-functionalized MNPs were successfully prepared via a one-pot process. Similarly, the resulting Fe3O4@SiO2@VPBA nanoparticles were all well-dispersed with near-spherical morphology. As shown in the inset of Fig. 1(b), the silica shell layer was an average 25 nm in thickness.
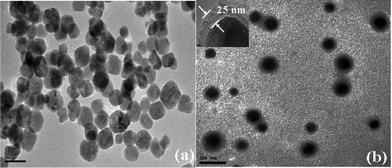 | ||
| Fig. 1 TEM images of the synthesized (a) Fe3O4 MNPs and (b) Fe3O4@SiO2@VPBA MNPs. | ||
Fourier transform infrared (FTIR) spectroscopy provides a direct proof for one-pot surface functionalization (Fig. 2(a)). The strong IR band at 585 cm−1 was characteristic of the Fe–O vibrations, while the band at 1627 cm−1 in curve a matched well with the free 1,6-hexanediamine, indicating the existence of the free –OH and –NH2 groups on the amino-functionalized nanoparticles.8 Compared to curve a, typical bands of Si–O–Si asymmetric/symmetric stretches at 1090 cm−1 and 795 cm−1 in curve b demonstrated the Fe3O4 MNPs were successfully coated with SiO2 shell. The peaks in 2963, 2930 and 2894 cm−1 were attributed to –CH3 and –CH2 groups. The band of 1700 cm−1 was attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond from the ester group. The bands of B–O adsorption at 1336 and 1378 cm−1 were raised, suggesting the VPBA was successfully copolymerized on the surface of nanoparticles in the one-pot process.
O bond from the ester group. The bands of B–O adsorption at 1336 and 1378 cm−1 were raised, suggesting the VPBA was successfully copolymerized on the surface of nanoparticles in the one-pot process.
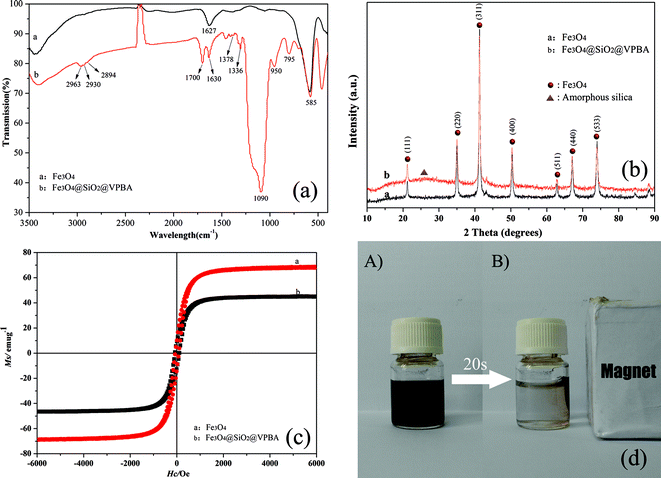 | ||
| Fig. 2 (a) FT-IR spectra of MNPs; (b) XRD patterns of MNPs; (c) Hysteresis loops of MNPs; (d) Magnetic separation-redispersion process of Fe3O4@SiO2@VPBA MNPs. | ||
The crystalline structure and phase purity of Fe3O4 nanoparticles with and without coating are determined by powder X-ray diffraction (XRD) as shown in Fig. 2(b). The positions and relative intensities of all diffraction peaks matched well with those from the JCPDS card (75–1610) for magnetite. The sharp, strong peaks confirmed the products were well crystallized and had high crystallinity after coating. The broadband located at 2θ = 25° can be assigned to the characteristic diffraction peak of amorphous silica shell.
Magnetic characterization at 300 K with a vibrating sample magnetometer shows that the saturation magnetization values of Fe3O4 and Fe3O4@SiO2@VPBA were 68.0 emu g−1 and 45.0 emu g−1, respectively. Such high saturation magnetization endued the MNPs with a fast response to an external magnetic field. It was seen from Fig. 2(d) that the Fe3O4@SiO2@VPBA nanoparticles were dispersed in water by vigorous shaking, resulting in a brown colored suspension. Very fast aggregation of the microspheres from their homogeneous dispersion was observed with the help of external magnet and the dispersed solution became clear. While redispersion occurred quickly once the magnet was removed. These results showed that the VPBA-functionalized nanoparticles possessed excellent magnetic responsivity and redispersibility, which was important in the practical manipulation.
