A nanohybrid of graphene oxide–fluorescein derived silyl ether for photocurrent generation triggered by F− ions†
Lan-Ya
Cheng
,
Ji
Zhou
,
Qi
Zou
,
Yi-Tao
Long
* and
He
Tian
*
Key Laboratory for Advanced Materials & Institute of Fine Chemicals, East China University of Science and Technology, Shanghai 200237, P. R. China.. Fax: (86)-21-64250032; E-mail: ytlong@ecust.edu.cn; tianhe@ecust.edu.cn
First published on 26th March 2012
Abstract
A graphene oxide-fluorescein derivative nanohybrid, which was based on the covalent functionalization of graphene oxide with a fluorescein-derived tert-butyldiphenylsilyl ether, demonstrated distinctive photocurrent depending on the cleavage of Si–O bond triggered by F− ions.
The hybridization of carbon nanomaterials and dye molecules plays an important role in the development of nanoscale optoelectronic devices.1 Graphene oxide (GO), as a novel carbon nanomaterial, has attracted increasing attention of the chemists due to its dispersibility and convenient chemical modification in solution phase.2 The nanohybrid of GO covalently functionalized with organic molecules was considered a promising methodology to make multifunctional devices,3 which can tune the properties of both GO and the organic molecule. Recently, great efforts have been contributed to exploit covalent GO nanohybrid for fluorescence biosensors,4 and nonlinear optics.5 However, the nanohybrid of GO functionalized with organic dyes for photoelectric conversion is still largely unexplored. To the authors' knowledge, the only example is a GO–azobenzene nanohybrid, which has been reported to exhibit outstanding photocurrent behavior upon UV irradiation.6 Considering the wide existence of sunlight energy and the significance of multi-tunable conditions in the development of complex optoelectronic devices, a novel nanohybrid of graphene oxide-fluorescein derived silyl ether (GO–SiFL) is demonstrated, which exhibited specific photoelectric conversion ability depending on the cleavage of the Si–O bonds within its structure and the white light irradiation.
To construct a nanohybrid involving a carbon nanomaterial and organic dye molecules used for photoelectric conversion, one key path is to design a donor–acceptor unit which can facilitate charge generation, separation and transport within the unit.7 In the nanohybrid GO–SiFL, GO is introduced as an electron acceptor since its feasibility has been revealed in several GO-dye hybrids.8 In the nanohybrid GO–SiFL, the fluorescein-derived tert-butyldiphenylsilyl ether (SiFL) is introduced as a tunable photo-excited electron donor because it combines the features of the Si–O bond and the fluorescein moiety. The Si–O bond, which can be sensitively and selectively cleaved by F− ions due to the high affinity of silicon towards F− ions,10 is utilized to control the absorption property of the fluorescein. The fluorescein without the silyl substitute, which not only has strong absorption and high fluorescence efficiency in the visible light region,9 but also exhibits definite photo-induced electron transfer (PET) to fullerene,11 is utilized as the white light harvester. It could be proved that the Si–O bond in the nanohybrid of GO–SiFL can be cleaved by F− ions, as shown in Scheme 1A, and the resulting FGO–SiFL would show photoelectric conversion ability in an electrochemical cell system according to the proposed electron transfer process shown in Scheme 1B.
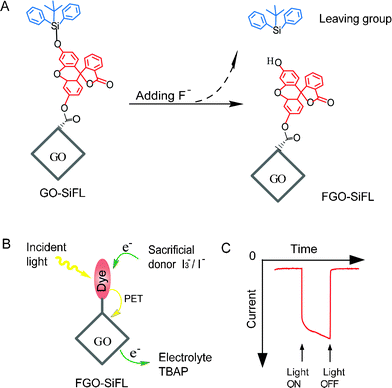 | ||
| Scheme 1 (A) Feasible reactive mechanism for GO–SiFL with addition of F− anions. The grey rhombus represents the GO moiety, the red structure represents the fluorescein moiety, and the blue structure represents the derived silyl moiety. (B) Proposed photoexcited electron transfer process of the FGO–SiFL in the electrochemical cell with addition of I3−/I− ions as the sacrificial donor and tetrabutylammonium perchlorate (TBAP) as the electrolyte. (C) The representative photocurrent response of FGO–SiFL under irradiation. | ||
The nanohybrid GO–SiFL was prepared according to scheme S1, ESI†. The GO was prepared as described in literature,12 the SiFL was synthesized via a one-step reaction between dihydroxyfluorane and tert-butyldiphenyl-chlorosilane, and GO–SiFL was synthesized through coupling SiFL with carboxyl activated GO (see ESI†). The morphologies of GO and GO–SiFL were observed by atomic force microscopy (AFM) and transmission electron microscopy (TEM). The AFM profile analysis showed the height of ∼1 nm for both GO and GO–SiFL (Figure S5, ESI†), which was consistent with other reports about single-layered graphene oxide sheets and hybrids.13 The TEM image of GO resembled crumpled silk veil waves, and the TEM image of GO–SiFL showed fluffy surface which was ascribed to covalent linkage between GO and SiFL (Figure S6, ESI†).
