Palladium-catalyzed aerobic oxidative C–H amination: synthesis of 2-unsubstituted and 2-substituted N-aryl benzimidazoles†
Rapolu Kiran
Kumar
and
Tharmalingam
Punniyamurthy
*
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati-781039, India. E-mail: tpunni@iitg.ernet.in; Fax: +91 0361 2690762; Tel: +91 0361 2582309
First published on 26th March 2012
Abstract
Palladium-catalyzed aerobic synthesis of 2-unsubstituted and 2-substituted N-aryl benzimidazoles has been described from N,N′-bis(aryl)amidines via oxidative C–H amination.
The recent advances in transition metal catalysis have led to the development of effective methods for the construction of carbon–heteroatom bonds.1 Among them, C–H activation protocols are attractive because they are atom economical and obviate the need for preactivated substrate precursors. Significant progress has been made using predominantly Ru,2 Rh,3 Pd,4 Ag5 and Cu6 based catalytic systems. Herein, we wish to report the first Pd-catalyzed direct synthesis of 2-unsubstituted and 2-substituted N-aryl benzimidazoles from N,N′-bis(aryl)amidines via oxidative C–H amination. The protocols are straightforward and the target molecules can be obtained using molecular oxygen as the terminal oxidant.
N-Aryl benzimidazoles are a class of prominent heterocyclic motifs that exhibit a wide range of biological properties such as Nek27a and lymphocyte specific kinase (Lck) inhibition (Fig. 1).7b In addition, they are substrate precursors for the preparation of an important class of N-heterocyclic carbenes (NHCs)8 that have been successfully utilized as ligands in organocatalysis as well as in transition metal catalysis.
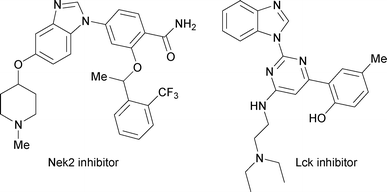 | ||
| Fig. 1 Some examples of pharmaceutically active N-aryl benzimidazoles. | ||
The classical approaches employed for the synthesis of the N-aryl benzimidazoles involve condensation followed by the oxidative cyclization of o-phenylenediamine with carboxylic acids or carboxylic acid derivatives (Scheme 1, route a).9 However, these processes often suffer due to the unavailability of suitably substituted substrate precursors. Furthermore, the oxidative cyclization requires the combination of strong acids and elevated temperature.
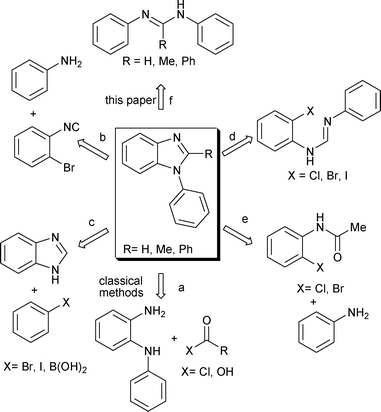 | ||
| Scheme 1 Methods available for the synthesis of N-aryl benzimidazoles: (a) classical method, (b–e) cross-coupling and (f) C–H amination. | ||
Some of these limitations have been circumvented by the recent development of cross-coupling reactions using transition metal catalysis.10–12 For example, Pd11a–b or Co11c or Cu11d–g based catalytic systems have been explored for domino reactions of amines with 2-bromophenyl isocyanides (Scheme 1, route b), the intermolecular C–N cross-coupling of benzimidazole with aryl boronic acids (Scheme 1, route c), the cyclization of 2-haloaryl amidines (Scheme 1, route d) or the cross-coupling reaction followed by condensation of 2-haloacetanilides with anilines to afford N-aryl benzimidazoles (Scheme 1, route e). Recently, the synthesis of benzimidazole has been shown via C–H activation, however, these methods are limited to the construction of N-methyl or N-unsubstituted benzimidazoles6d,f and not effective for the synthesis of N-arylbenzimidazoles. Development of new methods for the direct synthesis of N-aryl benzimidazole structural framework via C–H activation/C–N bond formation would thus valuable in organic synthesis (Scheme 1, route f).
