Synthesis and cytotoxic activities of novel hybrid compounds of imidazole scaffold-based 2-substituted benzofurans†
Wen-Jian
Song
,
Xiao-Dong
Yang
*,
Xiang-Hui
Zeng
,
Xiao-Liang
Xu
,
Gao-Lan
Zhang
and
Hong-Bin
Zhang
*
Key Laboratory of Medicinal Chemistry for Natural Resource (Yunnan University), Ministry of Education, School of Chemical Science and Technology, Yunnan University, Kunming, 650091, P. R. China. E-mail: zhanghbyd@gmail.com or xdyang@ynu.edu.cn; Fax: +86-871-5035538; Tel: +86-871-5031119
First published on 27th March 2012
Abstract
A series of novel hybrid compounds between 2-substituted benzofuran and imidazole have been prepared and evaluated in vitro against a panel of human tumor cell lines. The results show that the hybrid compounds were more selective towards an ovarian carcinoma cell line (Skov-3) and suggest that hybrid compounds bearing 2-substituted benzofuran and benzimidazole moieties, as well as imidazolium salts, were vital for modulating cytotoxic activity. The 2-substituted benzofuran imidazole hybrids 24 and 8 can serve as valuable leads for further structural modifications.
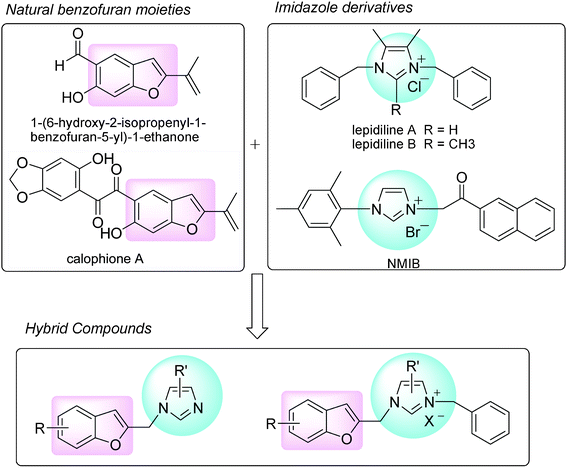
Cancer is a major burden of disease worldwide and is the leading cause of human mortality exceeded only by cardiovascular diseases.1 Therefore, development of new anticancer drugs and more effective treatment strategies for cancer are of the utmost importance.2 Natural products represent a significant source of inspiration for the design of structural analogues with improved pharmacological profiles in medicinal chemistry.3 Naturally occurring substituted benzofurans are an important class of biologically active oxygen-containing heterocycles. Natural products possessing the 2-substituted benzofuran moiety exhibit a broad range of biological and pharmacological activities such as antimicrobial, antiviral, antioxidant, antifungal, antiproliferative, anti-inflamanatory, antifeedant, anti-HIV and antiplatelet activities.4 Recently, naturally occurring benzofurans have been identified to possess anti-tumor activity.5 As exemplified in Scheme 1, 1-(6-hydroxy-2-isopropenyl-1-benzofuran-5-yl)-1-ethanone6 and calophione A7 are also 2-substituted benzofuran derived compounds exhibiting potent cytotoxic activities against human breast cancer cells and colon cancer cells.6,7
 | ||
| Scheme 1 Design of novel hybrid compounds. | ||
Imidazole and its derivatives have attracted considerable interest in recent years for their versatile properties in chemistry and pharmacology. Biological activities of imidazole derivatives have been reported to include antimicrobial and antifungal, antimuscarinic, thromboxane synthetase inhibition, antiinflamatory, antiarrhythmic, and plasmid DNA cleavage activities,8 especially anti-tumor activity.9 For example, two new imidazolium halides (Scheme 1), Lepidiline A and Lepidiline B, isolated from the roots of Lepidium meyenii, showed potent cytotoxic activity against human cancer cell lines.10 Recently, we have reported the synthesis of a series of novel hybrid compounds of imidazole moieties such as NMIB (Scheme 1) and their potential anti-tumor activity.11
Molecular hybridization as a drug discovery strategy involves the rational design of new chemical entities by the fusion of two drugs, both active compounds and/or pharmacophoric units recognized and derived from known bioactive molecules.12 Considering the anticancer activities of naturally occurring 2-substituted benzofurans as well as the potent cytotoxic activities of natural and synthetic imidazole derivatives, we were interested in synthesizing a number of new hybrid compounds bearing 2-substituted benzofuran and imidazole moieties (Scheme 1).
