Arborescent polystyrene-graft-poly(2-vinylpyridine) copolymers: solution polyelectrolyte behavior
Mario
Gauthier
* and
Abdul
Munam
Institute for Polymer Research, Department of Chemistry, University of Waterloo, 200 University Avenue West, Waterloo, Ontario N2L 3G1, Canada. E-mail: gauthier@uwaterloo.ca
First published on 23rd February 2012
Abstract
Branched polyelectrolyte precursors were obtained on a 100 g scale by coupling ‘living’ poly(2-vinylpyridine) (P2VP) macroanions with linear polystyrene and arborescent polystyrene substrates of generations G0 or G1, functionalized with acetyl groups. Arborescent copolymers with Mn ≈ 5000 or 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 side-chains and containing 87–98 mol% of P2VP were thus synthesized. These materials are characterized by a very compact structure, a narrow molecular weight distribution (Mw/Mn = 1.08–1.10), and a roughly geometric increase in branching functionality and molecular weight for successive generations. The copolymers are freely soluble in water and in polar organic solvents such as methanol and N,N-dimethylformamide (DMF) upon protonation by trifluoroacetic acid (TFA), a strong acid. The solutions obtained display properties typical of polyelectrolytes, including strong curvature in plots of reduced viscosity (ηsp/C) versus concentration (C) at low concentrations. The copolymers exhibit larger reduced viscosity increases in methanol than in DMF, and coil expansion apparently varies in the order G1 > G0 > G2. The reduced viscosity of the arborescent polyelectrolyte solutions is much lower than for linear P2VP samples of comparable molecular weights, however, due to the small dimensions and increased structural rigidity of the molecules. The addition of salts to the branched polyelectrolyte solutions further decreases their viscosity and the curvature of the reduced viscosity plots, presumably as a result of charge screening.
000 side-chains and containing 87–98 mol% of P2VP were thus synthesized. These materials are characterized by a very compact structure, a narrow molecular weight distribution (Mw/Mn = 1.08–1.10), and a roughly geometric increase in branching functionality and molecular weight for successive generations. The copolymers are freely soluble in water and in polar organic solvents such as methanol and N,N-dimethylformamide (DMF) upon protonation by trifluoroacetic acid (TFA), a strong acid. The solutions obtained display properties typical of polyelectrolytes, including strong curvature in plots of reduced viscosity (ηsp/C) versus concentration (C) at low concentrations. The copolymers exhibit larger reduced viscosity increases in methanol than in DMF, and coil expansion apparently varies in the order G1 > G0 > G2. The reduced viscosity of the arborescent polyelectrolyte solutions is much lower than for linear P2VP samples of comparable molecular weights, however, due to the small dimensions and increased structural rigidity of the molecules. The addition of salts to the branched polyelectrolyte solutions further decreases their viscosity and the curvature of the reduced viscosity plots, presumably as a result of charge screening.
I. Introduction
Polyelectrolytes exhibit solution properties very distinct from both neutral macromolecules and low molecular weight electrolytes.1 This is due to the combination of properties derived from long-chain macromolecules with those arising from electrostatic interactions. These properties are not simply superimposed, as there is a mutual influence of the characteristics of both components. The properties of polyelectrolytes in solution depend on different parameters including the degree of dissociation of the ionic groups, the solvent quality for the polymer backbone, the dielectric constant (polarity) of the solvent, and the presence of salts.2 Depending on solvent polarity, bound charges on the polyelectrolyte chains can lead to both intra- and intermolecular interactions that are stronger and of much longer range than for neutral macromolecules.3 A direct consequence of electrostatic interactions is the expansion of the polymer coil due to strong repulsion of the ionic groups. The polyelectrolyte effect is well-documented for linear polyelectrolytes, but it is less understood for branched polymers.Arborescent polymers4 provide an excellent opportunity to investigate the effects of branching in polyelectrolytes systematically, because they can be prepared with controllable side-chain molecular weights and branching functionalities. Arborescent polymers are derived from successive grafting reactions of well-defined polymeric building blocks, as illustrated graphically in Scheme 1. This “graft upon graft” approach makes it possible to obtain macromolecules with high branching functionalities and molecular weights in a few reaction steps, while maintaining narrow molecular weight distributions (Mw/Mn ≈ 1.1). The synthesis of arborescent polymers starts with the random introduction of coupling sites along a linear polymer chain. ‘Living’ polymer chains are then coupled with the linear substrate to generate a comb-branched (generation 0 or G0) polymer. Repetition of the functionalization and coupling reactions cycle leads to the subsequent generations (G1, G2, etc.) of arborescent polymers.
 | ||
| Scheme 1 Synthesis of an arborescent polymer (G0PS-g-P2VP) by successive grafting reactions. | ||
This grafting strategy has been applied to the synthesis of arborescent polystyrenes using cycles of acetylation and coupling with polystyryl anions, on both small (10–20 g)5 and large (100 g) scales.6 A modified small-scale procedure to graft poly(2-vinylpyridine) (P2VP) side-chains onto acetylated polystyrene substrates according to Scheme 1 was also developed to generate polystyrene-graft-poly(2-vinylpyridine) copolymers useful as dendritic polyelectrolyte precursors.7a We now achieved the large (100 g) scale synthesis of a series of arborescent copolymers incorporating P2VP segments grafted onto acetylated linear, comb-branched (G0), and G1 polystyrene substrates, by adapting the small-scale synthetic procedure previously reported. Two arborescent copolymer series, containing either Mn ≈ 5000 (P2VP5 series) or Mn ≈ 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 side-chains (P2VP30 series) were synthesized. Characterization results for the copolymers by size exclusion chromatography and 1H NMR spectroscopy are discussed. The P2VP copolymers are easily converted to polyelectrolytes upon protonation by a strong acid such as trifluoroacetic acid (TFA), and are freely soluble in water and in polar organic solvents such as methanol and N,N-dimethylformamide (DMF). The reduced viscosity (ηsp/C) of arborescent polyelectrolyte solutions was investigated as a function of concentration (C) and structure for the two families of P2VP copolymers synthesized.
