Facile synthesis of glass–silver nanodisk core–shell composite hollow spheres†
Xiao
Xie
ab,
Kaiqi
Yan
ab,
Zhenguo
An
a and
Jingjie
Zhang
*a
aTechnical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: jjzhang@mail.ipc.ac.cn; Fax: +86 10 82543691; Tel: +86 10 82543691
bGraduate School of the Chinese Academy of Sciences, Beijing, 100049, China
First published on 29th March 2012
Abstract
Novel glass–silver nanodisk core–shell composite hollow spheres were successfully fabricated by a simple aqueous solution route. The effects of some reaction parameters on the morphology of the silver layers were investigated. The unique structure of the as-obtained composite makes it promisingly practicable in catalytic or sensory fields.
In the past several decades, shape-controlled synthesis of metal nanoparticles has attracted considerable attention because there is a strong correlation between the shape and properties of nanoparticles.1–3 Silver is an especially attractive metal to examine on the nanoscale because of its wide applications in localized surface plasmon resonance (LSPR),4–6 surface-enhanced Raman scattering (SERS),7–9 and chemical and biological sensing5,10–13 resulting from the remarkable properties which depend on its size, crystallinity, shape and structure.9,14–18 Up to the present day, several methods have been developed for synthesizing silver nanoparticles in a variety of shapes, such as disks,19,20 rods,21 cubes22,23 and wires.24 Recently, there has been intense effort focused on silver nanodisks because they possess an extreme degree of anisotropy.25 Although control over nanoparticle morphology can be successfully realized through many methods, the reaction systems in these methods often contain various kinds of surfactants or polymers. Moreover, silver nanodisks can only disperse stably in solutions or emulsions in the presence of surfactants or polymers. Removal of the surfactants and polymers requires harsh conditions and complex procedures involving multiple washings, which is attributed to the size of the nanoparticles.26 This drawback may lead to a limited range of applications for silver nanoparticles, but it can be avoided by fabricating the nanostructure on micro-cores. Shell-nanoparticles can be well-dispersed on micro-cores rather than aggregating. Besides, core–shell particles often exhibit unique and tailored properties, which can be finely tuned by changing the parameters,27 and show great value for potential applications in various fields.28,29
In this communication we present a facile method to synthesize silver nanodisk coatings on hollow glass microspheres by a surfactantless wet procedure. The hollow glass spheres (HGS, silicate glass) used as the core particles were prepared by us using a high temperature smelting method. All agents were of analytical grade and used without further purification. First, the hollow glass spheres were activated through a two-step process using AgNO3 as an activator, according to the literature.30 In a typical synthesis procedure of the composite, an aqueous solution was prepared by dissolving sodium citrate (C6H5Na3O7·2H2O, 5.0 mmol L−1) in 50 ml distilled water. Then nitric acid (0.1 mol L−1) or NaOH solution (0.1 mol L−1) was added dropwise into this solution to adjust its pH value (4.0, sample B). After adding 0.25 mmol AgNO3 and 0.05 g activated hollow glass spheres, the mixture was stirred rapidly for 5 min. Meanwhile, 20 ml aqueous solution containing 0.25 mmol L-ascorbic acid was added dropwise into the mixture. Finally, the reaction mixture was further stirred for 60 min at room temperature. After the reaction was completed, the Ag-coated particles were then filtered off, rinsed three times with distilled water, and vacuum-dried at 40 °C.
Powder X-ray diffraction (XRD) was used to determine the chemical composition and crystal structure of the resulting products. Fig. 1 presents the XRD patterns of the pristine hollow glass spheres (sample A, curve a), the composite hollow spheres with silver nanodisk shells prepared at a pH value of 4.0 (sample B, curve b) and composite hollow spheres with silver-granular shells prepared at a pH value of 9.5 (sample C, curve c). Compared with the pristine glass spheres, five more sharp peaks, which can be definitely indexed to the (111), (200), (220), (311) and (222) crystalline planes, appeared in the XRD patterns of the composite spheres (sample B & sample C). This could be indexed as face-centered cubic phase silver (JCPDS No. 65-2871, space group Fm3m). A broad weak diffraction peak of the amorphous SiO2 phase, corresponding to the hollow glass spheres as the cores of the composites, was detected (sample B & sample C). The XRD analyses, therefore, reveal that the shell layer consisted of pure silver of high crystallinity.
