Chemically patterned porous silicon photonic crystals towards internally referenced organic vapour sensors
Martin J.
Sweetman
a and
Nicolas H.
Voelcker
*b
aSchool of Chemical and Physical Sciences, Flinders University, Bedford Park, SA 5042, Australia
bMawson Institute, University of South Australia, Mawson Lakes, SA 5095. E-mail: nico.voelcker@unisa.edu.au Fax +61 (8) 8302 5639; Tel: +61 (8) 8302 5508
First published on 26th March 2012
Abstract
The fabrication of chemically patterned porous silicon (pSi) photonic crystals towards their use as an internally referenced organic vapour sensor is reported. The sensor fabrication technique involves photoresist patterning and surface silanisation of pSi photonic crystals to produce patterns of hydrophobic and hydrophilic regions. The photonic response to the presence of organic vapour is monitored at the interface of these regions.
Chemical and biological sensors that utilise both the optical and physical properties of nanostructured pSi films are gaining increasing popularity.1 Optical properties such as room temperature photoluminescence and Fabry–Pérot fringes, both of which are sensitive to changes in the internal pore environment, allow the accurate detection of chemical and biological species.2 Tuneable pore diameter and depth, along with the high internal surface area (up to 800 m2 g−1), permit a large amount of chemical and/or biological species to be incorporated into the porous film at any one time.3 This greatly enhances detection limits and sensing capabilities. While the detection of biological species is a major focus of pSi based sensors, a range of chemical sensors for the detection of corrosive and hazardous vapours have also been developed. Various examples of organic vapour sensors have been reported, which exploit the optical and other physical properties of pSi films to improve the sensitivity and limits of detection.4 As pSi films can be easily functionalised with a large range of different chemical species, the effect of different surface functionality on organic vapour sensing has also been investigated.5 Functionalisation of pSi films is a key step when designing and fabricating robust, reusable and sensitive chemical sensors. Ruminski et al. recently described a pSi based sensor for the detection of HF(aq) incorporating an internal referencing capability.6 This was achieved by selectively functionalising part of the pSi film with polystyrene and hence protecting it from corrosion when exposed to HF(aq). As this sensor is based on irreversible chemical reactions, the possibility of reuse is limited.
The design and fabrication of pSi films for reusable organic vapour sensing, with an internal referencing component is reported here. A technique previously reported by our group to introduce distinctly different chemical functionality in microscale patterns on a pSi film was used here to fabricate surfaces suitable for the detection of organic vapour.7 pSi photonic crystals were chemically patterned with hydrophilic (3-aminopropyltriethoxy silane, APTES) and hydrophobic (pentafluorophenyl dimethylchlorosilane, PFPS) silane compounds. When this chemical patterning procedure was performed on pSi rugate filters (photonic crystal structures showing sinusoidal variation in refractive index), the resulting surface displayed two narrow optical reflectance peak maxima at different wavelengths on the two regions of the pattern.8 We hypothesised that by functionalising the surface with a hydrophobic and a hydrophilic silane, organic vapour would readily interact within the hydrophobic functionalised pores, but would not enter the hydrophilic pores. As air is displaced by organic vapour in the hydrophobic pores, the corresponding reflectance peak undergoes a red shift, due to the net increase in refractive index of the film.2 The position of the corresponding reflectance peak on the hydrophilic regions would remain constant and could therefore act as an internal reference to the shift of the hydrophobic reflectance peak. This could allow the effect of external factors such as ambient light, temperature and humidity to be corrected for.6
pSi rugate filters were produced from p-type, boron doped silicon (< 1 mΩcm), by anodic etching in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aqueous HF48% to EtOH, with a sinusoidal current, cycled from 11.5–34.6 mA cm−2, with a period of 12 s. The wafer was etched for 60 cycles (720 s), after which the HF was removed and rinsed with methanol, acetone and dichloromethane and dried under nitrogen. pSi surfaces were functionalised following a chemical patterning procedure previously reported by the authors.7 The surface functionalisation procedure is depicted in Fig. 1a and is briefly described in the following. Freshly etched surfaces were oxidised by exposure to ozone and initially functionalised with APTES. Positive tone photoresist was then spin-coated onto the surface, into which an array of circular features of 500 μm diameter, separated by 250 μm was developed. The exposed regions of the pSi surface were then treated with HF(aq) to completely remove the APTES and the resulting Si–H terminated surface was re-oxidised in ozone. The oxidised surface was then silanised with neat PFPS at 80 °C for 2 h. Finally, the surface was washed with copious amounts of acetone to remove unreacted silane and remaining photoresist.