000 side-chains (P2VP30 series) were synthesized. Characterization results for the copolymers by size exclusion chromatography and 1H NMR spectroscopy are discussed. The P2VP copolymers are easily converted to polyelectrolytes upon protonation by a strong acid such as trifluoroacetic acid (TFA), and are freely soluble in water and in polar organic solvents such as methanol and N,N-dimethylformamide (DMF). The reduced viscosity (ηsp/C) of arborescent polyelectrolyte solutions was investigated as a function of concentration (C) and structure for the two families of P2VP copolymers synthesized.
II. Experimental methods
II.1. Synthesis of arborescent copolymers
Linear, G0, and G1 partially acetylated polystyrene substrates were prepared by cycles of acetylation, grafting, and fractionation as described previously.6 The branched substrates were synthesized from a polystyrene core and polystyrene side-chains with Mn ≈ 5000. The copolymers were obtained by grafting P2VP macroanions with Mn ≈ 5000 or 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 onto the acetylated substrates. Detailed experimental protocols for the large (100 g) scale synthesis of the arborescent polystyrene-graft-poly(2-vinylpyridine) samples used in the present investigation have been reported elsewhere.7b
000 onto the acetylated substrates. Detailed experimental protocols for the large (100 g) scale synthesis of the arborescent polystyrene-graft-poly(2-vinylpyridine) samples used in the present investigation have been reported elsewhere.7b
II.2. Linear poly(2-vinylpyridine)
Linear poly(2-vinylpyridine) samples with Mn ≈ 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 230
000, 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 430
000, and 430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 were synthesized as reference materials for comparison of their polyelectrolyte behavior with the arborescent copolymers.
000 were synthesized as reference materials for comparison of their polyelectrolyte behavior with the arborescent copolymers.
II.3. Polymer characterization
The absolute number-average molecular weight (Mn) and polydispersity index (Mw/Mn) of the linear P2VP samples (reference materials) and the P2VP side-chains were determined by SEC laser light scattering (MALLS) analysis using a miniDAWN (3-angle) detector from Wyatt Technology operating at 632.8 nm. The SEC-MALLS system consisted of a Waters 510 HPLC pump coupled with a 500 × 10 mm2 Jordi DVB linear Mixed-Bed column (linear polystyrene molecular weight range 102–107) operated at room temperature. THF at a flow rate of 1 mL min−1 served as the eluent, and polymer concentration measurements were accomplished with a Waters 410 DRI detector. The molecular weights were obtained from the MALLS and DRI signals using the Astra Version 4.70.07 software package.Since the SEC-MALLS system used was dedicated exclusively to measurements in THF, absolute molecular weight measurements were not attempted for the graft copolymers. An alternative method was used to estimate the number-average molecular weight (Mn) of the copolymers, by combining the absolute Mn of the substrate (from SEC-MALLS analysis in pure THF) with the copolymer composition determined by 1H NMR analysis. The composition was determined using a Bruker-300 (300 MHz) nuclear magnetic resonance spectrometer in CDCl3 at a concentration of 5%.
II.4. Viscosity measurements
Flow times were determined for the pure solvents and the polymer solutions using a Cannon-Ubbelohde model 75 viscometer immersed in a water bath at 25.0 ± 0.2 °C. The polymer solutions were prepared by dissolving 0.2–0.4 g of vacuum-dried polymer (depending on the sample) at room temperature in 10 mL of solvent (DMF or methanol) with stirring for one day. The maximum concentration of the solutions was adjusted to either 2 or 4% w/v, depending on the viscosity of the sample. The solutions and the solvents were filtered through 5 μm PTFE membrane filters before adding the protonating agent, trifluoroacetic acid (TFA), within a range of 0.1 to 3 equiv TFA per 2VP unit. The solutions were subsequently stirred for one month to ensure homogeneity. For aqueous solutions, the copolymer was first dissolved in water containing the desired amount of TFA and the solution was stirred for one month prior to filtration. Series of five consistent (± 0.1 s) flow times were obtained for up to eleven different concentrations, using successive additions of solvent to the solution reservoir of the viscometer. Lithium chloride (LiCl) solutions at concentrations of 0.05 and 0.25 M in methanol and DMF were also added to the polyelectrolyte solutions protonated with 1.0 equiv TFA/2VP unit in some cases, to study the effect of added salt. In that case, a LiCl solution of identical concentration was used to dilute the polymer solution in the successive dilution procedure, to maintain a constant ionic strength at the different polymer concentrations. The reduced viscosity (ηsp/C) was determined as a function of concentration (C) for the linear P2VP homopolymers and for the copolymers of generations G0–G2 with Mn ≈ 5000 or 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 P2VP side-chains.
