A novel acid-driven, microwave-assisted, one-pot strategy toward rapid production of graphitic N-doped carbon nanoparticles-decorated carbon flakes from N,N-dimethylformamide and their application in removal of dye from water†
Sen
Liu
a,
Jingqi
Tian
ab,
Lei
Wang
a,
Yingwei
Zhang
a,
Yonglan
Luo
a,
Abdullah M.
Asiri
cd,
Abdulrahman O.
Al-Youbi
cd and
Xuping
Sun
*acd
aState Key Lab of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, Jilin, China. E-mail: sunxp@ciac.jl.cn; Fax: (+86) 431-85262065; Tel: (+86) 431-85262065
bGraduate School of the Chinese Academy of Sciences, Beijing 100039, China
cChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia
dCenter of Excellence for Advanced Materials Research, King Abdulaziz University, Jeddah 21589, Saudi Arabia
First published on 6th March 2012
Abstract
The present communication reports on a novel one-pot strategy toward rapid production of N-doped carbon nanoparticles-decorated carbon flakes (N-CNP-CFs) by microwave irradiation of N,N-dimethylformamide in the presence of H3PO4 for the first time. It suggests these N-CNP-CFs exhibit excellent ability to remove dye from water.
Recently, graphitic carbon materials (GCMs) have attracted much attention due to their high surface area, good electrical conductivity and chemical stability, which ensure their wide applications in catalysis, biosensing, energy storage, adsorption, fuel cells etc.1–4 Up to now, a wealth of GCMs with various structures, including nanosheets, nanotubes, nanoparticles, mesostructures, have been successfully prepared.5–8 In order to obtain graphitic carbon structures, several synthesis strategies have been developed, such as arc discharge,9 laser vaporization,10 plasma and thermal chemical vapor deposition11 and high temperature carbonization of carbon precursors in the absence and presence of metal catalysts, etc.12–16 However, all these above-mentioned methods suffer from drawbacks such as complex and time-consuming processes, severe synthetic conditions, and high cost, which limit their wide application. More recently, Liu et al. have prepared graphitic carbon plates by hydrothermal treatment of glucose solution in the presence of H2SO4 at 190 °C for 6 h.17 Unfortunately, a long reaction time is required for the formation of GCMs. Accordingly, developing simple and low-cost methods for rapid production of GCMs is highly desired.
It is well known that synthetic dyes are extensively used in many industries, such as textiles, paper, printing, food, and cosmetics, which generate a large amount of dye-containing wastewaters.18 From the point of view of environment protection, a lot of effort has been put into removing dyes from water.19 Recently, graphene-based carbon materials in particular have been used as effective adsorbents for removal of dyes due to their large surface area and π-rich structures.20–22 However, the methods for preparing such graphene-based carbons suffer from drawbacks such as time-consuming, complicated and expensive processes, low yield, high cost, and severe synthetic conditions. In this communication, we develop a novel one-pot strategy toward rapid production of graphitic N-doped carbon nanoparticles-decorated carbon flakes (N-CNP-CFs) by microwave irradiation of N,N-dimethylformamide (DMF) with the use of H3PO4 as a catalyst. We further demonstrate that the N-CNP-CFs thus obtained can be used as a very effective adsorbent for removal of dye from water.
DMF, H3PO4, and Rhodamine B (RhB) were purchased from Beijing Chemical Corp. All chemicals were used as received without further purification. The water used throughout all experiments was purified through a Millipore system. The N-CNP-CFs were prepared by microwave irradiation of DMF solution in the presence of H3PO4. In a typical synthesis, 1 mL of H3PO4 was added into 5 mL of DMF solution. After that, the mixture was directly placed in a domestic microwave oven and irradiated at 700 W for 2 min. The N-CNP-CFs were collected by washing with water. The adsorption experiments were conducted with N-CNPs-CFs as adsorbents for RhB (5 mg L−1) solutions in bottles at room temperature. The concentration of RhB in the solution was measured with a UV-vis recording spectrophotometer (UV5800).