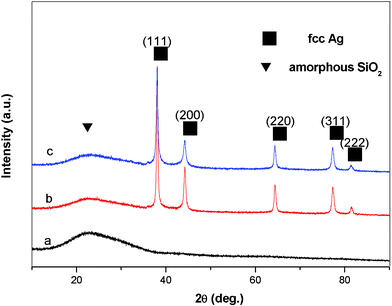 | ||
| Fig. 1 XRD patterns of (a) the pristine hollow glass spheres, (b) composite hollow spheres with silver-nanodisk shells, (c) composite hollow spheres with silver-granular shells. | ||
The size and morphology of the products were investigated by scanning electron microscopy (SEM). Fig. 2a–d show the typical SEM images at different magnifications of the as-obtained composite hollow spheres prepared at a pH value of 4.0 (sample B). The panoramic image of Fig. 2a clearly reveals that the majority of the hollow glass spheres were coated uniformly with compact silver shells. The magnified images of Fig. 2b and 2c indicate that the silver shells on the hollow glass spheres consist of nanodisks of ca. 30∼50 nm in thickness. These silver nanodisks of different shapes, varying from triangular disks to circular disks, present a disordered nature. The side length of the triangular disks reaches over 100 nm. A portion of the nanodisks grow almost perpendicular to the surface of the glass spheres, while others incline towards the core surface. The core–shell superstructure can be seen more clearly in the image of a fragment shown in Fig. 2d. It is thus clear that the silver shell thickness is slightly greater than 50 nm. These disks grew firmly from the surface of the hollow glass spheres. To further reveal the fine structure of the disk-like shell, TEM analysis was also carried out as shown in Fig. 2e. The magnified TEM images clearly indicate that the shell layer is formed by assembling the nanodisks. The dark space in the images may indicate that the bottom of the shell consists of overlapping nanodisks. The energy dispersive X-ray (EDX) spectrum presented in Fig. 2f shows a prominent presence of Ag within the shell layer, and therefore indicates successful Ag-coating of the hollow glass cores. Au comes from the electro-conductive coating required for SEM analysis. And we can notice the distribution of Ca, Na and Al, which are associated with the chemical composition of glass cores.
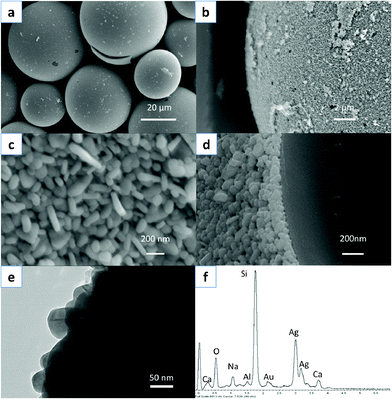 | ||
| Fig. 2 Integrated characterization of sample B (spheres with disk-like shells): (a–d) SEM images at different magnifications, (e) TEM image of the edge of the disk-like shell layer. (f) EDX spectrum of the example. | ||
In order to verify the structural anisotropy of the silver nanodisks, a UV-vis absorption experiment was carried out (Fig. 3). Ultrasonic processing was firstly applied to separate the disk-like nanoparticles from the core glass spheres in the presence of polyvinyl pyrrolidone (PVP). After filtration of the glass fragments and agglomeration particles, the silver nanoparticles dispersed evenly in aqueous solution under the stabilizing effect of PVP. The spectrum of the two samples simultaneously displayed a distinct peak centered at ∼400 nm, which could be attributed to the formation of some larger, spherical particles. The colloidal dispersion of nanoparticles separated from sample B exhibited two more peaks located at 330 and 851 nm. According to theoretical calculations by Schatz and co-workers,31 the peak located at 330 nm could be assigned to the out-of-plane quadrupole plasmon resonance mode. And the broad peak centered at ∼851 nm could be the in-plane dipole resonance band. In spite of the presence of spherical nanoparticles, the morphology of nanodisk-like nanoparticles was also confirmed.
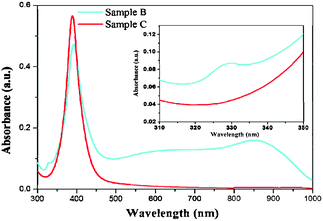 | ||
| Fig. 3 UV-vis absorption spectra of silver nanoparticles peeled from sample B and sample C. | ||
The particle size distribution of sample B was achieved by a particle size analyser (ESI,† Fig. S1). The curve shows that the average particle size is slightly smaller than 50 μm.