000 P2VP side-chains.
III. Results and discussion
III.1. Synthesis
The characteristics of the linear and branched polystyrene substrates used in the preparation of the graft copolymers are summarized in Table 1. The substrates were obtained from cycles of acetylation, grafting, and fractionation as described previously6 using Mn ≈ 5000 polystyrene core and side-chains with a narrow molecular weight distribution. These polymers were acetylated and coupled with poly(2-vinylpyridinyl)lithium macroanions having a number-average molecular weight Mn ≈ 5000 or 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to obtain the polystyrene-graft-poly(2-vinylpyridine) copolymers.7 Characterization results for these copolymers are provided in Table 2. The graft copolymer sample nomenclature used in Table 2 specifies their composition and structure. For example, G0PS-g-P2VP5 refers to a copolymer obtained by grafting Mn ≈ 5000 P2VP side-chains onto a G0 (once-grafted) arborescent polystyrene substrate, while G0PS-g-P2VP30 refers to the same substrate grafted with Mn ≈ 30
000 to obtain the polystyrene-graft-poly(2-vinylpyridine) copolymers.7 Characterization results for these copolymers are provided in Table 2. The graft copolymer sample nomenclature used in Table 2 specifies their composition and structure. For example, G0PS-g-P2VP5 refers to a copolymer obtained by grafting Mn ≈ 5000 P2VP side-chains onto a G0 (once-grafted) arborescent polystyrene substrate, while G0PS-g-P2VP30 refers to the same substrate grafted with Mn ≈ 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 P2VP side-chains.
000 P2VP side-chains.
| Polymer | Side chains | Substrate | |||
|---|---|---|---|---|---|
| M n b | M w/Mnb | M n c | M w/Mnc | f n d | |
| a Substrates obtained by cycles of acetylation, grafting, and fractionation using Mn ≈ 5000 polystyrene core and side-chains. b Absolute values determined by SEC analysis with a linear polystyrene standards calibration curve. c Absolute values determined by SEC-MALLS analysis. d Number of branches added in the last grafting reaction. | |||||
| PS (linear) | 5.2 × 103b | 1.06b | |||
| G0PS | 5500 | 1.06 | 9.7 × 104 | 1.03 | 17 |
| G1PS | 4900 | 1.07 | 1.1 × 106 | 1.03 | 205 |
| Sample | P2VP side-chains | Graft copolymer | Mol% P2VP e | ||||
|---|---|---|---|---|---|---|---|
| M n a | M w/Mna | M n b | M w/Mnc | M n c | f n d | ||
| a Absolute values determined by SEC-MALLS. b Estimated values determined by combining the absolute Mn of the substrate with the copolymer composition from 1HNMR analysis. c Apparent values determined by SEC analysis using a linear polystyrene standards calibration curve; G1PS-g-P2VP30 not eluted from the column in SEC analysis. d Number of P2VP branches added in the last grafting reaction. e P2VP content determined by 1H NMR spectroscopy analysis. | |||||||
| PS-g-P2VP5 | 5100 | 1.15 | 7.4 × 104 | 1.08 | 4.1 × 104 | 13 | 93 |
| G0PS-g-P2VP5 | 5500 | 1.15 | 1.1 × 106 | 1.08 | 1.1 × 105 | 182 | 91 |
| G1PS-g-P2VP5 | 6200 | 1.10 | 8.4 × 106 | 1.09 | 1.6 × 105 | 1180 | 87 |
| PS-g-P2VP30 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.08 | 2.6 × 105 | 1.08 | 1.1 × 105 | 8 | 98 |
| G0PS-g-P2VP30 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.08 | 2.4 × 106 | 1.10 | 2.3 × 105 | 83 | 96 |
| G1PS-g-P2VP30 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.07 | 1.1 × 107 | — | — | 303 | 90 |
The composition of the copolymers was determined by 1H NMR spectroscopy analysis after removal of the linear (non-grafted) P2VP contaminant from the crude product. The results obtained (Table 2, last column) indicate P2VP contents of 87–93 mol% for the P2VP5 series copolymers, and 90–98 mol% for the P2VP30 series.
The absolute number-average molecular weight (Mn) and polydispersity index (Mw/Mn) of the graft copolymers could not be determined by SEC-MALLS analysis, because the instrument was dedicated exclusively to analysis in pure THF. Consequently, an alternate method was used to estimate the absolute molecular weight of the copolymers, by combining the absolute Mn of the substrate with the copolymer composition from 1H NMR spectroscopy analysis. The absolute Mn values for the graft copolymers estimated by that method are reported in Table 2, Column 4.
Considering the significant errors involved in NMR analysis for copolymers with high (> 90 mol%) P2VP contents, the errors on the Mn values for these graft copolymers are also likely large. The apparent values determined by SEC analysis using a DRI detector are reported along with the estimated values for comparison, and it is clear that the apparent molecular weights are underestimated. This is due to the very compact structure of arborescent polymers, as confirmed by light scattering8 and viscosity9 measurements. The polydispersity index remains low (Mw/Mn ≤ 1.10) for successive generations of graft polymers.
The number-average branching functionality (fn) of the arborescent polymers, defined as the number of chains added in the last grafting reaction, was calculated from the equation
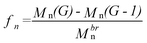 | (1) |
III.2. Viscosity of arborescent copolymer polyelectrolyte solutions
Branched polyelectrolytes are attractive because many of their physical properties, including the solution viscosity, differ markedly from their linear counterparts. It was for example reported that the dilute solution viscosity of branched polyelectrolytes with approximately eight side-chains, obtained by partial acetalization of the corresponding poly(vinyl alcohol)s with glyoxylic acid or o-phthalaldehydic acid, was much lower than for a linear polymer with the same degree of polymerization (Xn = 7000).10 Polyelectrolytes prepared by the sulfonation of star-branched polystyrenes with 13–18 arms were also used to investigate the effect of branching. In this case the hydrodynamic radius increased by only 20–40% upon decreasing the ionic strength of the solutions, while ca. 100% expansion was observed for the corresponding linear materials. This difference in behavior was attributed to the topology constraints present in the high branching functionality star polymers.11 The intrinsic viscosity of polyelectrolytes was also investigated in salt-free aqueous solutions by preparing a series of cross-linked (microgel) polystyrenesulfonates with molecular weights in the range 4.96 × 105–3.07 × 106. In contrast to linear chains, the viscosity of the cross-linked materials was molecular weight-independent below their overlap concentration C*.12 More recently, a pH-dependent study of dilute solutions of branched poly(methacrylic acid) (PMAA) was conducted in 10 vol% MeOH/H2O.13 In comparison with linear PMAA the influence of pH on viscosity was minimal in these branched polymers, with almost complete suppression of the polyelectrolyte effect attributed to the branched architecture of the molecules. The reduced viscosity of the branched polymers was also lower than for linear PMAA, the branched architecture precluding significant expansion of the molecules. Similar suppression of the polyelectrolyte effect was also reported for hyperbranched polyesters with terminal carboxylic acid groups at high added salt concentrations.14These results provide some hints of the trends to be expected for arborescent polyelectrolytes. The excellent control attained over the structure of arborescent polymers and their wide range of branching functionalities provide further opportunities to investigate the influence of branching in polyelectrolytes systematically.