Although benzofuran–triazole hybrid molecules through a heptyloxybenzene chain were synthesized and found to exhibit CYP26A1 inhibitory activity by Simons,13 to the best of our knowledge, no reports concerning anti-tumor activity for hybrid compounds between 2-substituted benzofuran and imidazole have been reported.
In the present research, we have designed and synthesized a series of novel hybrid compounds of imidazole scaffold-based 2-substituted benzofurans. The purpose of this study was to investigate the anti-tumor activity of benzofuran–imidazole hybrids, with the ultimate aim of developing novel potent anti-tumor agents.
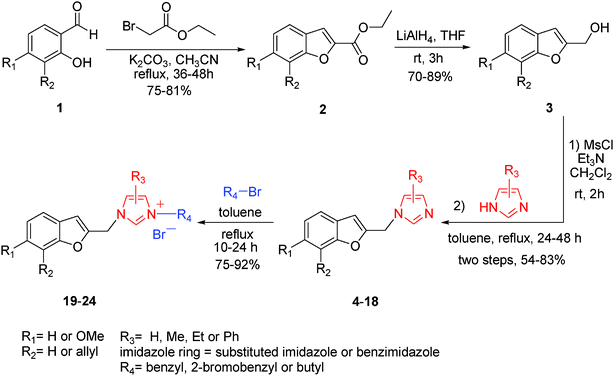
As shown in Scheme 2, substituted salicylaldehydes (1) were condensed with ethyl bromoacetate to afford benzofuran-2-carboxylate compounds (2, 75–81% yields).14 The benzofuran 2-carboxylate compounds 2 were reduced with LiAlH4 to the respective benzofuran 2-methanol compounds (3, 70–89% yields).15 Subsequently, the benzofuran 2-methanol compounds (3) were transformed via the mesylate to the respective fifteen 2-substituted benzofuran–imidazole hybrids (4–18) with various substituted imidazoles by refluxing under toluene with 54–83% yields (two steps).16 Finally, six benzofuran-based imidazolium salts (19–24) were prepared with excellent yields by reaction of 2-substituted benzofuran–imidazole hybrids with the corresponding alkyl bromides by refluxing under toluene (75–92% yields).17 The structures of hybrid compounds are shown in Table 1.
 | ||
| Scheme 2 Synthesis of hybrid compounds 4–21. | ||
| Compound | R1 | R2 | Imidazole ring | R4 | Skov-3 | HL-60 | MCF-7 |
|---|---|---|---|---|---|---|---|
| a Cytotoxicity as IC50 for each cell line, is the concentration of compound which reduced by 50% the optical density of treated cells with respect to untreated cells using the MTT assay. b Data represent the mean values of three independent determinations. | |||||||
| 4 | H | H | Imidazole | — | >40 | >40 | >40 |
| 5 | H | H | 2-Methyl-imidazole | — | >40 | >40 | >40 |
| 6 | H | H | 2-Ethyl-imidazole | — | >40 | >40 | >40 |
| 7 | H | H | 4-Methyl-imidazole | — | >40 | >40 | >40 |
| 8 | H | H | Benzimidazole | — | 9.5 | 8.4 | 11.8 |
| 9 | OMe | H | Imidazole | — | >40 | >40 | >40 |
| 10 | OMe | H | 2-Methyl-imidazole | — | >40 | >40 | >40 |
| 11 | OMe | H | 2-Ethyl-imidazole | — | >40 | >40 | >40 |
| 12 | OMe | H | Benzimidazole | — | >40 | >40 | >40 |
| 13 | H | Allyl | Imidazole | — | >40 | >40 | >40 |
| 14 | H | Allyl | 2-Methyl-imidazole | — | >40 | >40 | >40 |
| 15 | H | Allyl | 2-Ethyl-imidazole | — | >40 | >40 | >40 |
| 16 | H | Allyl | Benzimidazole | — | 7.9 | >40 | >40 |
| 17 | OMe | Allyl | Imidazole | — | 36.2 | >40 | >40 |
| 18 | OMe | Allyl | Benzimidazole | — | 9.3 | >40 | >40 |
| 19 | H | H | Imidazole | Benzyl | 20.8 | >40 | >40 |
| 20 | H | H | 2-Methyl-imidazole | Benzyl | 25.4 | >40 | >40 |
| 21 | H | H | 2-Ethyl-imidazole | Benzyl | 23.2 | >40 | >40 |
| 22 | H | H | 2-Ethyl-imidazole | 2-Bromobenzyl | 35.1 | >40 | >40 |
| 23 | H | H | 2-Ethyl-imidazole | Butyl | 39.5 | >40 | >40 |
| 24 | H | H | Benzimidazole | Benzyl | 9.1 | 7.4 | 12.5 |
| DDP | 8.9 | 5.5 | 13.0 | ||||
The cytotoxic potential of all newly synthesized hybrid compounds was evaluated in vitro against a panel of human tumor cell lines according to procedures described in the literature.18 The panel consisted of ovarian carcinoma (Skov-3), myeloid leukaemia (HL-60), and breast carcinoma (MCF-7). Cisplatin (DDP) was used as the reference drug. The results are summarized in Table 1 (IC50 value, defined as the concentrations corresponding to 50% growth inhibition).