The FTIR spectra of GO–SiFL, SiFL and GO are shown in Fig. 1A. In the spectrum of GO, the peak at 1714 cm−1 is assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of the carboxylic group on the GO moiety, which is in accordance with literature.14 In the spectrum of GO–SiFL, the C
O stretch of the carboxylic group on the GO moiety, which is in accordance with literature.14 In the spectrum of GO–SiFL, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch peak of the carboxylic group at 1718 cm−1 exhibits a blue shift of 4 cm−1 to that of GO, and two new broad peaks appear at 1220 cm−1 and 1153 cm−1 corresponding to the C–O characteristic stretching mode in the ester bond,15 which clearly indicates that the SiFL molecules are covalently bound to GO through ester linkage. The UV-vis spectrum of GO in DMF was shown as the black line in Fig. 1B, which displays a strong absorption band at 268 nm with a broad absorption background consistent with the report of a GO suspension solution in DMF.8 The UV-vis spectrum of SiFL is shown as the orange line in Fig. 1B, and displays an absorption peak at 277 nm. The UV-vis spectrum of GO–SiFL is shown as the red line in Fig. 1B, and displays a strong absorption at 277 nm along with a broad absorption background, which could be attributed to the absorption of SiFL moiety along with the GO moiety.
O stretch peak of the carboxylic group at 1718 cm−1 exhibits a blue shift of 4 cm−1 to that of GO, and two new broad peaks appear at 1220 cm−1 and 1153 cm−1 corresponding to the C–O characteristic stretching mode in the ester bond,15 which clearly indicates that the SiFL molecules are covalently bound to GO through ester linkage. The UV-vis spectrum of GO in DMF was shown as the black line in Fig. 1B, which displays a strong absorption band at 268 nm with a broad absorption background consistent with the report of a GO suspension solution in DMF.8 The UV-vis spectrum of SiFL is shown as the orange line in Fig. 1B, and displays an absorption peak at 277 nm. The UV-vis spectrum of GO–SiFL is shown as the red line in Fig. 1B, and displays a strong absorption at 277 nm along with a broad absorption background, which could be attributed to the absorption of SiFL moiety along with the GO moiety.
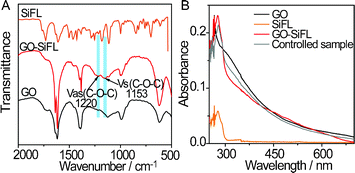 | ||
| Fig. 1 (A) FT-IR spectra of GO–SiFL (red), SiFL (orange) and GO (black). (B) UV-vis spectra of GO–SiFL (red, 35.1 mg L−1), SiFL (orange, 2.2 mg L−1), GO (black, 13.3 mg L−1) and the control sample (grey, 1.8 mg L−1 SiFL + 13.3 mg L−1 GO). | ||
The triggering property of F− ions on the Si–O bond cleavage within GO–SiFL was investigated via UV-vis absorption spectra and fluorescence spectra of GO–SiFL upon additions of excess F− (TBA+ salts) ions. In Fig. 2A, a strong absorption at around 500 nm appeared in the UV-vis spectrum of GO–SiFL (35.1 mg L−1, 3 mL in DMF) upon the addition of excess F− anions (1 mM, 100 μL in DMF), which was similar to the phenomena occurring in the UV-vis spectrum of SiFL (2.2 mg L−1, 3 mL in DMF) upon the addition of excess F− anions (1 mM, 100 μL in DMF). Correspondingly, a new emission peak at around 541 nm appeared in the emission spectra of GO–SiFL and SiFL (ex 519 nm) upon the addition of excess F− ions shown in Fig. 2B. These observations suggested the Si–O bonds in GO–SiFL and SiFL were triggered to cleave by F− anions, which could be ascribed to the high affinity of silicon toward F− ions.10 The mass results supported the Si–O bond cleavage in SiFL, which displayed the presence of a new molecular ion peak with m/z = 333.0756 assigned to the fluorescein segment and the absence of the molecular