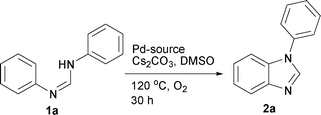
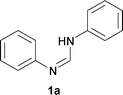
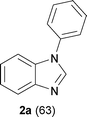
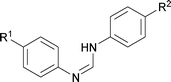
First, the optimization of the reaction conditions was carried out with N,N′-diphenyl formamidine 1a as a model substrate that can be readily prepared from aniline and triethylorthoformate (see the ESI†). The substrate 1a underwent C–H activation followed by intramolecular C–N bond formation to afford the desired N-phenyl benzimidazole 2a in 46% yield when the reaction was carried out in the presence of 10 mol % Pd(PPh3)2Cl2 and 2 equiv. Cs2CO3 in DMSO under molecular oxygen (Table 1, entry 1). Yield of the heterocycle 2a could be further increased to 63% when activated 4 Å molecular sieves were added as an additive (entry 2). Palladium sources such as Pd(PPh3)2(OAc)2, Pd(CH3CN)2Cl2 and Pd(PhCN)2Cl2 were less effective compared to Pd(PPh3)2Cl2 affording 2a in 10–30% yield (entries 3–5). In contrast, Pd(OAc)2 led to decomposition of the starting material 1a and no desired 2a was obtained. When 1a was subjected to 5 mol % Pd(PPh3)2Cl2, the yield of 2a dropped significantly (entry 6). Control experiments confirmed that 2a was not formed in the absence of the palladium catalyst (entry 7).
| Entry | Pd-source | Yield (%) a,b |
|---|---|---|
| a A mixture of N,N′-diphenyl formamidine 1a (0.5 mmol), Pd-source (10 mol %), Cs2CO3 (1 mmol) and 4 Å MS (50 mg) were stirred at 120 °C in DMSO (1 mL) under an oxygen balloon. b Isolated yield. c Without 4 Å MS. d Pd(PPh3)2Cl2 (5 mol %) used. n.d. = Not detected. | ||
| 1c | Pd(PPh3)2Cl2 | 46 |
| 2 | Pd(PPh3)2Cl2 | 63 |
| 3 | Pd(PPh3)2(OAc)2 | 30 |
| 4 | Pd(CH3CN)2Cl2 | 11 |
| 5 | Pd(PhCN)2Cl2 | 10 |
| 6d | Pd(PPh3)2Cl2 | 35 |
| 7 | — | n.d. |
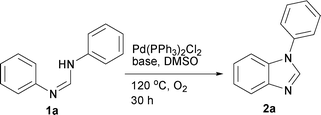
Among the bases screened, Cs2CO3, K2CO3, Ag2CO3, K3PO4 and Na2CO3, the former yielded the best results (Table 2, entries 1–5). The reactions with K2CO3, K3PO4, Na2CO3, Ag2CO3 and KOH gave 2a in 10–50% yield. In contrast, Ag2CO3 and KOH led to the decomposition of 1a and no product 2a was obtained. When the amount of Cs2CO3 was lowered to 1.5 equiv, the yield of 2a was dropped to 45% (entry 6).
| Entry | Base | Yield (%) a,b |
|---|---|---|
| a Substrate 1a (0.5 mmol), Pd(PPh3)2Cl2 (10 mol %), base (1 mmol) and 4 Å MS (50 mg) were stirred at 120 °C in DMSO (1 mL) under an oxygen balloon. b Isolated yield. c Cs2CO3 (0.75 mmol) used. n.d. = Not detected. | ||
| 1 | K2CO3 | 50 |
| 2 | Na2CO3 | 10 |
| 3 | K3PO4 | 40 |
| 4 | KOH | n.d. |
| 5 | Ag2CO3 | n.d. |
| 6c | Cs2CO3 | 45 |
The reaction using DMF provided 2a in moderate yield (Table 3, entries 1–4). In contrast, N-methyl-2-pyrrolidone (NMP), toluene and acetonitrile yielded inferior results. A similar result was obtained when the reaction was subjected to 70 °C or under N2 atmosphere (entries 5–6). However, a reaction with air led to the formation of 2a in moderate yield (entry 7). In summary, the optimized conditions in DMSO include Pd(PPh3)2Cl2 (10 mol %) and Cs2CO3 (2 equiv) at 120 °C for 30 h under an oxygen balloon.