Variations in the structure of arborescent copolymers can provide materials with a wide range of physical properties. Thus copolymers with short P2VP segments should be equivalent to a branched block copolymer with spherical symmetry (Fig. 1a). When long P2VP segments are grafted onto the polystyrene substrates, in contrast, the small dimensions of the substrate relatively to the outer branches yields a structure analogous to a star-branched homopolymer molecule (Fig. 1b). The compact structure of these copolymers should lead to interesting polyelectrolyte behavior upon protonation by a strong acid such as trifluoroacetic acid (TFA): on the one hand, expansion of the molecules may be favored when compared with linear poly(2-vinylpyridine) samples, due to the close proximity of ionic groups within the molecules. On the other hand, branching also increases the structural rigidity of the macromolecules, which may hinder conformation changes and oppose the electrostatic repulsive forces. Indications of this type of behavior were already obtained in preliminary investigations on polystyrene-graft-P2VP15a and arborescent polystyrene-graft-poly(methacrylic acid)15b polyelectrolytes using dynamic light scattering measurements. In the current investigation, the relative importance of electrostatic repulsions and structural rigidity was probed by examining the solution properties of arborescent polyelectrolytes as a function of molecular structure, protonation level, solvent type, and salt addition. To this end, the reduced viscosity (ηsp/C) of arborescent polyelectrolyte solutions was compared as a function of polyelectrolyte concentration (C) for the two families of P2VP copolymers synthesized and for linear P2VP samples under the same conditions.
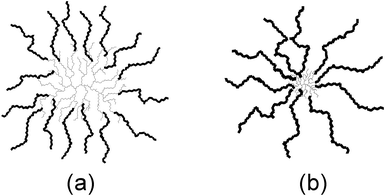 | ||
| Fig. 1 Comparison of structures obtained when a G1 polystyrene substrate is grafted with (a) short and (b) long P2VP side-chains. | ||
The arborescent polystyrene-graft-poly(2-vinylpyridine) copolymers are soluble in polar solvents (DMF, methanol) and act as a weak base able to acquire a net charge by protonation of the pyridine ring. The copolymers are soluble in acidic aqueous solutions for the same reason, even though the neutral copolymers are insoluble in pure water. The apparent pKa of such a polymer is difficult to define,16 because it depends on factors such as the degree of protonation of the chains and the background electrolyte concentration. However it was shown previously that linear P2VP, when dissolved in water with an equivalent concentration of a strong acid (HCl), had a protonation level of at least 70%.17
III.3. Influence of protonation level and solvent type
The properties of the copolymer solutions in methanol will be considered as a starting point, using sample G0PS-g-P2VP5 as an example. Methanol is a polar protic solvent with a dielectric constant (ε = 33) sufficient to induce significant dissociation of the counterions from the protonated P2VP chains. As a result, the arborescent P2VP copolymers display classical polyelectrolyte behavior even at low protonation levels (0.1 equiv TFA/2VP unit). The influence of the protonation level on solution viscosity, depicted in Fig. 2, is characterized by a marked polyelectrolyte effect due to chain expansion at low concentrations. The upswing is clearly visible up to 1.0 equiv TFA/2VP unit, but becomes less pronounced when excess TFA (3.0 equiv TFA/2VP unit) is added. Chain expansion is presumably suppressed under these conditions due to charge screening, as a result of partial ionization of the excess TFA.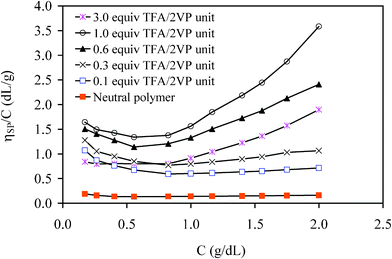 | ||
| Fig. 2 Reduced viscosity of G1 copolymer {G0PS-g-P2VP5} solutions protonated by TFA in methanol at 25 °C. | ||
An upturn is also observed in the high concentration range, as the viscosity becomes much larger than for the neutral copolymer. While high reduced viscosity values relatively to neutral polymers are characteristic for polyelectrolytes, the origin of the upturn at high concentrations is not so clear. For example, Hara et al.3 observed such viscosity enhancements at high concentrations for sulfonated polystyrene ionomers in a relatively low polarity solvent (THF) and attributed them to dominant intermolecular association of the ion pairs along the chains, but this effect was not observed in DMF. Entanglement formation could be a tempting explanation for viscosity enhancement in neutral polymer solutions, but chain entanglement in polyelectrolyte solutions is normally assumed to occur at high concentrations as compared to neutral polymers.2 Finally, while TFA is a relatively strong acid (pKa ≈ 0.2), it is still much weaker than mineral strong acids such as HCl (pKa ≈ −4). Any TFA not contributing to protonation of the P2VP segments in the copolymers may therefore give rise to ionic strength fluctuations due to variations in its ionization level. Intermolecular association, if present, may be partly suppressed by excess TFA due to charge screening resulting from the ionization of free TFA molecules. Consequently, while the following discussion will generally assume that an upturn in a ηsp/C vs. C plot in the high concentration range is related to intermolecular association, it should be kept in mind that alternate interpretations for this phenomenon are possible.