UV-vis spectra were obtained on a UV5800 Spectrophotometer. Raman spectrum was obtained on a J-Y T64000 Raman spectrometer with 514.5 nm wavelength incident laser light. X-ray photoelectron spectroscopy (XPS) analysis was measured on an ESCALAB MK II X-ray photoelectron spectrometer using Mg as the exciting source. Transmission electron microscopy (TEM) measurements were made on a HITACHI H-8100 electron microscope (Hitachi, Tokyo, Japan) with an accelerating voltage of 200 kV. The sample for TEM characterization was prepared by placing a drop of colloidal solution on a carbon-coated copper grid and dried at room temperature. Scanning electron microscopy (SEM) measurements were made on a XL30 ESEM FEG scanning electron microscope at an accelerating voltage of 20 kV. Powder X-ray diffraction (XRD) data were recorded on a Rigaku D/MAX 2550 diffractometer with Cu–Kα radiation (λ = 1.5418 Å).
Large amounts of black powders were obtained after microwave irradiation of the mixture of H3PO4 and DMF for 2 min. Fig. 1a shows the low magnification SEM image of the products thus obtained, revealing that the products consist of a flake about several micrometers in size. Large amounts of NPs are also observed on the surface of the flake, which is further evidenced by the high magnification SEM image, as shown in Fig. 1b. The corresponding energy-dispersed spectrum (EDS) shown in Fig. 1c shows that the peaks of C and N elements are observed, indicating the products are formed from DMF. The other peaks of O, Na, Si, In and Sn elements originate from the indium tin oxide (ITO)-coated glass substrate used. It should be noted that there is no peak attributed to element P in the final products.
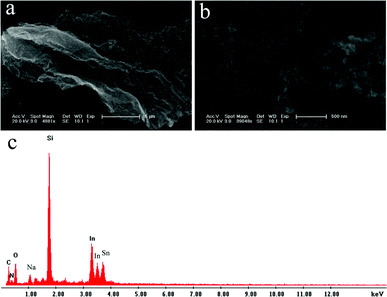 | ||
| Fig. 1 (a) Low, (b) high magnification SEM images, and (c) corresponding EDS of the products thus obtained. | ||
Fig. 2 shows the TEM images of the products. The low magnification TEM image (Fig. 2a) indicates the product is a micrometer-size flake. The corresponding high magnification TEM image (Fig. 2b) indicates there are large amounts of NPs on the surface of the carbon flake. All these observations further confirm the formation of N-CNP-CFs by this novel method.
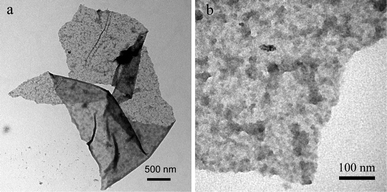 | ||
| Fig. 2 (a) Low, and (b) high magnification TEM images of the products thus obtained. | ||
XPS has been proven to be useful to analyze the chemical environment of carbon atoms in the carbon-based materials. Fig. 3a shows the C1s XPS spectrum of N-CNP-CFs thus obtained. It is seen that three peaks at 284.6, 285.7 and 287.5 eV were observed, which are attributed to C–C, C–N (sp2) and C–N (sp3), respectively, indicating the formation of N-containing carbon materials.23,24 The elemental analysis result exhibits that the N content in the N-CNP-CFs is about 3.67%. The presence of element N in N-CNP-CFs is also confirmed by the N 1s spectrum, as shown in Fig. S1. It is seen that two peaks at 398.5 and 401.0 eV are observed, which are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C and N–H bands, respectively. Thus, there are large amounts of NH2 functional groups attached to the graphitic flakes and a small amount of N atoms in graphitic carbon framework.25 Raman spectroscopy is a common approach used to investigate the graphitic carbon materials.23Fig. 3b shows the Raman spectrum of N-CNP-CFs. It is seen that an intense G band located at 1598 cm−1 is observed, which is attributed to an E2g mode of carbon closely related to the vibration in all sp2-bonded carbon atoms in a two dimensional hexagonal lattice.26 Furthermore, a D band located at 1343 cm−1 is also observed, arising from a breathing mode of κ-point photons of A1g symmetry, which is related to the disorder or defects of the structure.26 It is also found that the peak at about 2700 cm−1 attributed to the 2D mode band in graphene is not observed, indicating the formation of graphitic carbon materials, not graphene-like carbon materials. These results are in agreement with the observations of graphitic carbon plates reported by other researchers.17Fig. 3c shows the XRD pattern of N-CNP-CFs thus obtained. It is seen that two obvious peaks at 24.1 and 42.3° were observed, which are attributed to the reflections of graphitic planes (002) and (101), respectively.3Fig. 3d shows the UV-vis spectrum of the aqueous dispersion of N-CNP-CFs. It is seen that the samples exhibit a broad absorption from UV light to visible light. The electron diffraction pattern of N-CNP-CFs (Fig. S2) shows a poor crystalline structure, which is different from that of graphene, further confirming the formation of graphitic carbon materials. All these observations indicate the formation of graphitic N-CNP-CFs.