ion peak of SiFL with m/z = 571.1948 (Fig. S4, ESI†). In comparison, no such phenomenon was observed in the UV-vis spectrum of GO (13.3 mg L−1, 3 mL in DMF) upon the addition of excess F− ions, and an identical phenomenon was observed in the UV-vis spectrum of the controlled sample (1.8 mg L−1 SiFL + 13.3 mg L−1 GO, 3 mL in DMF) upon the addition of excess F− ions, further supporting the Si–O bond cleavage in GO–SiFL above. In Fig. 2B, the resulting fluorescence intensity for GO–SiFL was much weaker than those for SiFL and the controlled sample, indicating that the luminescence quenching ability of GO on the excited state of the fluorescein moiety in the nanohybrid of GO–SiFL, which was in accord with the observation in other GO-organic dye hybrids.8
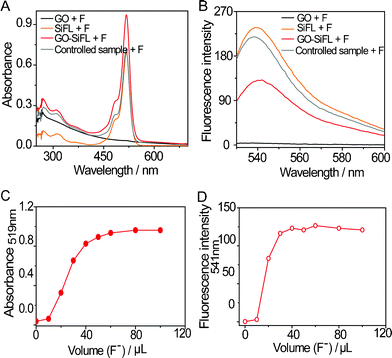 | ||
| Fig. 2 (A) Effects of the addition of 100 μL of F− ions on the UV-vis spectra of GO–SiFL (red), SiFL (orange), GO (black) and the control sample (grey) in DMF, respectively. (B) Effects of the addition of 100 μL of F− ions on the fluorescence emission spectra (ex 519 nm) of GO–SiFL (red), SiFL (orange), GO (black) and the controlled sample (grey) in DMF, respectively. (C) The absorption changes at 519 nm resulted from the F− titration on GO–SiFL in DMF. (D) The emission changes at 541 nm resulted from the F− titration on GO–SiFL in DMF. Concentrations: GO–SiFL, 35.1 mg L−1; SiFL, 2.2 mg L−1; GO, 13.3 mg L−1; and the controlled sample, 1.8 mg L−1 SiFL + 13.3 mg L−1 GO; F−/Cl−/Br−/I− anions, 1 mM in DMF. | ||
The reactive stoichiometry of the Si–O bond cleavage within GO–SiFL was further studied via the F− anion titration experiments on their UV-vis and fluorescence emission spectra (Fig. S7–S8, ESI†; and Fig. 2C–2D). As a comparison, the same experiments were operated on SiFL (Fig. S9–S12, ESI†). It was found that the Si–O bond cleavage triggered by F− anions was fast and quantifiable in both SiFL and GO–SiFL. The stoichiometric results for SiFL indicated that the reaction was accomplished at a ratio of around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between F− anions and SiFL, which was in accordance with the reaction mechanism in other reports.9 The stoichiometric results for GO–SiFL can not be obtained due to the uncertainty of its molecular weight, but the experimental phenomenon above strongly indicates that the Si–O bond in GO–SiFL can be cleaved by F− anions, much as SiFL.
1 between F− anions and SiFL, which was in accordance with the reaction mechanism in other reports.9 The stoichiometric results for GO–SiFL can not be obtained due to the uncertainty of its molecular weight, but the experimental phenomenon above strongly indicates that the Si–O bond in GO–SiFL can be cleaved by F− anions, much as SiFL.
The selectivity of F− ions triggered on the Si–O bond cleavage within GO–SiFL was investigated through comparative experiments involving Cl−, Br− and I− (TBA+ salts) anions. No obvious change was observed in the absorption spectra or the emission spectra for GO–SiFL and SiFL upon the addition of Cl−/Br−/I− anions (Fig. S13–S16, ESI†), indicating the definite selectivity of F− anions on the Si–O bond cleavage in GO–SiFL and SiFL. The color changes of GO–SiFL upon the addition of various ions were shown as Fig. 3A. The F− anions changed the sample color from black to light red. The fluorescence images of GO–SiFL before and after the addition of various anions were shown in Fig. 3B. The F− anions changed the sample from non-fluorescent to green fluorescent excited at 519 nm. The investigations suggested that the Si–O bond in GO–SiFL could be selectively cleaved by F− anions.