The reduced viscosity of the G0PS-g-P2VP5 copolymer (of generation 1 overall) is compared in Fig. 3 for three different solvents (methanol, DMF, and water) containing 1.0 equiv TFA/2VP unit. These solvents differ in terms of dielectric constant and character: Polarity increases within the series methanol (ε = 33) < DMF (ε = 37) < water (ε = 80), DMF being aprotic while the other two are protic solvents. It can be seen that viscosity enhancement (particularly in the high concentration range) correlates with solvent polarity (methanol > DMF > water), suggesting that intermolecular association is most significant in the lower polarity solvents. The upturn in the low concentration range, linked to coil expansion, is only visible in the two protic solvents due to their stronger ionizing power. The lack of upturn in DMF can be explained by the stronger counterion/polyion interactions and smaller electrostatic repulsive interactions due to the counterions, the latter “shielding” the polyion charges. Electrostatic repulsive interactions between the charges on the chain decrease under these conditions and lead to an overall decrease in molecular dimensions, inducing in turn a decrease in reduced viscosity. The interactions between the counterions and the polyion are thus stronger due to the lower ionizing power of DMF.
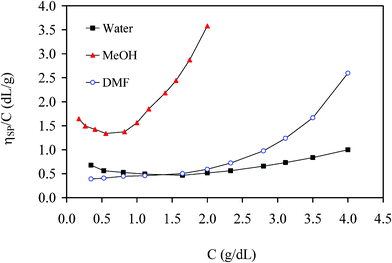 | ||
| Fig. 3 Reduced viscosity of G1 copolymer {G0PS-g-P2VP5} in different solvents with 1.0 equiv TFA/2VP unit at 25 °C. | ||
III.4. Influence of polymer structure
After investigating the influence of the protonation level and solvent type on the dilute solution properties of an arborescent copolymer of overall generation 1 (G0PS-g-P2VP5), the influence of the copolymer structure on the solution properties was examined by comparing the behaviors of the two families of P2VP copolymers in DMF and in methanol at constant protonation level (1.0 equiv TFA/2VP unit). The trends observed will also be compared with measurements on linear P2VP samples of different molecular weights under the same conditions.The reduced viscosity of arborescent polyelectrolytes with short P2VP side-chains (Mn ≈ 5000, P2VP5 sample series) of different generations in DMF and in methanol is compared in Fig. 4a and 4b, respectively. The corresponding curves for copolymers with long P2VP side-chains (Mn ≈ 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, P2VP30 sample series) are displayed in Fig. 4c and 4d, respectively. The overlap concentration C* = 1/[η] provided for each sample in the figure legends was estimated using the Fedors equation,18 where η and ηo are the viscosities of the solution and the pure solvent, respectively, and Cm is a polymer-specific constant parameter:
000, P2VP30 sample series) are displayed in Fig. 4c and 4d, respectively. The overlap concentration C* = 1/[η] provided for each sample in the figure legends was estimated using the Fedors equation,18 where η and ηo are the viscosities of the solution and the pure solvent, respectively, and Cm is a polymer-specific constant parameter:
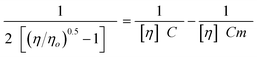 | (2) |
 | ||
| Fig. 4 Comparison of the reduced viscosity of arborescent copolymers with 5 K P2VP side-chains in (a) DMF, (b) methanol, and 30 K P2VP side-chains in (c) DMF, (d) methanol, with 1.0 equiv TFA/2VP unit at 25 °C. | ||
The uncertainties reported on C* were determined from the standard deviation on the slope. Other methods suggested to determine the overlap concentration of polyelectrolytes such as using the Fuoss equation,19 or experimentally as the concentration where the viscosity of the solution becomes twice as large as that of the pure solvent2 (η = 2ηo) were also explored but yielded similar results in terms of trends among the different samples.
The reduced viscosity of all the arborescent polyelectrolyte samples is systematically higher in methanol than in DMF over the whole concentration range investigated. In analogy to Fig. 3 the low-concentration upturn, linked to coil expansion, is more pronounced in methanol due to its higher ionizing power, but it is also more significant for the copolymers with the longer P2VP30 side-chains. The viscosity enhancement in the high concentration range, when present, correlates with the solvent polarity as before [methanol (ε = 33) > DMF (ε = 37)], intermolecular association being most favored in the less polar solvents and at higher polymer concentrations. Interestingly, the behavior of copolymers based on the G0PS substrate (G0PS-g-P2VP5 and G0PS-g-P2VP30; generation G1 overall) is very different from copolymers based on linear PS (PS-g-P2VP5, PS-g-P2VP30) and G1PS substrates (G1PS-g-P2VP5, G1PS-g-P2VP30) in both solvents. The G1 copolymers generally display the largest upturn in the low concentration range (except for G0PS-g-P2VP5 in DMF), and the strongest association in the high concentration range (except for G0PS-g-P2VP30 in methanol). The viscosity enhancement at low concentrations, linked to molecular expansion under the influence of electrostatic repulsive forces along the chain, is clearly most significant for the G1 copolymers. The repulsive electrostatic forces are opposed by the elastic forces due to the loss of entropy as the chains are forced to stretch. Conformation changes and chain deformation should be more difficult for branched molecules than for linear polymers, due to increased steric crowding and possibly to initial partial stretching of the chains. On the other hand, the charge density per unit volume of the molecules should be higher for branched polyelectrolytes than for linear polyelectrolytes, due to their more compact structure. This could either enhance electrostatic repulsions and molecular expansion, or else favor counterion condensation, enhancing ion pair formation and intramolecular association. The extent of chain expansion and intermolecular association must, therefore, depend on the delicate balance of polymer–solvent interactions, the extent of ionic group dissociation, and intramolecular elastic forces. On the basis of the results obtained, it appears that molecular expansion and intermolecular association are most favored for copolymers of generation G1 overall (G0PS-g-P2VP5 and G0PS-g-P2VP30). The other copolymers apparently have very little ability to associate intermolecularly over the concentration range examined (the reduced viscosity curves being remarkably flat in the high concentration range), as is normally expected for polyelectrolytes.