| Entry | Solvent | Yield (%) a,b |
|---|---|---|
| a Substrate 1a (0.5 mmol), Pd(PPh3)2Cl2 (10 mol %), Cs2CO3 (1 mmol) and 4 Å MS (50 mg) were stirred at 120 °C in solvent (1 mL) under an oxygen balloon. b Isolated yield. c Reaction T = 70 °C. d Under N2 . e Under air. n.d. = Not detected. | ||
| 1 | DMF | 45 |
| 2 | NMP | n.d. |
| 3 | toluene | n.d. |
| 4 | CH3CN | n.d. |
| 5c | DMSO | n.d. |
| 6d | DMSO | n.d. |
| 7e | DMSO | 35 |
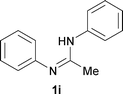
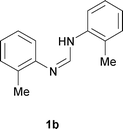
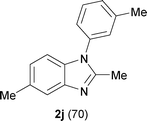
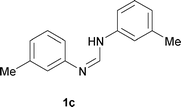
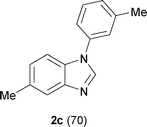
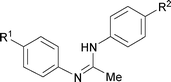
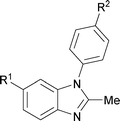
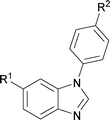
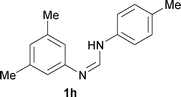
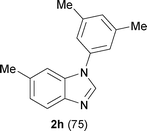
Next, the scope of the procedure was studied for the reactions of the substituted N,N′-bis(aryl)formamidines (Table 4, entries 1–7). N,N′-Bis(phenyl)formamidine 1b with methyl substituents at the o-position of both the phenyl rings did not cyclize, which may be due to the steric hindrance of the methyl groups (entry 2). However, the symmetrical substrates 1c–g with methyl, ethyl, fluoro and 2-propyl substituents at m- as well as the p- positions of both the phenyl rings cyclized at the hindered aryl C–H bonds to give the respective N-aryl benzimidazoles 2c–g as a single regioisomer in 63–75% yield (entries 3–7). Similarly, unsymmetrical N,N′-bis(aryl)formamidine having methyl substituents at the m- and p-positions 1h could be converted into benzimidazole 2h in 75% yield (entry 8).
| Entry | Substrate | Time (h) | Product (yield, %)ab | Entry | Substrate | Time (h) | Product (yield, %)ab |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: a substrate 1a–p (0.5 mmol), Pd(PPh3)2Cl2 (10 mol %), Cs2CO3 (1 mmol) and 4 Å MS (50 mg) were stirred at 120 °C under an O2 balloon. b Isolated yield. | |||||||
| 1 |
|
30 |
|
9 |
|
48 |
|
| 2 |
|
30 |
|
10 |
|
60 |
|
| 3 |
|
36 |
|
|
|
||
|
|
11 | R1, R2 = Et 1k | 48 | 2k (68) | ||
| 12 | R1, R2 = F 1l | 30 | 2l (75) | ||||
| 13 | R1, R2 = Me 1m | 48 | 2m (75) | ||||
| 14 | R1, R2 = i-Pr 1n | 50 | 2n (78) | ||||
| 4 | R1, R2 = Et 1d | 36 | 2d(66) | 15 |
|
60 |
|
| 5 | R1, R2 = F 1e | 38 | 2e (74) | ||||
| 6 | R1, R2 = Me 1f | 30 | 2f (63) | ||||
| 7 | R1, R2 = i-Pr 1g | 48 | 2g (75) | ||||
| 8 |
|
30 |
|
16 |
|
60 |
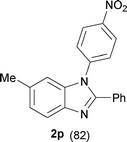
|
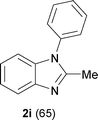
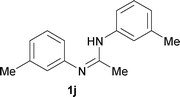
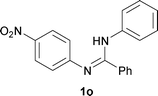
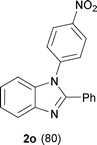
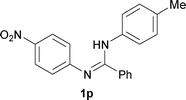
