Highly specific triple-fragment aptamer for optical detection of cocaine†
Ruxing
Zou‡
a,
Xinhui
Lou‡
*a,
Huichao
Ou‡
b,
Ying
Zhang
a,
Wenjie
Wang
a,
Min
Yuan
cd,
Ming
Guan
a,
Zhaofeng
Luo
b and
Yueying
Liu
*a
aDepartment of Chemistry, Capital Normal University, Xisanhuan North Rd. 105, Beijing, 100048, China. E-mail: xlou9999@yahoo.com; Fax: 8610 68902320; Tel: 8610 68902491-808
bUniversity of Science and Technology of China (USTC), Hefei National Laboratory for Physical Sciences at the Microscale, School of Life Sciences, Hefei 230027, China
cState Key Laboratory of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Science, Changning Rd. 865, Shanghai, China
dGraduate School of the Chinese Academy of Sciences, Beijing, China
First published on 26th April 2012
Abstract
For the first time an anti-cocaine aptamer was cut into three fragments, which were still able to specifically assemble with cocaine and render the sensitive and highly selective optical detection of cocaine. The assembly process possesses significantly different thermodynamic signature compared to the interactions involved monolithic aptamer and double-fragment aptamer.
Aptamers1 as prime sensor candidates are particularly attractive in that they can be selected in vitro, rationally designed, chemically synthesized, and readily adapted to sequence- (and hence signal-) amplification methods.2 The rational design strategies of aptamers highly depend on their binding structures with targets, such as stem-loops,3 pseudo-knots,4 G-quartets,2 and three-way junctions.5 Among those reported scaffolds, the three-way junctions have received considerable interest due to their well-demonstrated applications in biologically important targets, such as cocaine, oncoprotein platelet-derived growth factor (PDGF),6 and steriods,7 in the construction of controllable DNA nanoarchitectures,8 and importance in biological processes.
The cocaine aptamer has a typical three-way junction, which is composed of three stem loops that meet at a three-way junction when binding with cocaine.2 The cocaine aptamer has been used as an ideal and representative target for testing new analytical techniques. These techniques can be roughly divided into two categories. The first group of methods utilize single aptamer chains, referred as monolithic aptamers (MAs), which undergo analyte-dependent conformational changes, resulting in signal changes corresponding to the amount of cocaine.9,10 The second group of methods utilize double-fragment aptamers (DFAs), in which MAs are cut into two pieces.11,12 The physical separation of two strands and the flexibility for probe modification open up many opportunities for the development of novel amplification pathways13 and other applications14 that are not capable with MAs.
We report here a new split mode of aptamer, namely, a triple-fragment aptamer (TFA), in which for the first time an anti-cocaine aptamer was cut into three fragments (Fig. 1A). Compared to DFA, TFA has two more open terminals available for labeling, which would certainly render more choice for probe design and optimization. TFA also has the potential to be used as a three way-joint in the nanostructure assembly for two dimensional patterns. Both MA and DFA could only be used for constructing one dimensional structures.
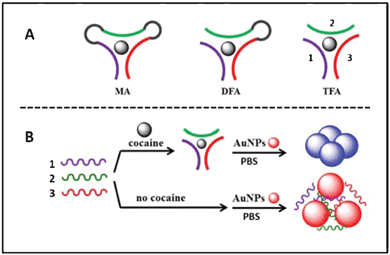 | ||
| Fig. 1 Schematic drawings of (A) different designs of assay probes for cocaine detection: (left) monolithic aptamer (MA), (middle) double-fragment aptamer (DFA), and (right) triple-fragment aptamer (TFA); (B) gold nanoparticle based optical cocaine detection using TFA. The same oligonucletide sequences were drawn in the same colors. | ||
An anti-cocaine aptamer was respectively split into two fragments or three fragments that were designed by inspection of the aptamer's predicted secondary structure, which suggested that cuts at the loop sites would not impede cocaine binding.9,11 Of note, for precise comparison of different probe designs, the DFA and the TFA share the same binding sequences with MA except for the removal of a couple of bases at each connection part (Table 1).