All copolymer molecules with P2VP30 side-chains have a stronger tendency to expand at low concentrations (under the influence of electrostatic repulsions) than their P2VP5 analogues, presumably due to the increased flexibility of the longer side-chains. When comparing the two copolymer series in the same solvent, the reduced viscosity is always higher for the P2VP30 series with the long, flexible side-chains than the P2VP5 series with shorter, stiffer side-chains. This is in agreement with a study of the solution properties of star-branched polyelectrolytes,20 sodium polyacrylate stars with the same branching functionality (same number of arms) always displaying enhanced molecular expansion for increasing branch molecular weights.
With the exception of the P2VP5 sample series in DMF, the reduced viscosity increases to different extents when the polyelectrolyte concentration decreases. The lack of upturn observed in the low concentration range for the P2VP5 series copolymers in DMF (Fig. 4a), a solvent with a comparatively lower ionizing power than methanol, suggests a more collapsed chain conformation due to enhanced counterion condensation. Stronger counterion–polyion interactions lead to lower electrostatic repulsive forces and less pronounced chain expansion. Since the copolymers in the P2VP30 series have more flexible side-chains than the P2VP5 series, the upturn due to coil expansion is more clearly visible (Fig. 4c). The reduced viscosity enhancement within each series is found to vary in the order (G1 > G0 > G2) for the P2VP5 series in DMF in the higher concentration range, whereas it varies in the order (G1 ≈ G0 > G2) in the lower concentration range (Fig. 4a), and (G1 > G0 > G2) in methanol over the entire concentration range examined (Fig. 4b). For the P2VP30 series it varies in the order (G1 > G0 ≥ G2) in DMF (Fig. 4c) and (G1 > G0 ≥ G2) in methanol (Fig. 4d) over the whole concentration range investigated. Within each sample series, coil expansion is therefore most pronounced for the G1 samples as compared to G0 and G2. This is presumably due to the relatively low steric crowding level experienced by the G1 molecules, in combination with a compact molecular size.
Within each series of arborescent copolymers, the reduced viscosity is lowest for the G2 samples. This effect is again attributed to the enhanced structural rigidity of these molecules. Previous investigations of arborescent polymers7,8,15 confirmed the very compact structure of these graft polymers, with hydrodynamic volumes over 10 times lower than linear polymers of comparable molecular weight. In a morphology investigation of arborescent polystyrenes using fluorescence quenching techniques, it was also demonstrated that the inner portion of the molecules was less accessible to quencher molecules than chains in the outer layer.21 The fraction of less accessible material was found to increase for higher generation polymers. Small-angle neutron scattering measurement on arborescent polystyrene22 further confirmed the high chain density in the core portion of the molecules relatively to the outer layer. This enhanced structural rigidity explains the smaller reduced viscosity increases observed for the G2 samples.
All the trends among the different samples, discussed above in terms of reduced viscosity, are also reflected in the overlap concentrations C* reported in the legends of Fig. 4: polymers with higher C* values (more compact structures) also have lower reduced viscosities. Conversely C* is lowest for the G1 copolymer samples in most cases, due to their more expanded structure.
III.5. Comparison with linear P2VP samples and added salt effects
Three linear poly(2-vinylpyridine) samples P2VP65, P2VP230, and P2VP430, with Mn ≈ 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 230
000, 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 430
000, and 430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively, were synthesized as reference materials for comparison of their polyelectrolyte behavior with the arborescent copolymers. Narrow molecular weight distributions were obtained in all cases, as determined by SEC-MALLS analysis (P2VP65: Mn = 65
000, respectively, were synthesized as reference materials for comparison of their polyelectrolyte behavior with the arborescent copolymers. Narrow molecular weight distributions were obtained in all cases, as determined by SEC-MALLS analysis (P2VP65: Mn = 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.06; P2VP230: Mn = 230
000, Mw/Mn = 1.06; P2VP230: Mn = 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.08; P2VP430: Mn = 430
000, Mw/Mn = 1.08; P2VP430: Mn = 430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 1.09).
000, Mw/Mn = 1.09).