N–C and N–H bands, respectively. Thus, there are large amounts of NH2 functional groups attached to the graphitic flakes and a small amount of N atoms in graphitic carbon framework.25 Raman spectroscopy is a common approach used to investigate the graphitic carbon materials.23Fig. 3b shows the Raman spectrum of N-CNP-CFs. It is seen that an intense G band located at 1598 cm−1 is observed, which is attributed to an E2g mode of carbon closely related to the vibration in all sp2-bonded carbon atoms in a two dimensional hexagonal lattice.26 Furthermore, a D band located at 1343 cm−1 is also observed, arising from a breathing mode of κ-point photons of A1g symmetry, which is related to the disorder or defects of the structure.26 It is also found that the peak at about 2700 cm−1 attributed to the 2D mode band in graphene is not observed, indicating the formation of graphitic carbon materials, not graphene-like carbon materials. These results are in agreement with the observations of graphitic carbon plates reported by other researchers.17Fig. 3c shows the XRD pattern of N-CNP-CFs thus obtained. It is seen that two obvious peaks at 24.1 and 42.3° were observed, which are attributed to the reflections of graphitic planes (002) and (101), respectively.3Fig. 3d shows the UV-vis spectrum of the aqueous dispersion of N-CNP-CFs. It is seen that the samples exhibit a broad absorption from UV light to visible light. The electron diffraction pattern of N-CNP-CFs (Fig. S2) shows a poor crystalline structure, which is different from that of graphene, further confirming the formation of graphitic carbon materials. All these observations indicate the formation of graphitic N-CNP-CFs.
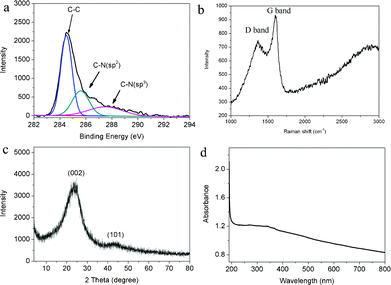 | ||
| Fig. 3 The (a) C1s spectrum, (b) Raman spectrum, (c) XRD pattern and (d) UV-vis spectrum of N-CNP-CFs thus obtained. | ||
We also performed one control experiment by microwave irradiation of DMF in the absence of H3PO4. It is found that no carbon powders are formed even with a longer irradiation time of 5 min. In our previous work, fluorescent carbon dots (CNDs) were obtained by microwave irradiation of DMF in the presence of acids such as chlorosulfonic acid (CSA), H2SO4, HCl, and HNO3.27 In our prerent study, N-CNP-CFs were obtained when H3PO4 was used as a catalyst, indicating that H3PO4 plays an important role in the formation of N-CNP-CFs. It is well-known that DMF can decompose into CO and dimethylamine under high temperature with the use of acids as catalysts,28 organic amines can be polymerized into carbon dots under microwave irradiation in the presence of acids,29 and H3PO4 is an effective catalyst for formation of carbon plates under microwave synthesis.30 The use of H3PO4 is key to the formation of N-CNP-CFs, that is, the formation of N-CNP-CFs from DMF is driven by H3PO4 serving as a catalyst. It should be noted that the content of element N in N-CNP-CFs (3.67%) is lower than that of DMF (19.18%), which may be attributed to the loss of N atoms during the polymerization reaction as well as the CO involved in the reaction. However, the exact formation mechanism of N-CNP-CFs is not completely understood at the present time and requires further study.