1 aqueous HF48% to EtOH, with a sinusoidal current, cycled from 11.5–34.6 mA cm−2, with a period of 12 s. The wafer was etched for 60 cycles (720 s), after which the HF was removed and rinsed with methanol, acetone and dichloromethane and dried under nitrogen. pSi surfaces were functionalised following a chemical patterning procedure previously reported by the authors.7 The surface functionalisation procedure is depicted in Fig. 1a and is briefly described in the following. Freshly etched surfaces were oxidised by exposure to ozone and initially functionalised with APTES. Positive tone photoresist was then spin-coated onto the surface, into which an array of circular features of 500 μm diameter, separated by 250 μm was developed. The exposed regions of the pSi surface were then treated with HF(aq) to completely remove the APTES and the resulting Si–H terminated surface was re-oxidised in ozone. The oxidised surface was then silanised with neat PFPS at 80 °C for 2 h. Finally, the surface was washed with copious amounts of acetone to remove unreacted silane and remaining photoresist.
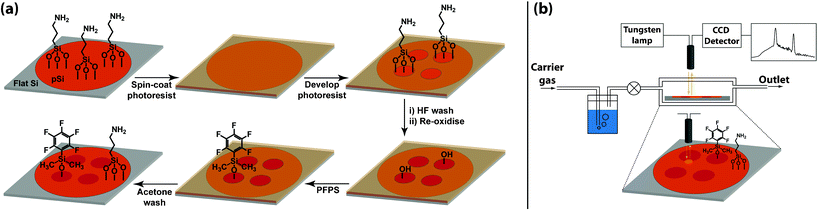 | ||
| Fig. 1 (a) Surface fabrication procedure for the patterned incorporation of two different silane compounds on a pSi rugate surface. (b) Organic vapour sensor setup, illustrating flow cell and IRS monitoring at a chemical interface on the pSi surface. | ||
Fig. 2a displays two attenuated total internal reflectance IR (ATR-IR) microspectra of a modified pSi surface, one from the APTES functionalised region and one from the PFPS functionalised region. In the case of APTES, a small vibrational peak corresponding to C–H bond stretching vibrations can be seen at 2910 cm−1, along with a prominent peak from the primary amine at 1535 cm−1.9 The PFPS spectrum displays a sharp peak at 1510 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration within the phenyl ring of the silane.10 Another conspicuous peak at 1160 cm−1 is attributed to the C–F stretching vibration.10 In both spectra, a broad peak centred around 1050 cm−1 from Si–O stretching vibrations is present.11 An IR microscopy map of a part of the pSi surface patterned with APTES and PFPS (Fig. 2b) shows four circular features. The map displays integrated area under the C
C stretching vibration within the phenyl ring of the silane.10 Another conspicuous peak at 1160 cm−1 is attributed to the C–F stretching vibration.10 In both spectra, a broad peak centred around 1050 cm−1 from Si–O stretching vibrations is present.11 An IR microscopy map of a part of the pSi surface patterned with APTES and PFPS (Fig. 2b) shows four circular features. The map displays integrated area under the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrational peak at 1510 cm−1, for each pixel. As expected, the PFPS signal is prominent and of even intensity within the circular regions, and absent in the surrounding APTES functionalised areas. This indicates that the patterning procedure generated a chemical pattern of high fidelity without cross-reactions.
C stretching vibrational peak at 1510 cm−1, for each pixel. As expected, the PFPS signal is prominent and of even intensity within the circular regions, and absent in the surrounding APTES functionalised areas. This indicates that the patterning procedure generated a chemical pattern of high fidelity without cross-reactions.