| Name | Sequences (5′→3′) | Description |
|---|---|---|
| Probe 1 | GGGAGTCAAGAACAAAGTTCTTCAATGAAG TGTGGGACGACA(42 bp) | One fragment used in MA |
| Probe 2 | GTTCTTCAATGAAGTGTGGGACGACA(26 bp) | One fragment used in DFA |
| Probe 3 | GGGAGTCAAGAAC(13 bp) | One fragment used in DFA and TFA |
| Probe 4 | AGTGGGACGACA(12 bp) | One fragment used in TFA |
| Probe 5 | GTTCTTCAAT(10 bp) | One fragment used in TFA |
| Probe 5-1 | CAAGAAGATT(10 bp) | Four fragments used for cocaine selective test in colorimetric method |
| Probe 5-2 | CAACCTCAAA(10 bp) | |
| Probe 5-3 | GTTCT![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) CAAT(10 bp) CAAT(10 bp) |
|
| Probe 5-4 | GTTCTT![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) AAT(10 bp) AAT(10 bp) |
The binding affinities of MA (probe 1), DFA (probe 2 and 3), and TFA (probe 3, 4 and 5) with cocaine at different temperatures were, respectively, measured through isothermal titration measurements (ITC). The thermodynamic parameters of cocaine binding are shown in Table 2 and representative ITC data is shown in Fig. S1, S2 (ESI†). As expected, at 25 °C MA had the highest binding affinity of 18.0 ± 0.6 μM and DFA binds cocaine with an affinity of 120.6 ± 9.8 μM. Both affinity values are in line with the previous reports of 9 μM15 and ~200 μM.11 For TFA, no binding was observed when the same titration conditions were used. However, an affinity of 111.2 ± 19.1 μM was measured when the low c conditions (ESI†) were attempted at 4 °C. At 37 °C, DFA lost its affinity to cocaine and MA was still able to bind cocaine with an affinity of 35.4 ± 0.7 μM. Two categories of DNA-small molecule binding interactions have been classified according to their thermodynamic signatures.16 Groove binders display small negative or positive enthalpy with a favorable binding entropy, while intercalators have unfavorable entropy and negative enthalpy values. Quite interestingly, for both MA and DFA studied here, cocaine binding is an enthalpically driven process offset by an unfavorable entropic penalty, which fits this interaction into the “intercalator” category. In contrast, for TFA, cocaine binding displays about 10 or 20 times smaller negative enthalpy compared to MA and DFA, and with favorable binding entropy. The thermodynamic parameters would put the interaction in the “groove binder” category, in which no DNA rearrangement in structure is involved. In addition, it has been reported that the cocaine binding affinity of MAs dramatically decreases as NaCl concentration increases.15 In contrast, we observed that TFA has the highest affinity under the same salt concentration as the selection occurs and the higher or lower salt concentrations only cause the slight decreases of the binding affinity (238.1 ± 3.0 μM at 500 mM NaCl, 150.4 ± 13.5 μM without NaCl) (Fig. S3, ESI†).