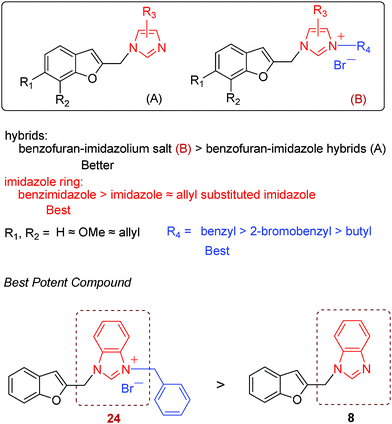
As shown in Table 1, all 2-substituted benzofuran–imidazole hybrids lacked activity against the HL-60 and MCF-7 tumor cell lines investigated at a concentration of 40 μg ml−1 (except compounds 8 and 24). However, the hybrid compounds were more selective towards ovarian carcinoma cell line (Skov-3).
As for Skov-3 cell lines, the structures of the hybrid compounds have an obvious influence on the cytotoxic activities. In terms of the benzofuran ring and imidazole ring, the 2-substituted benzofuran–imidazole hybrids 4–18 with no substituent or a methoxy or an allyl group at the benzofuran ring, as well as with an imidazole ring or alkyl substituted-imidazole ring (methyl or ethyl) were almost inactive (IC50 > 40 μg ml−1). However, when a benzimidazole ring was used instead of a imidazole ring, hybrid compounds displayed similar cytotoxic activity in vitro compared with DDP (with an IC50 value of 9.5 μg ml−1, 7.9 μg ml−1 and 9.3 μg ml−1 for compounds 8, 16 and 24, except compound 12).
Compared with the above hybrid compounds 4–18, benzofuran-based imidazolium salts 19–24 exhibited higher cytotoxic activity. Most of this kind of derivatives showed moderate activity. Similarly, the hybrid salt with a benzimidazole ring (compound 24, with IC50 value of 9.1 μg ml−1) exhibited a higher cytotoxic activity than the hybrid salts with an imidazole ring or alkyl substituted-imidazole ring (compounds 19–23). Compared with compounds 21–23 bearing the same substituents at position 1 and 2 of the imidazole ring, the cytotoxic activity of the hybrid salt with a benzyl substituent at position 3 of the imidazole ring (R4 group) was higher than that of the hybrid compounds with the other alkyl substituents.
The results suggest that 2-substituted benzofuran–imidazole hybrids bearing benzimidazole moieties, as well as imidazolium salts at the imidazolyl-3 position with a benzyl group, were vital for modulating cytotoxic activity. The structure–activity relationship (SAR) results were summarized in Scheme 3.
 | ||
| Scheme 3 Structure–activity relationship of hybrid compounds. | ||
In conclusion, a number of novel hybrid compounds between 2-substituted benzofuran and imidazole have been prepared in this research and evaluated in vitro against a panel of human tumor cell lines. The results show that the hybrid compounds were more selective towards ovarian carcinoma cell lines (Skov-3) and suggest that hybrid compounds bearing 2-substituted benzofuran and benzimidazole moieties, as well as imidazolium salts, were vital for modulating cytotoxic activity. The 2-substituted benzofuran–imidazole hybrids 24 and 8 can be considered promising leads for further structural modifications guided by the valuable information derivable from our detailed SARs.