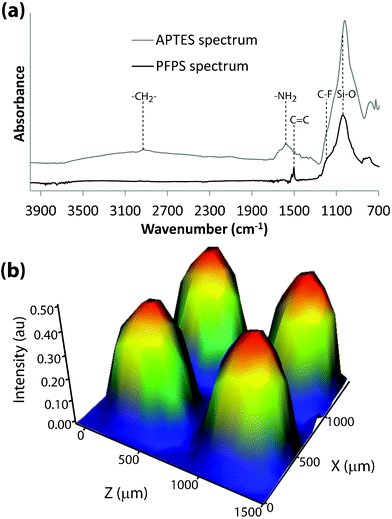 | ||
Fig. 2 (a) ATR-IR spectra from the APTES and PFPS functionalised regions on the pSi surface, respectively. (b) IR map of an array of four circular spots, displaying the intensity of the integrated peak area for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of the phenyl ring in the PFPS. C stretching vibrations of the phenyl ring in the PFPS. | ||
The two silanes selected for this patterning method were chosen specifically, in order to incorporate highly hydrophobic fluorinated surface chemistry (PFPS), surrounded by more hydrophilic amine-functional surface chemistry (APTES). Water contact angle measurements for APTES and PFPS functionalised pSi surfaces were measured as 37 ± 3° and 104 ± 11° for APTES and PFPS, respectively, which are comparable to reported values in the literature.10
The photonic response from the pSi rugate filters fabricated by the described patterning method displays two reflectance peak maxima at 623 nm and 795 nm for the PFPS and APTES functionalised regions, respectively (Fig. 3a, inset). The pronounced peak separation of 172 nm is due to the HF treatment step during fabrication, where the oxide layer in the pores within the circular features is removed. This leads to pore widening which lowers the net refractive index in those regions.12 The decrease in refractive index results in a blue shift of the reflectance peak maximum.13,14
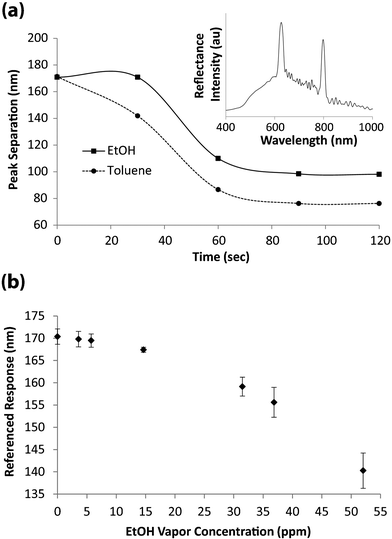 | ||
| Fig. 3 (a) Peak separation of reflectance peaks over time, upon exposure to saturated toluene and EtOH vapour atmospheres. The inset displays a representative reflectance spectrum from the pSi photonic crystal, acquired at the interface between chemically distinct regions. (b) Rugate filter peak separation for different EtOH vapour concentrations (n = 3). | ||
Since the circular features and the surrounding pSi display separate rugate filter reflectance peak maxima, both peaks can be observed simultaneously in a single reflectance spectrum by monitoring the reflectance at the interface between the two chemically distinct regions using fibre-optics interferometric reflectance spectroscopy (IRS). This technique is illustrated schematically in Fig. 1b. The ability to monitor both of the reflectance peak maxima simultaneously allows interactions of chemical species within the pores of one region to be monitored (as a shift in the reflectance peak maximum), while the second peak can be used as an internal reference.
The sensor response in saturated vapour atmospheres was monitored by placing the sensor in a closed vessel containing a small amount (∼2 mL) of organic solution (toluene or ethanol). The effect of exposing patterned pSi rugate filters to saturated toluene or ethanol atmospheres is demonstrated in Fig. 3a. Here, the reflectance spectrum at the interface of the two chemically distinct regions on the rugate filter (shown in the inset in Fig. 3a), was monitored over time and the peak separation according to eqn. (1) was recorded.
| Peak Separation = λAPTES − λPFPS | (1) |
It can be observed that the peak separation plateaus out after approximately 80 s of exposure. In both cases, the peak separation decreases over time as the PFPS peak red-shifts to wavelengths closer to that of the APTES peak. The red shift is caused by the changed refractive index of the pSi film upon replacement of air within the pores with organic vapour of higher refractive index. The change in peak separation is more pronounced for toluene compared to ethanol. This difference is due to the higher refractive index of toluene, which as it replaces air in the pores causes a greater red shift in the reflectance peak maxima. Several replicate measurements were taken (n = 6) and the sensor responses were within 1% demonstrating high reproducibility of this sensor.