| Model | Kd (μM) | ΔH (kcal mol−1) | −TΔS (kcal mol−1) |
|---|---|---|---|
| a Data acquired at 25 °C, b 37 °C and c 4 °C in 25 mM Tris (pH 8.0), 150 mM NaCl solution. Kd: dissociation constant; H: enthalpy; S: entropy ; T: temperature. | |||
| MAa | 18.0 ± 0.6 | −11.4 ± 0.3 | 4.9 |
| MAb | 35.4 ± 0.7 | −9.4 ± 2.3 | 3.1 |
| DFAa | 120.6 ± 9.8 | −22.2 ± 7.2 | 16.8 |
| TFAc | 111.2 ± 19.1 | −1.4 ± 0.2 | −3.7 |
With the purpose to test the potential use of the TFA in cocaine detection, we constructed a very simple gold nanoparticle (AuNP) based optical sensor (Fig. 1B).17 The method is based on the observation that single stranded DNA (ssDNA) stabilize unmodified AuNPs better than double stranded DNA (dsDNA). The assay was designed such that, in the presence of cocaine, the dsDNA supramolecular structure would form and the unmodified AuNPs would aggregate upon the addition of the salt solution, giving rise to a colorimetric change from red to purple or blue.18 We observed a significant colorimetric transition for the assay containing cocaine (Fig. S4, ESI†), but not cocaine analogues— ecogonine methyl ester (EME) and benzoyl ecgonine (BE) (Fig. 2B). In addition, the co-presence of BE and EME at equal or ten times the concentration of cocaine did not interfere with the successful detection of 100 μM cocaine. Color changes and UV-vis absorption spectra were similar to those of the standard cocaine sample (Fig. S5, ESI†). In order to further confirm that the binding between TFA and cocaine was specific, a series of control sequences were tested. Specifically, we replaced probe 5 by probe 5-1, probe 5-2, probe 5-3 and probe 5-4, respectively (Table 1). None of assays using these control sequences could result in the aggregation of AuNPs under the same assay conditions used for TFA (Fig. 2C). Please note that, for probe 5-3 and probe 5-4, there is only one nucleotide difference from probe 5. Our observation is consistent with the reports on the importance of nucleotide identity in cocaine binding for MA.15 It's really amazing that TFA with such short lengths was still able to bind cocaine with such high specificity.
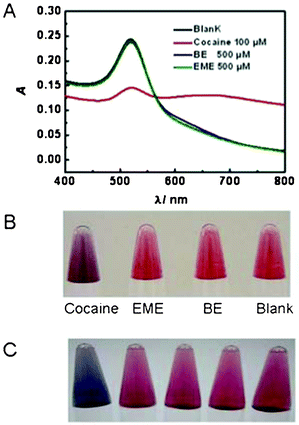 | ||
| Fig. 2 The UV-Vis spectra (A) and visual detection (B–C) of AuNP-based cocaine assays using TFA. A) Selectivity for cocaine over two analogues, ecgonine methyl ester (EME) and benzoyl ecgonine (BE). B) Visual detection of cocaine over EME and BE. C) From left to right, assays with TFA (probe 3, 4, and 5) and the control sequences (probe 5 in TFA was replaced by probe 5-1, probe 5-2, probe 5-3 and probe 5-4, respectively) in the presence of cocaine. | ||
The sensor showed a gradual color change from red to blue as the concentrations of cocaine were increased from 0 to 200 μM with slightly better sensitivity and broader color gradient than the previously reported DFA assay (Fig. 3). The shorter fragments stabilized unmodified AuNPs better than the longer fragments or strands at the same molar concentrations (Fig. S6, ESI†). Compared to DFA and MA assays, TFA assays have a bigger stability difference than the AuNPs between the blank and the cocaine saturated state, corresponding to the broadened color gradients and smaller errors.
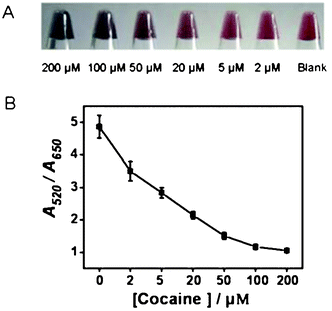 | ||
| Fig. 3 The visual detection photos (A) and titration curves (B) of cocaine assays using TFA. The errors were calculated from three repeated experiments under the same conditions. | ||
The sensor worked well in complex samples and 100 μM cocaine in human urine or filtered serum was readily detected with the color change of AuNPs similar to that of a standard cocaine sample in buffer (Fig. S7, ESI†).
The strategy is also shown to be generic by selectively detecting of adenosine triphosphate (ATP) in the sub-micromolar range (Fig. S8, ESI†). Please note that the colorimetric AuNP-based approach is not a good detection platform for ATP because all nucleoside triphosphates including ATP, guanosine triphosphate (GTP), cytidine triphosphate (CTP), and uridine triphosphate (UTP), are able to stabilize the unmodified AuNPs by nonspecifically absorbing onto AuNPs.19 However, we demonstrated that the colorimetric detection still worked at low ATP concentrations (0–2 μM). We also looked into the specificity of the TFA probes by conducting colorimetric detection of GTP, CTP, and UTP under the same conditions. No noticeable color change was observed in the presence of GTP, which has the most similar structure to ATP. A little color change was observed for UTP, which could be due to the fact that among all NTPs, UTP is the worst stabilizer for AuNPs. The experiments demonstrated that TFA probes for ATP still retain good affinity and specificity.