The protocol was also compatible for the cyclization of N,N′-bis(aryl)acetamidines and N,N′-bis(aryl)benzamidines to afford 2- substituted N-aryl benzimidazoles (Table 2, entries 9–16). For example, bis(aryl)acetamidine 1i proceeded via cyclization to give 2-methyl N-aryl benzimidazole 2i in 65% yield (entry 9). Likewise, the symmetrical bis(aryl)acetamidines 1j–n with methyl, ethyl, fluoro and 2-methyl substituents at m- as well as the p-positions of both the phenyl rings underwent reactions to give the corresponding 2-methyl N-aryl benzimidazoles 2j–n in 68–78% yield (entries 10–14). Furthermore, the unsymmetrical N,N′- bis(aryl)benzamidines 1o–p having methyl and nitro groups at the p-position of one of the phenyl rings could be cyclized to give the respective 1,2-diaryl benzimidazoles 2o–p in 80–82% yield. Recrystallization of 2n and 2p in CH2Cl2 gave crystals whose structures were confirmed unambiguously by single crystal X-ray analysis (see the ESI†).
In summary, palladium-catalyzed C–H aerobic oxidative amination of bis(aryl)amidines have been developed to afford N-aryl benzimidazoles. The reaction provided a general route for the synthesis of 2-unsubstituted as well as 2-alkyl/-aryl substituted N-aryl benzimidazoles.
Acknowledgements
We thank Department of Science and Technology, New Delhi, and Council of Scientific and Industrial Research, New Delhi, for generous financial support. One of us (K.K.) thanks Indian Institute of Technology Guwahati for Senior Research Fellowship.References
- For some recent examples, see: (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (b) S. Wurtz and F. Glorius, Acc. Chem. Res., 2008, 41, 1523 CrossRef; (c) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS; (d) J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS; (e) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS; (f) M. Meldal and C. W. Tornoe, Chem. Rev., 2008, 108, 2952 CrossRef CAS; (g) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS; (h) T. R. M. Rauws, B. U. W. Maes, Chem. Soc. Rev. DOI: 10.1039/c1cs15236j Search PubMed; (i) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef CAS; (j) D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27 RSC; (k) J. P. Wolfe, S. Wagaw, J-F. Marcoux and S. L. Buchwald, Acc. Chem. Res., 1998, 31, 805 CrossRef CAS; (l) J. F. Hartwig, Angew. Chem., Int. Ed., 1998, 37, 2046 CrossRef CAS; (m) J. F. Hratwig, Acc. Chem. Res., 1998, 31, 852 CrossRef; (n) N. Guimond, S. I. Gorelsky and K. Fagnou, J. Am. Chem. Soc., 2011, 133, 6449 CrossRef CAS; (o) D. R. Stuart, P. Alsabeh, M. Kuhn and K. Fagnou, J. Am. Chem. Soc., 2010, 132, 18326 CrossRef CAS; (p) R. J. Lundgren, B. D. Peters, P. G. Alsabeh and M. Stradiotto, Angew. Chem., Int. Ed., 2010, 49, 4071 CrossRef CAS; (q) P. G. Alsabeh, R. J. Lundgren, L. E. Longobardi and M. Stradiotto, Chem. Commun., 2011, 47, 6936 RSC.
- For examples, see: (a) A. K. M. Long, R. P. Yu, G. H. Timmer and J. F. Berry, J. Am. Chem. Soc., 2010, 132, 12228 CrossRef; (b) S. K.-Y. Leung, W.-M. Tsui, J.-S. Huang, C.-M. Che, J.-L. Liang and N. Zhu, J. Am. Chem. Soc., 2005, 127, 16629 CrossRef CAS; (c) W. G. Shou, J. Li, T. Guo, Z. Lin and G. Jia, Organometallics, 2009, 28, 6847 CrossRef CAS.