It should be noted that, like graphene, the CFs are carbon-based flakes and π-rich structures and hence have a large surface area and strong π–π interactions with π-rich organic molecules. The carbon NPs on the surface of a CF further increase its surface area. Thus, it is expected that such N-CNP-CFs can be used as a very effective adsorbent for adsorption of dye. We chose RhB as a model dye for this demonstration. In order to investigate the removal of RhB from water, N-CNP-CFs (7 mg) were immersed into an aqueous solution of RhB (1.0 × 10−5 M, 20 ml) for a designated time. Fig. 4a shows that UV-vis spectra of the adsorbed solution at various times. It is seen that the UV-vis absorbance intensity greatly decreases about 90% for the first 2.5 min, indicating the fast adsorption rate of N-CNP-CFs toward dye. It is also found that the adsorption process reaches an equilibrium state in 60 min. Fig. 4b shows the corresponding C/C0versus time plots for the adsorption of RhB by N-CNPs-CFs. It should be noted that N-CNP-CFs exhibit a much faster adsorption rate and higher adsorption capacity than Cu2O/reduced graphene oxide nanocomposites reported before.21 A comparison of the performance of our newly prepared materials with already reported results regarding the performance of adsorption of RhB by graphene oxide (GO) and activated carbon (AC) is shown in Table S1. Although N-CNPs-CFs exhibit a lower adsorption capacity than those of GO and AC, they show a much faster adsorption rate than those of GO and AC. To further examine the adsorption ability of N-CNPs-CFs for RhB, a lower amount of adsorbents was used. Fig. S3 shows the UV-vis spectra and corresponding C/C0versus time plots for the adsorption of RhB in the presence of 2 mg of N-CNPs-CFs. It is seen that the adsorption capacity decreases to 48% with decreasing the amounts of N-CNPs-CFs. It should be noted that the adsorption rate is also very fast during the first 5 min.
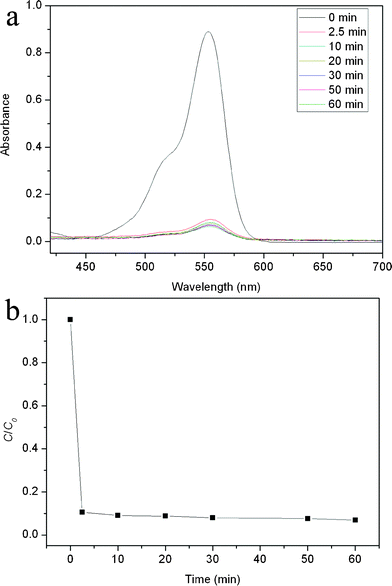 | ||
| Fig. 4 (a) The UV-vis spectra and (b) the C/C0versus time plots for adsorption of RhB solution by N-CNPs-CFs (RhB: 5 mg L−1; N-CNPs-CFs: 350 mg L−1). | ||
In summary, microwave irradiation of DMF in the presence of H3PO4 has been proven to be a simple method for the rapid production of N-CNP-CFs. Such N-CNP-CFs have been further used as a very effective adsorbent with a fast adsorption rate and high adsorption capacity to remove dye from water. Our study is important because it provides us with a novel one-pot strategy toward rapid preparation of carbon materials on a large scale for applications.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21175129) and the National Basic Research Program of China (No. 2011CB935800).References
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS.
- H. Xie, Z. Wu, S. H. Overbury, C. Liang and V. Schwartz, J. Catal., 2009, 267, 158 CrossRef CAS.
- D. W. Wang, F. Liu, M. Liu, G. Q. Lu and H.-M. Cheng, Angew. Chem., Int. Ed., 2007, 47, 373 CrossRef.
- J. N. Wang, Y. Z. Zhao and J. J. Niu, J. Mater. Chem., 2007, 17, 2251 RSC.
- S. Liu, J. Tian, L. Wang, H. Li, Y. Zhang and X. Sun, Macromolecules, 2010, 43, 10078 CrossRef CAS.