In conclusion, we have demonstrated a new type of aptamer probe design, TFA, and its potential use in biosensing. TFA broadens the repertoire of probe designs and would provide extraordinary opportunities for the future development of novel detection approaches and for nanostructure constructions. Even though the TFA demonstrated in this work has low affinity to cocaine and the assays need to be conducted at low temperature (4 °C), the affinity should be able to be improved by strategies such as increasing the stem length as reported.15
This work was supported by National Natural Science Foundation (20975108, 21105066, 21004056).
References
- (a) S. E. Osborne and A. D. Ellington, Chem. Rev., 1997, 97, 349–370 CrossRef CAS; (b) X. H. Lou, J. R. Qian, Y. Xiao, L. Viel, A. E. Gerdon, E. T. Lagally, P. Atzberger, T. M. Tarasow, A. J. Heeger and H. T. Soh, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 2989–2994 CrossRef CAS; (c) S. M. Shamah, J. M. Healy and S. T. Cload, Acc. Chem. Res., 2008, 41, 130–138 CrossRef CAS; (d) S. S. Oh, K. Plakos, X. Lou, Y. Xiao and H. T. Soh, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14053–14058 CrossRef CAS.
- (a) D. Li, S. P. Song and C. H. Fan, Acc. Chem. Res., 2010, 43, 631–641 CrossRef CAS; (b) A. Vallee-Belisle and K. W. Plaxco, Curr. Opin. Struct. Biol., 2010, 20, 518–526 CrossRef CAS.
- J. Y. Cho, K. Hamasaki and R. R. Rando, Biochemistry, 1998, 37, 4985–4992 CrossRef CAS.
- C. Wilson, J. Nix and J. Szostak, Biochemistry, 1998, 37, 14410–14419 CrossRef CAS.
- (a) T. Kato, K. Yano, K. Ikebukuro and I. Karube, Nucleic Acids Res., 2000, 28, 1963–1968 CrossRef CAS; (b) L. S. Green, D. Jellinek, R. Jenison, A. Ostman, C. H. Heldin and N. Janjic, Biochemistry, 1996, 35, 14413–14424 CrossRef CAS; (c) W. Chiuman and Y. Li, PLoS One, 2007, 2, e1224 Search PubMed.
- A. Csordas, A. E. Gerdon, J. D. Adams, J. Qian, S. S. Oh, Y. Xiao and H. T. Soh, Angew. Chem., Int. Ed., 2010, 49, 355–358 CrossRef CAS.
- (a) E. Green, M. J. Olah, T. Abramova, L. R. Williams, D. Stefanovic, T. Worgall and M. N. Stojanovic, J. Am. Chem. Soc., 2006, 128, 15278–15282 CrossRef CAS; (b) O. Reinstein, M. A. D. Neves, M. Saad, S. N. Boodram, S. Lombardo, S. A. Beckham, J. Brouwer, G. F. Audette, P. Groves, M. C. J. Wilce and P. E. Johnson, Biochemistry, 2011, 50, 9368–9376 CrossRef CAS.
- (a) D. Shu, Y. Shu, F. Haque, S. Abdelmawla and P. Guo, Nat. Nanotechnol., 2011, 6, 658–667 CrossRef CAS; (b) I. T. Seemann, V. Singh, M. Azarkh, M. Drescher and J. S. Hartig, J. Am. Chem. Soc., 2011, 133, 4706–4709 CrossRef CAS.
- M. N. Stojanovic, P. de Prada and D. W. Landry, J. Am. Chem. Soc., 2001, 123, 4928–4931 CrossRef CAS.