The pSi surface was then exposed to different concentrations of organic vapour by bubbling a carrier gas (purified air) through toluene or different solutions of ethanol in ultrapure MilliQ H2O. Reflectivity spectra at the chemical interface were measured every min for a period of 20 min after which the reflectance peak positions at all concentrations had stabilised. The peak positions were recorded and the peak separation determined according to eqn. (1). After each exposure, the sensor surface was regenerated by flowing purified air over the surface for a minimum of 2 min. Each sensor run was conducted using the same, continuous flow rate, with the temperature maintained at 22 °C. Vapour concentrations (in ppm) were calculated from gas cell IR absorbance measurements. In Fig. 3b, the peak separation at increasing EtOH vapour concentration is plotted. As the vapour concentration increases, the photonic peak separation between the PFPS and APTES functionalised regions decreases. From the graph in Fig. 3b, a limit of detection for ethanol vapour of approximately 15 ppm can be derived which is on par with other pSi based ethanol vapour sensors.15 However, our sensor features internal referencing capability.
In conclusion, we have described a robust, reusable organic vapour sensor based on a pSi rugate filter, where two photonic peaks from two chemically distinct regions on the rugate filter as a detector and internal reference. The fabrication and functionalisation methods used here impart a high degree of stability to this pSi based sensor. Coupled with the non-destructive sensing technique, pSi films will be stable over long periods of time, allowing repeated and reproducible sensing. Future experiments will focus on calibration of the internal reference to factors such as humidity and temperature, as well as investigating different combinations of silanes to impart a degree of selectivity (to different organic vapours) on the sensor platform. We anticipate that this platform may be readily modified and adapted, based on the chemistry of the silanes used to functionalise the pSi surface, for the detection of more specialised chemical or biological species.
The authors would like to acknowledge financial support from the Australian Research Council.
References
- A. Jane, R. Dronov, A. Hodges and N. H. Voelcker, Trends Biotechnol., 2009, 27, 230–239 CrossRef CAS.
- K. A. Kilian, L. M. H. Lai, A. Magenau, S. Cartland, T. Bocking, N. Di Girolamo, M. Gal, K. Gaus and J. J. Gooding, Nano Lett., 2009, 9, 2021–2025 CrossRef CAS.
- S. P. Low, K. A. Williams, L. T. Canham and N. H. Voelcker, Biomaterials, 2006, 27, 4538–4546 CrossRef CAS.
- J. Gao, Y. Gao, Y. Y. Li and M. J. Sailor, Langmuir, 2002, 18, 2229–2233 CrossRef CAS.
- T. Gao, J. Gao and M. J. Sailor, Langmuir, 2002, 18, 9953–9957 CrossRef CAS.
- A. M. Ruminski, G. Barillaro, C. Chaffin and M. J. Sailor, Adv. Funct. Mater., 2011, 21, 1511–1525 CrossRef CAS.
- M. J. Sweetman, C. J. Shearer, J. G. Shapter and N. H. Voelcker, Langmuir, 2011, 27, 9497–9503 CAS.
- F. Cunin, T. A. Schmedake, J. R. Link, Y. Y. Li, J. Koh, S. N. Bhatia and M. J. Sailor, Nat. Mater., 2002, 1, 39–41 CrossRef CAS.
- M. Hiraoui, M. Guendouz, N. Lorrain, A. Moadhen, L. Haji and M. Oueslati, Mater. Chem. Phys., 2011, 128, 151–156 CrossRef CAS.
- Y. L. Khung, M. A. Cole, S. J. P. McInnes and N. H. Voelcker, P Soc Photo-Opt Ins, Canberra, 2008.
- S. Aouida, M. Saadoun, K. Ben Saad, M. F. Boujmil and B. Bessais, Phys. Chem. News, 2006, 27, 55–58 CAS.
- S. Létant and M. J. Sailor, Adv. Mater., 2000, 12, 355–359 CrossRef.
- S. Labbe-Lavigne, S. Barret, F. Garet, L. Duvillaret and J. L. Coutaz, J. Appl. Phys., 1998, 83, 6007–6010 CrossRef CAS.
- C. Pacholski, M. Sartor, M. J. Sailor, F. Cunin and G. M. Miskelly, J. Am. Chem. Soc., 2005, 127, 11636–11645 CrossRef CAS.
- S. H. Park, D. Seo, Y. Y. Kim and K. W. Lee, Sens. Actuators, B, 2010, 147, 775–779 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