- S. Lijima, Nature, 1991, 354, 56 CrossRef.
- M. Ishihara, F. Kokai, A. Nakayama, A. Kosjio, T. Shimazu, A. Tanaka, S. Sasaki and Y. Koga, Carbon, 2007, 45, 880 CrossRef CAS.
- K. K. R. Datta, B. V. S. Reddy, K. Ariga and A. Vinu, Angew. Chem., Int. Ed., 2010, 49, 5961 CrossRef CAS.
- T. W. Ebbesen and P. M. Ajayan, Nature, 1992, 358, 220 CrossRef CAS.
- Y. Ma, Z. Hu, K. Huo, Y. Lu, Y. Liu, J. Hu and Y. Chen, Carbon, 2005, 43, 1667 CrossRef CAS.
- J. Kong, A. M. Cassell and H. Dai, Chem. Phys. Lett., 1998, 292, 567 CrossRef CAS.
- M. Sevilla, C. Salinas, M. Lecea, T. Valdés-Solís, S. Morallón and A. B. Fuertes, Phys. Chem. Chem. Phys., 2008, 10, 1433 RSC.
- P. F. Fulvio, R. T. Mayes, X. Wang, S. M. Mahurin, J. C. Bauer, V. Presser, J. McDough, Y. Gogotsi and S. Dai, Adv. Funct. Mater., 2011, 21, 2208 CrossRef CAS.
- Z. Wu, Y. Wang, D. Gu, D. Feng, Z. Chen, B. Tu, P. A. Webley and D. Zhao, Small, 2009, 5, 2738 CrossRef CAS.
- A. H. Lu, W. C. Li, N. Matoussevitch, B. Spliethoff, H. Bönnemann and F. Schüth, Chem. Commun., 2005, 98 RSC.
- X. Zou, G. Li, Y. Wang, J. Zhao, C. Yan, M. Guo, L. Li and J. Chen, Chem. Commun., 2011, 47, 1066 RSC.
- M. Liu, C. Wang and X. Wang, J. Mater. Chem., 2011, 21, 15197 RSC.
- V. K. Gupta, I. Ali and M. D. Suhas, J. Colloid Interface Sci., 2003, 265, 257 CrossRef CAS.
- E. Forgacs, T. Cserháti and G. Oros, Environ. Technol., 2004, 30, 953 CAS.
- C. K. Ramesha, A. V. Kumara, H. B. Muralidhara and S. Sampath, J. Colloid Interface Sci., 2011, 361, 270 CrossRef.
- B. Li, H. Cao, G. Yin, Y. Lu and J. Yin, J. Mater. Chem., 2011, 21, 10645 RSC.
- B. Li, H. Cao and G. Yin, J. Mater. Chem., 2011, 21, 13765 RSC.
- S. Liu, J. Tian, L. Wang and X. Sun, Carbon, 2011, 49, 3158 CrossRef CAS.
- S. Liu, J. Tian, L. Wang, Y. Luo, J. Zhai and X. Sun, J. Mater. Chem., 2011, 21, 11726 RSC.
- X. Zou, G. Li, Y. Wang, J. Zhao, C. Yan, M. Guo, L. Li and J. Chen, Chem. Commun., 2011, 47, 1066 RSC.
- X. Li, H. Wang, J. T. Robinson, H. Sanchez, G. Diankov and H. Dai, J. Am. Chem. Soc., 2009, 131, 15939 CrossRef CAS.
- S. Liu, L. Wang, J. Tian, J. Zhai, Y. Luo, W. Lu and X. Sun, RSC Adv., 2011, 1, 951 RSC.
- J. Muzart, Tetrahedron, 2009, 65, 8313 CrossRef CAS.
- S. Liu, J. Tian, L. Wang, Y. Luo and X. Sun, RSC Adv., 2012, 2, 411 RSC.
- S. Mitra, S. Chandra, P. Patra, P. Pramanik and A. Goswami, J. Mater. Chem., 2011, 21, 17638 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20381b/ |
| This journal is © The Royal Society of Chemistry 2012 |