- (a) B. R. Baker, R. Y. Lai, M. S. Wood, E. H. Doctor, A. J. Heeger and K. W. Plaxco, J. Am. Chem. Soc., 2006, 128, 3138–3139 CrossRef CAS; (b) Y. Zhao, X. W. He and X. B. Yin, Chem. Commun., 2011, 47, 6419–6421 RSC; (c) Y. Xiang and Y. Lu, Nat. Chem., 2011, 3, 697–703 CrossRef CAS; (d) C. H. Lu, J. A. Li, M. H. Lin, Y. W. Wang, H. H. Yang, X. Chen and G. N. Chen, Angew. Chem., Int. Ed., 2010, 49, 8454–8457 CAS; (e) C. P. Ma, W. S. Wang, Q. Yang, C. Shi and L. J. Cao, Biosens. Bioelectron., 2011, 26, 3309–3312 CrossRef CAS; (f) J. W. Liu, D. Mazumdar and Y. Lu, Angew. Chem., Int. Ed., 2006, 45, 7955–7959 CrossRef CAS; (g) D. Zheng, R. Zou and X. Lou, Anal. Chem., 2012, 84, 3554–3560 CrossRef CAS.
- M. N. Stojanovic, P. de Prada and D. W. Landry, J. Am. Chem. Soc., 2000, 122, 11547–11548 CrossRef CAS.
- (a) R. Freeman, Y. Li, R. Tel-Vered, E. Sharon, J. Elbaz and I. Willner, Analyst, 2009, 134, 653–656 RSC; (b) C. C. Wu, L. Yan, C. M. Wang, H. X. Lin, C. Wang, X. Chen and C. J. Yang, Biosens. Bioelectron., 2010, 25, 2232–2237 CrossRef CAS; (c) X. L. Zuo, Y. Xiao and K. W. Plaxco, J. Am. Chem. Soc., 2009, 131, 6944–6945 CrossRef CAS.
- (a) R. Freeman, E. Sharon, R. Tel-Vered and I. Willner, J. Am. Chem. Soc., 2009, 131, 5028–5029 CrossRef CAS; (b) A. K. Sharma and J. M. Heemstra, J. Am. Chem. Soc., 2011, 133, 12426–12429 CrossRef CAS; (c) J. L. He, Z. S. Wu, H. Zhou, H. Q. Wang, J. H. Jiang, G. L. Shen and R. Q. Yu, Anal. Chem., 2010, 82, 1358–1364 CrossRef CAS.
- (a) T. H. Nguyen, L. J. Steinbock, H. J. Butt, M. Helm and R. Berger, J. Am. Chem. Soc., 2011, 133, 2025–2027 CrossRef CAS; (b) X. W. Xu, J. Zhang, F. Yang and X. R. Yang, Chem. Commun., 2011, 47, 9435–9437 RSC.
- M. A. D. Neves, O. Reinstein, M. Saad and P. E. Johnson, Biophys. Chem., 2010, 153, 9–16 CrossRef CAS.
- M. A. D. Neves, O. Reinstein and P. E. Johnson, Biochemistry, 2010, 49, 8478–8487 CrossRef CAS.
- J. Zhang, L. H. Wang, D. Pan, S. P. Song, F. Y. C. Boey, H. Zhang and C. H. Fan, Small, 2008, 4, 1196–1200 CrossRef CAS.
- H. X. Li and L. Rothberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14036–14039 CrossRef CAS.
- (a) X. H. Lou, Y. Xiao, Y. Wang, H. J. Mao and J. L. Zhao, ChemBioChem, 2009, 10, 1973–1977 CrossRef CAS; (b) M. Liu, M. Yuan, X. Lou, H. Mao, D. Zheng, R. Zou, N. Zou, X. Tang and J. Zhao, Biosens. Bioelectron., 2011, 26, 4294–4300 CrossRef CAS; (c) W. Zhao, T. Lee, S. Leung and I. Hsing, Langmuir, 2007, 23, 7143–7147 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: experimental section, isothermal titration calorimetry data, TEM images, spectra and photographs for cocaine detection in its analogue mixture and in complex matrix (urine and serum), ATP detection using TFA probes. See DOI: 10.1039/c2ra20307c/ |
| ‡ Authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2012 |