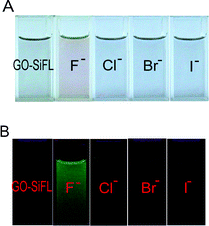 | ||
| Fig. 3 (A) Color changes of GO–SiFL with addition of 100 μL F−, Cl−, Br− and I− anions in DMF, respectively. (B) Fluorescence images of GO–SiFL before and after additions of 100 μL F−, Cl−, Br− and I− anions in DMF. Concentrations: GO–SiFL, 35.1 mg L−1; F−/Cl−/Br−/I− anions, 1 mM in DMF. | ||
To evaluate the possibility of electron transfer between the GO moiety and the excited fluorescein moiety, the energy levels of fluorescein and GO were calculated using the corresponding electrochemical redox (Fig. S19–S20, ESI†) and optical data according to the literature method, respectively.16 The fluorescein moiety was obtained from 2.2 mg L−1 SiFL upon addition of F− ions (FSiFL). As shown in Fig. 4, the lowest unoccupied molecular orbital (LUMO) of fluorescein was more negative than the conduction band (CB) of GO, which allowed the injection of the photoexcited electrons from fluorescein to GO. The highest occupied molecular orbital (HOMO) level of fluorescein was more positive than the redox potential of I3−/I−, which allowed the regeneration of fluorescein through obtaining the electrons of I3−/I−. All these results confirmed the thermodynamically feasibility of photoexcited electron transfer from the fluorescein to GO.
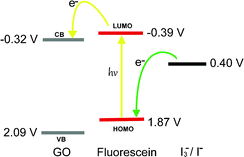 | ||
| Fig. 4 The energy level diagram illustrating the potential electron transfer process between GO and fluorescein moiety in FGO–SiFL. | ||
Based on the selective triggering ability of F− ion on the Si–O bond cleavage in GO–SiFL, and the potential electron transfer ability from the excited fluorescein moiety to the GO moiety, the photocurrent response of GO–SiFL under irradiation was investigated in a thin layer spectroelectrochemical cell. The thickness of the measured solution was around 0.5 mm. The i-t plot was recorded using a three-electrode electrochemical system. An ITO substrate was used as the working electrode with a working area of 0.25 cm2, and two silver wires were used as the reference and counter electrodes, respectively. For all the experiments, the sample volume was 500 μL, and the added anion volume was 40 μL. 0.1 M TBAP in DMF was used as electrolyte, including 0.05 M I3−/I−ions as the sacrificial electron donors. To minimize the effect of applied potential on the photocurrent response, the open circuit potential value of the system, + 0.35 V, was utilized as the applied potential. 1 mM F−/Cl−/Br−/I− anions were prepared in DMF utilizing the corresponding TBA+ salts. The photocurrent performance of GO–SiFL, GO, SiFL and the controlled sample was recorded in the absence and presence of F− anions, respectively, seen as Fig. 5A. It was easy to find that GO and SiFL did not show a definite photocurrent response whether excess F− ions were added or not. While, obvious photocurrent signals with an intensity of ∼460 nA were observed for GO–SiFL upon the addition of excess F− anions, indicating photoinduced electron transfer (PET) from the excited fluorescein moiety to the GO moiety. In the presence of excess F− anions, the control sample also displayed obvious photocurrent response with an intensity of ∼20 nA, which was less than 1/10 of the photocurrent value of the GO–SiFL, which suggested that the electron transfer efficiency from the fluorescein to GO was enhanced through the covalent linkage between GO and SiFL.