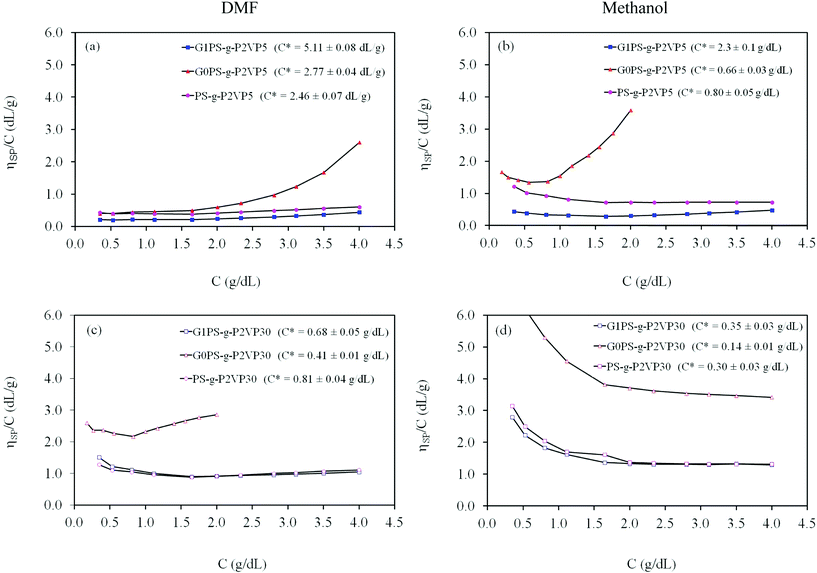
Comparisons with linear P2VP samples in DMF and in methanol are provided in Fig. 5a and 5b for the P2VP5 samples, and in Fig. 5c and 5d for the P2VP30 samples, respectively, using different scales to take into account the higher reduced viscosity of the linear P2VP samples. The corresponding overlap concentrations of the samples are also provided in the figure legends. Irrespective of the solvent type used, it is clear that the reduced viscosity of the arborescent copolymers is only comparable to linear P2VP for samples of relatively low molecular weights (P2VP30, P2VP65), while it is much lower than for linear samples with Mn = 230 K (P2VP230) and 430 K (P2VP430). This is particularly interesting when considering that the graft copolymers have absolute molecular weights ranging from Mn = 7.4 × 104–1.1 × 107. The variations in C* likewise reflect these trends, being much lower for the linear high molecular weight polymers than for the branched samples. The upturn in the reduced viscosity at low concentrations is also much more important for the P2VP230 and P2VP430 linear samples than for any of the arborescent polyelectrolytes. This confirms the strong suppression of molecular expansion observed for the branched polyelectrolytes, presumably due to their more rigid structure. These results are again consistent with findings reported for four- and six-arm star-branched sodium polyacrylate solutions:20 Molecular expansion was invariably more important for linear sodium polyacrylate samples with molecular weights comparable to the star-branched systems, and decreased as the branching functionality of the stars increased. This was explained by the lower degrees of freedom available for the chains in star polymers of higher branching functionality. It is interesting to note the similar trends observed among the arborescent polyelectrolytes, in spite of their molecular weight up to 27 times larger than the linear P2VP samples used in the comparison.
 | ||
| Fig. 5 Comparison of the reduced viscosity of linear P2VP and arborescent copolymer generations based on 5 K P2VP side-chains (a) DMF, (b) methanol, and 30 K P2VP side-chains (c) DMF, (d) methanol, with 1.0 equiv TFA/2VP unit at 25 °C. | ||
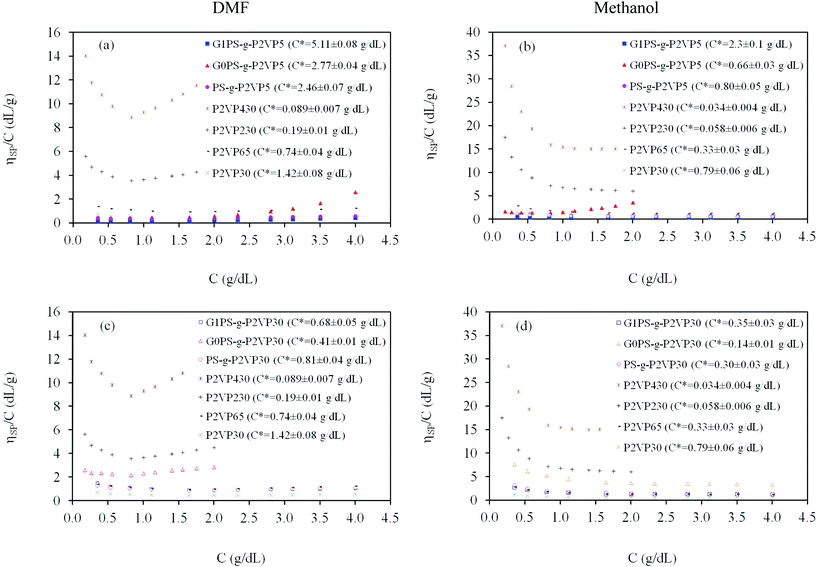
Finally, the influence of added salts on the viscosity of arborescent polyelectrolyte solutions is depicted in Fig. 6a and 6b for DMF and methanol, respectively. This behavior is characteristic of polyelectrolyte solutions in general: the concave curves almost revert to straight lines in the presence of a salt, becoming similar to neutral polymer solutions. It is well-known that for polyelectrolytes, counterion binding (condensation) within the polymer coil is enhanced in the presence of salts.23 According to Le Châtelier's principle, counterion binding should be favored as the concentration of salt is increased. This leads to decreased repulsive interactions between the ionic moieties along the polymer chains, a smaller molecular size, and a lower reduced viscosity.
 | ||
| Fig. 6 Effect of LiCl on the viscosity of an arborescent copolymer of generation G1 overall {G0PS-g-P2VP5} with 1.0 equiv TFA/2VP unit at 25 °C: (a) DMF, (b) methanol. | ||
IV. Concluding remarks
The large (100 g) scale synthesis of dendritic polyelectrolyte precursors was accomplished by grafting living poly(2-vinylpyridinyl)lithium onto linear and branched polystyrene substrates randomly functionalized with acetyl groups. Two arborescent copolymer families with Mn ≈ 5000 or 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 P2VP side-chains and containing 87–98 mol% P2VP were thus synthesized. A narrow molecular weight distribution (Mw/Mn = 1.08–1.10) was maintained for graft copolymers of successive generations. These materials are characterized by a very compact structure and a roughly geometric increase in branching functionality and molecular weight for each generation.
000 P2VP side-chains and containing 87–98 mol% P2VP were thus synthesized. A narrow molecular weight distribution (Mw/Mn = 1.08–1.10) was maintained for graft copolymers of successive generations. These materials are characterized by a very compact structure and a roughly geometric increase in branching functionality and molecular weight for each generation.