- For some examples, see: (a) J. Du Bois, Org. Process Res. Dev., 2011, 15, 758 CAS; (b) D. N. Zalatan and J. Du Bois, J. Am. Chem. Soc., 2009, 131, 7558 CrossRef CAS; (c) P. M. Wehn and J. Du Bois, Org. Lett., 2005, 7, 4685 CrossRef CAS; (d) A. Norder, P. Herrmann, E. Herdtweck and T. Bach, Org. Lett., 2010, 12, 3690 CrossRef CAS; (e) D. E. Olson and J. Du Bois, J. Am. Chem. Soc., 2008, 130, 11248 CrossRef CAS; (f) D. N. Zalatan and J. Du Bois, J. Am. Chem. Soc., 2008, 130, 9220 CrossRef CAS; (g) C. G. Espino, K. W. Fiori, M. Kim and J. Du Bois, J. Am. Chem. Soc., 2004, 126, 15378 CrossRef CAS.
- For some studies, see: (a) K. Inamoto, T. Saito, M. Katsuno, T. Sakamoto and K. Hiroya, Org. Lett., 2007, 9, 2931 CrossRef CAS; (b) J. A. Jordan-Hore, C. C. C. Johansson, M. Gulias, E. M. Beck and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 16184 CrossRef CAS; (c) T.-S. Mei, X. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 10806 CrossRef CAS; (d) G. T. Rice and M. C. White, J. Am. Chem. Soc., 2009, 131, 11707 CrossRef CAS; (e) L. Wu, S. Qiu and G. Liu, Org. Lett., 2009, 11, 2707 CrossRef CAS; (f) W. C. P. Tsang, N. Zheng and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 14560 CrossRef CAS; (g) Q. Xiao, W.-H. Wang, G. Liu, F.-K. Meng, J.-H. Chen, Z. Yang and Z.-J. Shi, Chem.–Eur. J., 2009, 15, 7292 CrossRef CAS; (h) J. J. Neumann, S. Rakshit, T. Droge and F. Glorius, Angew. Chem., Int. Ed., 2009, 48, 6892 CrossRef CAS; (i) E. J. Yoo, S. Ma, T.-S. Mei, K. S. L. Chan and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 7652 CrossRef CAS; (j) R. I. McDonald, P. B. White, A. B. Weinstein, C. P. Tam and S. S. Stahl, Org. Lett., 2011, 13, 2830 CrossRef CAS; (k) G. Yang and W. Zhang, Org. Lett., 2012, 14, 268 CrossRef CAS; (l) S. W. Youn, J. H. Bihn and B. S. Kim, Org. Lett., 2011, 13, 3738 CAS; (m) R. K. Kumar, M. A. Ali and T. Punniyamurthy, Org. Lett., 2011, 13, 2102 CrossRef CAS; (n) X. Ye, G. Liu, B. V. Popp and S. S. Stahl, J. Org. Chem., 2011, 76, 1031 CrossRef CAS; (o) B. Haffemayer, M. Gulias and M. J. Gaunt, Chem. Sci., 2011, 2, 312 RSC; (p) K. J. Fraunhoffer and M. C. White, J. Am. Chem. Soc., 2007, 129, 7274 CrossRef CAS; (q) A. M. Wagner and M. S. Sanford, Org. Lett., 2011, 13, 288 CrossRef CAS; (r) A. D. Satterfield, A. Kubota and M. S. Sanford, Org. Lett., 2011, 13, 1076 CrossRef CAS; (s) S. A. Reed and M. C. White, J. Am. Chem. Soc., 2008, 130, 3316 CrossRef CAS.
- (a) S. H. Cho, J. Y. Kim, S. Y. Lee and S. Chang, Angew. Chem., Int. Ed., 2009, 48, 9127 CrossRef CAS; (b) C-G. Yang, N. W. Reich, Z. Shi and C. He;, Org. Lett., 2005, 7, 4553 CrossRef CAS.