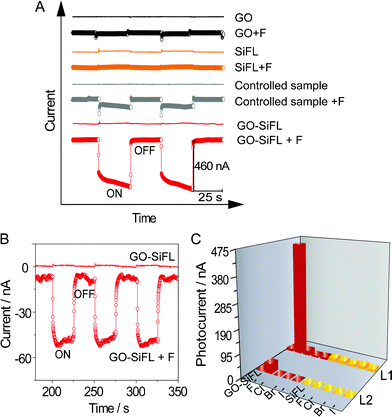 | ||
| Fig. 5 (A) The effects of excess F− anions on the photocurrent responses of GO–SiFL, GO, SiFL and the controlled sample illuminated by white light under the applied potential of + 0.35 V. (B) The effect of excess F− anions on the photocurrent response of GO–SiFL illuminated by homogeneous light 509 nm under the applied potential of + 0.35 V. (C) Three-dimensional column diagram representing the photocurrent response of GO–SiFL (red) and SiFL (orange) upon the addition of excess F−, Cl−, Br− and I− anions illuminated by L1 and L2, respectively. L1 represents white light, L2 represents 509 nm. Concentrations: GO–SiFL, 35.1 mg L−1 in 0.1 M TBAP DMF solution; SiFL, 2.2 mg L−1 in 0.1 M TBAP DMF solution; the controlled sample, 1.8 mg L−1 SiFL + 13.3 mg L−1 GO; F−/Cl−/Br−/I− anions, 1 mM in DMF. For all the experiments in (A), (B) and (C), the sample volume is 500 μL, and the added anions volume is 40 μL. | ||
To further confirm that the photocurrent was generated by the photo-excited electrons of the fluorescein moiety, the photocurrent performance of GO–SiFL upon addition of F− anions was consequently studied under irradiation of homogeneous light at 509 nm, which was close to the maximum absorption wavelength 519 nm shown in Fig. 2A. In Fig. 5B, GO–SiFL did not show an obvious photocurrent response under irradiation, while the sample, upon addition of F− ions, displayed an obvious photocurrent with an intensity of ∼45 nA illuminated by 509 nm, which was similar to the observations under irradiation of white light. All these observations suggested that there was an efficient electron or energy transfer from the excited fluorescein moiety to GO in the system of GO–SiFL upon addition of F− ions.
No obvious photocurrent was found upon addition of Br−, Cl− and I− ions, consistent with the spectroscopy experimental deduction above. The same experiments were also carried out on SiFL and SiFL upon addition of F−/Cl−/Br−/I− anions. No obvious photocurrent was observed in these systems, as seen in Fig. 5C, further suggesting that the GO moiety played an important role of photo-excited electron acceptor in the electron-transfer process.
In conclusion, we have successfully demonstrated a nanohybrid of GO–SiFL, which can be selectively triggered to cleave its Si–O bond by F− anions and consequently exhibit specific photocurrent (∼460 nA) under white light irradiation, resulting from the PET from the photo-excited fluorescein moiety to the GO moiety. This work not only processes a novel GO-organic dye nanohybrid as a potential candidate for tunable optoelectronic devices, but also provides a feasible way to design and to develop a highly efficient photoelectric conversion system based on the hybridization of GO and organic dyes.
We greatly appreciate the support of the National Science Fund for Distinguished Young Scholars (21125522), the National Natural Science Foundation of China (91027035, 21105028), the Fundamental Research Funds for the Central Universities (WK1013002), the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20100074120017) and the Shanghai Municipal Natural Science Foundation (No. 11ZR1408900).
References
- (a) K. Yanagi and H. Kataura, Nat. Photonics, 2010, 4, 200 CrossRef CAS; (b) J. Matsui, M. Mitsuishi, A. Aoki and T. Miyashita, Angew. Chem., Int. Ed., 2003, 42, 2272 CrossRef CAS; (c) Y. Zhou, L. Wang, J. Wang, J. Pei and Y. Cao, Adv. Mater., 2008, 20, 3745 CrossRef CAS.
- (a) S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217 CrossRef CAS; (b) D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015 CrossRef CAS.
- (a) J. Balapanuru, J.-X. Yang, S. Xiao, Q. Bao, M. Jahan, L. Polavarapu, J. Wei, Q.-H. Xu and K. Loh, Angew. Chem., Int. Ed., 2010, 49, 6549 CrossRef CAS; (b) C.-H. Lu, H.-H. Yang, C.-L. Zhu, X. Chen and G.-N. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785 CrossRef CAS; (c) S. He, B. Song, D. Li, C. Zhu, W. Qi, Y. Wen, L. Wang, S. Song, H. Fang and C. Fan, Adv. Funct. Mater., 2010, 20, 453 CrossRef CAS; (d) H. Dong, W. Gao, F. Yan, H. Ji and H. Ju, Anal. Chem., 2010, 82, 5511 CrossRef CAS.