For the purpose of studying the formation and assembly process of silver nanodisks, time-dependent experiments have been carried out. The morphology transmutation of the silver assemblies over time has been studied by SEM. The SEM images of the samples obtained at different reaction times are shown in Fig. 4a–c. During the process of activation, Sn2+ ions firstly get adsorbed onto the particle surface forming a uniform layer. Once the glass particle surface has adsorbed Sn2+ ions homogeneously, the addition of a silver salt leads to the selective redox reaction on the surface. The [Ag (NH3)2]+ ions are reduced to silver.30 Then Ag0 can act as a catalyst for the subsequent deposition of silver. As we can see from the SEM image in Fig. S2,† the silver nanoparticles could only adhere to the surface of the glass cores. Moreover, the disk-like morphology was not formed. After the process of activation, the colour of the HGS changes from white to light-brown due to the presence of silver nanoparticles. It can be observed from the SEM image (Fig. 4a) that these Ag seeds disperse on the surface of the glass sphere. The silver activated hollow glass spheres, as the Ag-seeded cores, were believed to be of great importance for the nucleation and growth of the silver crystallites.32 Then these small nanoparticles tend to form larger ones, along with the deposition of silver nanocrystals. As shown in Fig. 4b, sub-micro particles formed on the core surface during this process. As the reaction proceeded, these sub-micro particles continued to grow. It was reported that citrate ions were the key component in this morphological formation of nanodisks bounded by {111} facets.33 A preferential interaction of the citrate ions with the {111} facets of silver nanocrystals leads to the formation of disk-like morphology by inhibiting growth on the {111} plane. Fig. 4c demonstrates that a disk-like morphology was finally formed. According to the results above, we consider that the formation of the disk-like silver particles on the hollow glass spheres experiences a complex process including the reduction of Ag+ to silver seeds on the surface of glass cores, the directed deposition of the silver atoms, and the formation of silver nanodisks. The possible process is schematically shown in Fig. 5.
 | ||
| Fig. 4 SEM images of the products at different reaction stages: (a) after activation, (b) 30 min, (c) 60 min. | ||
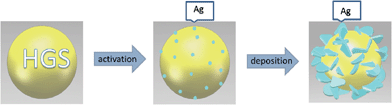 | ||
| Fig. 5 Schematic illustration of the possible formation process of the core–shell spheres. | ||
In the present system, the pH of the sodium citrate solution is found to be undoubtedly crucial to the growth of the silver crystallites, and further determines the morphology of the final products. Samples prepared under different conditions have been investigated in detail to reveal the relationship between the morphology and the pH values of the sodium citrate solution. The SEM images in Fig. 6a–d show the typical morphologies of 4 samples prepared at different pH values with other reaction conditions constant. As shown in Fig. 6a, the silver shells aggregate to form a granular shell. The reductant we used was L-ascorbic acid (AsA). Commonly, addition of a strong acid or base changes the dissociation degree of AsA. The addition of nitric acid suppresses the reducing reaction of Ag+.34 Therefore, disk-like crystals slowly grow in the presence of seeds on the core surface. The presence of sodium hydroxide leads to a high AsA− concentration due to the promotion effect of dissociation of AsA. The pH value determines not only the reducing capacity of L-ascorbic acid but also the citrate ion (C6H7O7−)–citrate acid (C6H8O7) molar ratio. This molar ratio finally determines the coordination capabilities of carboxyl groups with silver surfaces.33 Therefore, a suitable pH value could kinetically control the aggregation and growth of the silver crystallites by influencing the production rate of the silver atoms, and further determines the morphology of the shell layer.
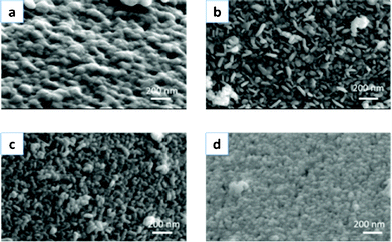 | ||
| Fig. 6 SEM images of the silver shell obtained under different pH values: (a) 2.5, (b) 4.0, (c) 7.5, (d) 9.5. | ||
Conclusions
In summary, we present a wet chemical method for the synthesis of glass–silver nanodisk core–shell composite hollow spheres. The results revealed that it was possible to control the morphology of the products by monitoring the components of the reaction solution properly. The silver nanodisk shell composite products are expected to show potential applications as a conduction filler, in catalysis or in sensory applications. More in-depth investigations are still under way to further understand the detailed influences of other factors on the formation and assembly of the disk-like nanocrystals.Acknowledgements
This work was supported by the National Natural Science Foundation of China (project No. 51102248&50901083) and the Knowledge Innovation Project of Chines Academy of Sciences (KJCX2-EW-L02-4).References
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS.
- G. Schmid, Chem. Rev., 1992, 92, 1709–1727 CrossRef CAS.