- For some examples, see: (a) K.-S. Masters, T. R. M. Rauws, A. K. Yadav, W. A. Herrebout, B. Van der Veken and B. U. W. Maes, Chem.–Eur. J., 2011, 17, 6315 CrossRef CAS; (b) L. Zhang, G. Y. Ang and S. Chiba, Org. Lett., 2010, 12, 3682 CrossRef CAS; (c) D. Monguchi, T. Fujiwara, H. Furukawa and A. Mori, Org. Lett., 2009, 11, 1607 CrossRef CAS; (d) G. Brasche and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 1932 CrossRef CAS; (e) X. Wang, Y. Jin, Y. Zhao, L. Zhu and H. Fu, Org. Lett., 2012, 14, 452 CrossRef CAS; (f) M. M. Guru, M. A. Ali and T. Punniyamurthy, J. Org. Chem., 2011, 76, 5295 CrossRef CAS.
- For some examples, see: (a) S. Solanki, P. Innocenti, C. Mas-Droux, K. Boxall, C. Barillari, R. L. M. Van Montfort, G. W. Aherne, R. Bayliss and S. Hoelder, J. Med. Chem., 2011, 54, 1626 CrossRef CAS; (b) M. Sabat, J. C. VanRens, M. J. Laufersweiler, T. A. Brugel, J. Maier, A. Golebiowski, B. De, V. Easwaran, L. C. Hsieh, R. L. Walter, M. J. Mekel, A. Evdokimov and M. J. Janusz, Bioorg. Med. Chem. Lett., 2006, 16, 5973 CrossRef CAS.
- For some examples, see: (a) A. Chan and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 5334 CrossRef CAS; (b) K. Iwamoto, M. Hamaya, N. Hashimoto, H. Kimura, Y. Suzuki and M. Sato, Tetrahedron Lett., 2006, 47, 7175 CrossRef CAS; (c) A. Miyashita, Y. Suzuki, K. Iwamoto and T. Higashino, Chem. Pharm. Bull., 1994, 42, 2633 CrossRef CAS.
- Y. Wang, K. Sarris, D. R. Sauer and S. W. Djuric, Tetrahedron Lett., 2006, 47, 4823 CrossRef CAS.
- For recent reviews on benzimidazole synthesis, see: L. C. R. Carvalho, E. Fernandes and M. M. B. Marques, Chem.–Eur. J., 2011, 17, 12544 CrossRef CAS and references cited therein..
- For examples on the synthesis of N-aryl benzimidazoles, see: (a) C. T. Brain and S. A. Brunton, Tetrahedron Lett., 2002, 43, 1893 CrossRef CAS; (b) N. Zheng, K. W. Anderson, X. Huang, H. N. Nguyen and S. L. Buchwald, Angew. Chem., Int. Ed., 2007, 46, 7509 CrossRef CAS; (c) P. Saha, M. A. Ali, P. Ghosh and T. Punniyamurthy, Org. Biomol. Chem., 2010, 8, 5692 RSC; (d) A. V. Lygin and A. de Meijere, Eur. J. Org. Chem., 2009, 5138 CrossRef CAS; (e) M. T. Wentzel, J. B. Hewgley, R. M. Kamble, P. D. Wall and M. C. Kozlowski, Adv. Synth. Catal., 2009, 351, 931 CrossRef CAS; (f) P. Saha, T. Ramana, N. Purkait, M. A. Ali, R. Paul and T. Punniyamurthy, J. Org. Chem., 2009, 74, 8719 CrossRef CAS; (g) J. Zhu, H. Xie, Z. Chen, S. Li and Y. Wu, Chem. Commun., 2009, 2338 RSC; (h) K. Hirano, A. T. Biju and F. Glorius, J. Org. Chem., 2009, 74, 9570 CrossRef CAS.
- For an example on the synthesis of N-alkyl benzimidazoles, see: (a) C. T. Brain and J. T. Seer, J. Org. Chem., 2003, 68, 6814 CrossRef CAS; (b) N. Zheng and S. L. Buchwald, Org. Lett., 2007, 9, 4749 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental procedure, CCDC 863007 (2n) and 863008 (2p) and NMR spectra (1H and 13C) See DOI: 10.1039/c2ra20328f/ |
| This journal is © The Royal Society of Chemistry 2012 |