The specificity of VPBA-functionalized MNPs for glycoproteins was evaluated by incubation with glycosylated ovalbumin (OVA) and mucin (Muc) at different concentrations, separately. Similarly, bovine serum albumin (BSA) and lysozyme (Lyz) within the same concentration range were used as negative controls to estimate nonspecific interactions. Fig. 3(a) showed that all proteins except Muc could reach saturation in the studied concentration ranges and the saturation adsorption capacity of OVA was estimated to be 320 mg g−1, 4–7 times higher than those obtained for BSA and Lyz, which were 45 and 70 mg g−1, respectively. The results revealed that the functionalized MNPs have higher selectivity for glycoproteins than nonglycoproteins. In contrast to the multistep modifications described in our previous work,10 the VPBA-functionalized MNPs prepared by the one-pot process showed lower nonspecific adsorptions for nonglycoproteins, indicating the superior advantages of one-pot synthesis. Since the isoelectric point of OVA and Muc is ∼4.6 and ∼3.2, both the glycoproteins and VPBA-functionalized MNPs (VPBA, pKa 8.8) will be negatively charged in PBS buffer at pH 9.0. In such case, the molecular interactions (e.g. hydrogen bonding/electrostatic interaction) are negligible. The high binding amount was mainly attributed to the specific interaction between phenylboronic acid and cis-diol groups in basic media. It should be noted that the binding capacity of Muc was 2 times less than that of OVA in the studied ranges (Fig. 3(a)), obviously, steric hindrance effect of Muc with high molecular weight (Mw > 200 kDa) can respond to the low binding capacity. Nevertheless, the maximum binding capacity (Qmax) of Muc calculated by Langmuir equation was 357 mg g−1, which is comparable the Qmax of OVA (370 mg g−1). The result validated that the VPBA-functionalized MNPs have an unbiased affinity toward all kinds of glycoproteins. In addition, the adsorption kinetics of all proteins on MNPs were examined. As shown in Fig. 3(b), the binding amount of glycoproteins increased very quickly in the first 45 min and slowed down after 2 h. However, BSA and Lyz show little change in adsorption amount. This result is consistent with our previous work10 and could be explained by the glycoproteins easily reacting with phenylboronic acid via the specific binding at the beginning. With the saturation of binding sites, glycoproteins diffused randomly onto the surface of MNPs like nonglycoproteins.
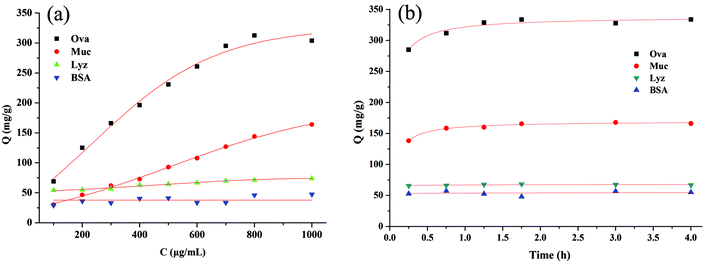 | ||
| Fig. 3 Adsorption isotherms (a) and kinetics (b) for all proteins on MNPs. MNPs: 1 mg; V: 1.0 mL; incubation time: 3 h; other conditions see ESI†. | ||
The quality and the specificity of the VPBA-functionalized MNPs were demonstrated by selective enrichment of immunoglobulin G (IgG), known as a glycoprotein, from model protein mixture. Fig. 4(a) showed the SDS-PAGE electropherogram of original protein mixture (Lane 2) and eluent after enrichment (Lane 3). It is well known that IgG are heterotetramers consisting of an equimolar ratio of heavy chain (HC) and light chain (LC) polypeptides. Lane 2 exhibited three bands, corresponding to BSA, IgG HC and IgG LC, respectively. In lane 3, the band intensity of HC and LC were increased 4.8-fold and 13.7-fold, respectively (Fig. S1 in ESI†). IgG HC are N-glycosylated, so the functionalized MNPs capture IgG molecule through the interaction between phenylboronic acid and the N-glycosylated HC. The enrichment efficiency of LC is higher than HC, suggesting that HC was difficult to elute completely. Although a visible band of BSA still remained in the eluent after being eluted several times with 50/50 v/v % ACN/H2O, the recovery of BSA was much less than 3%.