- (a) Y. Liu, J. Zhou, X. Zhang, Z. Liu, X. Wan, J. Tian, T. Wang and Y. Chen, Carbon, 2009, 47, 3113 CrossRef CAS; (b) Z. Liu, Y. Xu, X. Zhang, X. Zhang, Y. Chen and J. Tian, J. Phys. Chem. B, 2009, 113, 9681 CrossRef CAS; (c) X.-L. Zhang, X. Zhao, Z.-B. Liu, Y.-S. Liu, Y.-S. Chen and J.-G. Tian, Opt. Express, 2009, 17, 23959 CrossRef CAS.
- X. Zhang, Y. Feng, P. Lv, Y. Shen and W. Feng, Langmuir, 2010, 26, 18508 CrossRef CAS.
- (a) C. Schulz-Drost, V. Sgobba, C. Gerhards, S. Leubner, R. M. K. Calderon, A. Ruland and D. M. Guldi, Angew. Chem., Int. Ed., 2010, 49, 6425 CrossRef CAS; (b) D. Long, H. Lin and I. G. Scheblykin, Phys. Chem. Chem. Phys., 2011, 13, 5771 RSC; (c) N. K. Subbaiyan, I. Obraztsov, C. A. Wijesinghe, K. Tran, W. Kutner and F. D'Souza, J. Phys. Chem. C, 2009, 113, 8982 CrossRef CAS; (d) J. Roncali, Chem. Soc. Rev., 2005, 34, 483 RSC.
- (a) Y. Xu, Z. Liu, X. Zhang, Y. Wang, J. Tian, Y. Huang, Y. Ma, X. Zhang and Y. Chen, Adv. Mater., 2009, 21, 1275 CrossRef CAS; (b) N. Karousis, A. S. D. Sandanayaka, T. Hasobe, S. P. Economopoulos, E. Sarantopoulou and N. Tagmatarchis, J. Mater. Chem., 2011, 21, 109 RSC.
- (a) X.-F. Yang, S.-J. Ye, Q. Bai and X.-Q. Wang, J. Fluoresc., 2007, 17, 81 CrossRef CAS; (b) M. Nakaya, M. Oshima, T. Takayanagi, S. Motomizu and H. Yamashita, Talanta, 2011, 84, 1361 CrossRef CAS.
- (a) J. F. Zhang, C. S. Lim, S. Bhuniya, B. R. Cho and J. S. Kim, Org. Lett., 2011, 13, 1190 CrossRef CAS; (b) S. Y. Kim and J.-I. Hong, Org. Lett., 2007, 9, 3109 CrossRef CAS; (c) X.-F. Yang, H. Qi, L. Wang, Z. Su and G. Wang, Talanta, 2009, 80, 92 CrossRef CAS.
- (a) B. Jing, D. Zhang and D. Zhu, Tetrahedron Lett., 2000, 41, 8559 CrossRef CAS; (b) W. Zhan and K. Jiang, Langmuir, 2008, 24, 13258 CrossRef CAS.
- (a) H. A. Becerril, J. Mao, Z. F. Liu, R. M. Stoltenberg, Z. N. Bao and Y. S. Chen, ACS Nano, 2008, 2, 463 CrossRef CAS; (b) Z. F. Liu, Q. Liu, X. Y. Zhang, Y. Huang, Y. F. Ma, S. G. Yin and Y. S. Chen, Adv. Mater., 2008, 20, 3924 CrossRef CAS.
- (a) C. Gómez-Navarro, R. T. Weitz, A. M. Bittner, M. Scolar, A. Mews, M. Burghard and K. Kern, Nano Lett., 2007, 7, 3499 CrossRef; (b) X. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203 CrossRef CAS; (c) C. Peng, W. Hu, Y. Zhou, C. Fan and Q. Huang, Small, 2010, 6, 1686 CrossRef CAS.
- Y. H. Ding, P. Zhang, Q. Zhuo, H. M. Ren, Z. M. Yang and Y. Jiang, Nanotechnology, 2011, 22, 215601 CrossRef CAS.
- D. Yu, Y. Yang, M. Durstock, J.-B. Baek and L. Dai, ACS Nano, 2010, 4, 5633 CrossRef CAS.
- R. Stalder, C. Grand, J. Subbiah, F. So and J. R. Reynolds, Polym. Chem., 2012, 3, 89 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: Material synthesis and characterization; comparative experiments of Cl−, Br− and I− ions. See DOI: 10.1039/c2ra20370g/ |
| This journal is © The Royal Society of Chemistry 2012 |