- A. R. Tao, S. Habas and P. Yang, Small, 2008, 4, 310–325 CrossRef CAS.
- T. Linnert, P. Mulvaney and A. Heinglein, J. Phys. Chem., 1993, 97, 679–682 CrossRef CAS.
- A. J. Haes and R. P. Van Duyne, J. Am. Chem. Soc., 2002, 124, 10596–10604 CrossRef CAS.
- J. Mock, M. Barbic, D. Smith, D. Schultz and S. Schultz, J. Chem. Phys., 2002, 116, 6755 CrossRef CAS.
- S. Nie and S. R. Emory, Science, 1997, 275, 1102 CrossRef CAS.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667–1670 CrossRef CAS.
- R. Jin, Y. C. Cao, E. Hao, G. S. Métraux, G. C. Schatz and C. A. Mirkin, Nature, 2003, 425, 487–490 CrossRef CAS.
- B. Liedberg, C. Nylander and I. Lunstrom, Sens. Actuators, 1983, 4, 299–304 CrossRef CAS.
- F. Frederix, J. M. Friedt, K. H. Choi, W. Laureyn, A. Campitelli, D. Mondelaers, G. Maes and G. Borghs, Anal. Chem., 2003, 75, 6894–6900 CrossRef CAS.
- A. D. McFarland and R. P. Van Duyne, Nano Lett., 2003, 3, 1057–1062 CrossRef CAS.
- A. Tao, F. Kim, C. Hess, J. Goldberger, R. He, Y. Sun, Y. Xia and P. Yang, Nano Lett., 2003, 3, 1229–1233 CrossRef CAS.
- C. J. Murphy and N. R. Jana, Adv. Mater., 2002, 14, 80 CrossRef CAS.
- B. Wiley, Y. Sun, B. Mayers and Y. Xia, Chem.–Eur. J., 2005, 11, 454–463 CrossRef CAS.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668–677 CrossRef CAS.
- I. O. Sosa, C. Noguez and R. G. Barrera, J. Phys. Chem. B, 2003, 107, 6269–6275 CrossRef CAS.
- L. M. Liz-Marzán, Langmuir, 2006, 22, 32–41 CrossRef.
- M. Maillard, S. Giorgio and M. P. Pileni, J. Phys. Chem. B, 2003, 107, 2466–2470 CrossRef CAS.
- Y. Zhu and W. Geng, J. Phys. Chem. C, 2008, 112, 8545–8547 CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Chem. Commun., 2001, 617–618 RSC.
- D. Yu and V. W. W. Yam, J. Am. Chem. Soc., 2004, 126, 13200–13201 CrossRef CAS.
- Q. Zhang, W. Li, L.-P. Wen, J. Chen and Y. Xia, Chem.–Eur. J., 2010, 16, 10234–10239 CrossRef CAS.
- B. H. Hong, S. C. Bae, C. W. Lee, S. Jeong and K. S. Kim, Science, 2001, 294, 348 CrossRef CAS.
- I. Pastoriza-Santos and L. M. Liz-Marzán, J. Mater. Chem., 2008, 18, 1724–1737 RSC.
- K. K. Caswell, C. M. Bender and C. J. Murphy, Nano Lett., 2003, 3, 667–669 CrossRef CAS.
- X. Sun and Y. Li, Angew. Chem., Int. Ed., 2004, 43, 597–601 CrossRef.
- K. Aslan, M. Wu, J. R. Lakowicz and C. D. Geddes, J. Am. Chem. Soc., 2007, 129, 1524–1525 CrossRef CAS.
- S. R. Guo, J. Y. Gong, P. Jiang, M. Wu, Y. Lu and S. H. Yu, Adv. Funct. Mater., 2008, 18, 872–879 CrossRef CAS.
- S. Shukla, S. Seal, Z. Rahaman and K. Scammon, Mater. Lett., 2002, 57, 151–156 CrossRef CAS.
- R. Jin, Y. W. Cao, C. A. Mirkin, K. Kelly, G. C. Schatz and J. Zheng, Science, 2001, 294, 1901 CrossRef CAS.
- S. Chen and D. L. Carroll, J. Phys. Chem. B, 2004, 108, 5500–5506 CrossRef CAS.
- Y. Sun and Y. Xia, Adv. Mater., 2003, 15, 695–699 CrossRef CAS.
- T. Fukuyo and H. Imai, J. Cryst. Growth, 2002, 241, 193–199 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20293j/ |
| This journal is © The Royal Society of Chemistry 2012 |