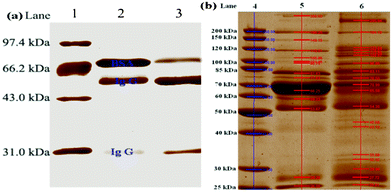 | ||
| Fig. 4 SDS-PAGE analysis of (a) a mixture of model proteins and (b) human serum without treatment and eluate after binding to MNPs. Lane (1) marker; (2) mixture of BSA (3 μg) and Ig G (6 μg); (3) the eluate; (4) marker; (5) human serum (diluted 50-fold); (6) the eluate; Loading amount of sample: 10 μL. | ||
The applicability of the functionalized MNPs was further evaluated by selective capture of glycoproteins from healthy human serum. As presented in Fig. 4(b), the separation of human serum without treatment (lane 5) revealed 14 major bands, ranging in molecular weight from 350 kDa to 26 kDa, including the high-abundance human serum albumin (HSA), IgG, as well as transferrin human (Trf), etc. Lane 6 exhibited 19 major bands after the sequential treatment and elution, in which nonglycosylated HSA was almost removed, but the known glycoproteins such as IgG and Trf were reappeared. Furthermore, some new bands in Lane 6 were also observed, i.e., 295.9 kDa, 123.8 kDa, 113.8 kDa, as well as 45.5–32.3 kDa. Although the unknown glycoproteins need to be further identified by MS, the preliminary results validated the practicability of the phenylboronic acid-functionalized MNPs for the selective isolation and enrichment of glycoproteins.
In summary, a novel one-pot method was firstly introduced to synthesize phenylboronic acid-functionalized MNPs. This method was simple, convenient and time-saving. The resultant MNPs exhibited high binding capacity and specificity toward glycoproteins. Moreover, the successful applications in the selective isolation and enrichment of glycoproteins from model proteins and human serum suggested that the purposed method could be expected to be an alternative solution for glycoproteomics.
Acknowledgements
This project was financially supported by National Basic Research Program of China (2010CB732403), the NSFC (20103514120002), the Doctoral Fund of Ministry of Education (20103514120002), Natural Science Foundation of Fujian Province (2010J01039), foundation of Fujian Educational Committee (JA10030), Technology Development Fund of Fuzhou University (2010-XQ-07), and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1116).References
- L. Krishnamoorthy and L. K. Mahal, ACS Chem. Biol., 2009, 4, 715–732 CrossRef CAS.
- P. Hägglund, J. Bunkenborg, F. Elortza, J.O. Nørregaard and P. Roepstorff, J. Proteome Res., 2004, 3, 556–566 CrossRef.
- R. Chen, X. N. Jiang, D. G. Sun, G. H. Han, F. J. Wang, M. L. Ye, L.M. Wang and H. F. Zou, J. Proteome Res., 2009, 8, 651–661 CrossRef CAS.
- S. Feng, N. Yang, S. Ennathur, S. Goodison and M. Bman, Anal. Chem., 2009, 81, 3776–3783 CrossRef CAS.
- L. B. Ren, Z. Liu, Y. C. Liu, P. Dou and H. Y. Chen, Angew. Chem., Int. Ed., 2009, 48, 6704–6707 CrossRef CAS.
- (a) Z. A. Lin, J. L. Pang, H. H. Yang, Z. W. Cai, L. Zhang and G. N. Chen, Chem. Commun., 2011, 47, 9675–9677 RSC; (b) Y. C. Liu, L .B. Ren and Z. Liu, Chem. Commun., 2011, 47, 5067–5069 RSC; (c) L. Liang and Z. Liu, Chem. Commun., 2011, 47, 2255–2257 RSC.
- A. J. Tong, A. Yamauchi, T. Hayashita, Z. Y. Zhang, B. D. Smith and N. Teramae, Anal. Chem., 2001, 73, 1530–1536 CrossRef CAS.
- L.Y. Wang, J. Bao, L. Wang, F. Zhang and Y. D. Li, Chem.–Eur. J., 2006, 12, 6341–6347 CrossRef CAS.
- (a) W. Zhou, N. Yao, G. P. Yao, C. H. Deng, X. M. Zhang and P. Y. Yang, Chem. Commun., 2008, 43, 5577–5579 RSC; (b) D. Loo, A. Jones and M. M. Hill, J. Proteome Res., 2010, 9, 5496–5500 CrossRef CAS; (c) J. A. Ferreira, A. L. Daniel-da-Silva, R. M. P. Alves, D. Duarte, I. Vieira, L. L. Santos, R. Vitorino and F. Amado, Anal. Chem., 2011, 83, 7035–7043 CrossRef CAS.
- Z. A. Lin, J. N. Zheng, F. Lin, L. Zhang, Z. W. Cai and G. N. Chen, J. Mater. Chem., 2011, 21, 518–524 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional figures. See DOI: 10.1039/c2ra20167d |
| This journal is © The Royal Society of Chemistry 2012 |