The polyelectrolyte behavior of the arborescent polystyrene-graft-poly(2-vinylpyridine) copolymers was investigated. These materials are freely soluble in polar solvents including methanol, DMF, and water upon protonation by strong acids such as trifluoroacetic acid. The copolymer solutions display properties typical of polyelectrolytes, including strong curvature in plots of reduced viscosity (ηsp/C) versus concentration (C) at both low and high concentrations. In comparing the influence of the solvent type used, the reduced viscosity of both copolymer series (P2VP5 and P2VP30) was always higher in methanol than in DMF, due its stronger ionizing power. In the same solvent, the reduced viscosity was also higher for copolymers with the long, flexible P2VP30 side-chains as compared to the stiffer P2VP5 copolymers. The reduced viscosity was found to decrease within each sample series in the order G1 > G0 > G2. Molecular expansion, as reflected in the reduced viscosity and the overlap concentration C*, is apparently more pronounced for the G1 copolymers than the G0 and G2 copolymers, presumably due to the balance of enhanced charge repulsions and increased structural rigidity within the compact molecules. In comparison with linear P2VP samples, however, the reduced viscosity of arborescent polyelectrolyte solutions is much lower, in spite of their much higher molecular weights. The addition of salt to the branched polyelectrolyte solutions decreases their viscosity and suppresses the curvature of the reduced viscosity plots, presumably due to charge screening effects of the type observed in linear polyelectrolyte systems.
Acknowledgements
We acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) for this work.References
- (a) For an overview of the properties of polyelectrolytes, including branched structures, see for example: Polyelectrolytes: Science and Technology, ed. M. Hara, Dekker: New York, 1993 Search PubMed; (b) F. M. Gray, Polymer Electrolytes, RSC Materials Monographs, Royal Society of Chemistry, London, 1997 Search PubMed; (c) F. A. M. Leermakers, M. Ballauff and O. V. Borisov, Langmuir, 2008, 24, 10026 CrossRef CAS; (d) F. Samadi, B. A. Wolf, Y. Guo, A. Zhang and A. D. Schlüter, Macromolecules, 2008, 41, 8173 CrossRef CAS; (e) Y. Xu, F. Plamper, M. Ballauff and A. H. E. Müller, Adv. Polym. Sci., 2010, 228, 1 CrossRef CAS; (f) D. V. Pergushov, O. V. Borisov, A. B. Zezin and A. H. E. Müller, Adv. Polym. Sci., 2011, 241, 131 CrossRef.
- A. V. Dobrynin and M. Rubinstein, Prog. Polym. Sci., 2005, 30, 1049 CrossRef CAS.
- M. Hara, J.-L. Wu and A. H. Lee, Macromolecules, 1988, 21, 2214 CrossRef CAS.
- S. J. Teertstra and M. Gauthier, Prog. Polym. Sci., 2004, 29, 277 CrossRef CAS.
- J. Li and M. Gauthier, Macromolecules, 2001, 34, 8918 CrossRef CAS.
- A. Munam and M. Gauthier, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5742 CrossRef CAS.
- (a) M. Gauthier, J. Li and J. Dockendorff, Macromolecules, 2003, 36, 2642 CrossRef CAS; (b) A. Munam, Ph.D. Thesis, University of Waterloo: Waterloo, 2007 Search PubMed.
- M. Gauthier, M. Möller and W. Burchard, Macromol. Symp., 1994, 77, 43 CrossRef CAS.
- M. Gauthier, W. Li and L. Tichagwa, Polymer, 1997, 38, 6363 CrossRef CAS.
- S. Ichiro, S. Yasuyoshi and T. Shizuo, Kobunshi Kagaku, 1962, 19, 663 CrossRef.
- J. W. Mays, Polymer Commun., 1990, 31, 170 CAS.
- M. Antonietti, A. Briel and S. Förster, Macromolecules, 1997, 30, 2700 CrossRef CAS.
- S. Graham, P. A. G. Cormack and D. C. Sherrington, Macromolecules, 2005, 38, 86 CrossRef CAS.
- S. R. Turner, F. Walter, B. I. Voit and T. H. Mourey, Macromolecules, 1994, 27, 1611 CrossRef CAS.
- (a) R. A. Kee and M. Gauthier, Macromolecules, 2002, 35, 6526 CrossRef CAS; (b) R. A. Kee and M. Gauthier, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2335 CrossRef CAS.
- E. A. Bekturov and Z. Kh. Bakauova, Synthetic Water-Soluble Polymers in Solution, Huethhig and Wepf: Basel, 1986; Chapter 2 Search PubMed.
- G. Muller, C. Ripoll and E. Selegny, Eur. Polym. J., 1971, 7, 1373 CrossRef CAS.
- (a) R. F. Fedors, Polymer, 1979, 20, 225 CrossRef CAS; (b) S. Dragan and L. Ghimici, Polymer, 2001, 42, 2887 CrossRef CAS; (c) I. Ydens, S. Moins, P. Degée and P. Dubois, Eur. Polym. J., 2005, 41, 1502 CrossRef CAS.
- R. M. Fuoss and U. P. Strauss, J. Polym. Sci., 1948, 3, 246 CrossRef CAS.
- D. Moinard, R. Borsali, D. Taton and Y. Gnanou, Macromolecules, 2005, 38, 7105 CrossRef CAS.
- R. S. Frank, G. Merkle and M. Gauthier, Macromolecules, 1997, 30, 5397 CrossRef CAS.
- S. Choi, R. M. Briber, B. J. Bauer, A. Topp, M. Gauthier and L. Tichagwa, Macromolecules, 1999, 32, 7879 CrossRef CAS.
- F. Oosawa, Polyelectrolytes, Dekker: New York, 1971; Chapter 7 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