Acknowledgements
This work was supported by grants (30960460, 21062026, 2010GA014 and 2009CB522300) from the National Natural Science Foundation of China, Yunnan Province and the National Basic Research Program of China (973 Program).References
- H. Varmus, Science, 2006, 312, 1162 CrossRef CAS.
- C. M. Haskell, in Cancer treatment, W.B. Saunders Company, Philadelphia, PA, 5th edn, 2001, ch. 1 Search PubMed.
- F. Belluti, G. Fontana, L. D. Bo, N. Carenini, C. Giommarelli and F. Zunino, Bioorg. Med. Chem., 2010, 18, 3543 CrossRef CAS.
- (a) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS; (b) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644 CrossRef CAS; (c) X. Jiang, W. Liu, W. Zhang, F. Jiang, Z. Gao, H. Zhuang and L. Fu, Eur. J. Med. Chem., 2011, 46, 3526 CrossRef CAS; (d) V. Kuete, D. C. Fozing, W. F. G. D. Kapche, A. T. Mbaveng, J. R. Kuiate, B. T. Ngadjui and B. M. Abegaze, J. Ethnopharmacol., 2009, 124, 551 CrossRef CAS; (e) S. H. Kim, H. S. Pak, M. S. Lee, Y. J. Cho, Y. S. Kim, J. T. Hwang, M. J. Sung, M. S. Kim and D. Y. Kwon, Biochem. Biophys. Res. Commun., 2008, 372, 108 CrossRef CAS; (f) E. M. Wenzig, W. Oleszek, A. Stochmal, O. Kunert and R. Bauer, J. Agric. Food Chem., 2008, 56, 8885 CrossRef CAS; (g) H. Saroyobudiono, L. D. Juliawaty, Y. M. Syah, S. A. Achmad, E. H. Hakim, J. Latip and I. M. Said, J. Nat. Med., 2008, 62, 195 CrossRef CAS; (h) T. Kálai, G. Várbiró, Z. Bognár, A. Pálfi, K. Hantó, B. Bognár, E. Osz, B. Sümegi and K. Hideg, Bioorg. Med. Chem., 2005, 13, 2629 CrossRef; (i) M. Halabalaki, N. Aligiannis, Z. Papoutsi, S. Mitakou, P. Moutsatsou, C. Sekeris and A.-L. Skaltsounis, J. Nat. Prod., 2000, 63, 1672 CrossRef CAS; (j) S. M. Bakunova, S. A. Bakunov, T. Wenzler, T. Barszcz, K. A. Werbovetz, R. Brun, J. E. Hall and R. R. Tidwell, J. Med. Chem., 2007, 50, 5807 CrossRef CAS.
- (a) N. T. Dat, X. J. Jin, K. Lee, Y. S. Hong, Y. H. Kim and J. J. Lee, J. Nat. Prod., 2009, 72, 39 CrossRef CAS; (b) E. S. Katsanou, M. Halabalaki, N. Aligiannis, S. Mitakou, A. L. Skaltsounis, X. Alexi, H. Pratsinis and M. N. Alexis, J. Steroid Biochem. Mol. Biol., 2007, 104, 228 CrossRef CAS.
- S. Van Miert, S. Van Dyck, T. J. Schmidt, R. Brun, A. Vlietinck, G. Lemiere and L. Pieters, Bioorg. Med. Chem., 2005, 13, 661 CrossRef CAS.
- S. Ganapaty, G. V. K. Srilakshmi, S. T. Pannakal, H. Rahman, H. Laatsch and R. Brun, Phytochemistry, 2009, 70, 95 CrossRef CAS.
- (a) E. E. Alberto, L. L. Rossato, S. H. Alves, D. Alves and A. L. Braga, Org. Biomol. Chem., 2011, 9, 1001 RSC; (b) A. Vik, E. Hedner, C. Charnock, L. W. Tangen, Ø. Samuelsen, R. Larsson, L. Bohlinb and L. L. Gundersen, Bioorg. Med. Chem., 2007, 15, 4016 CrossRef CAS; (c) Q. L. Li, J. Huang, Q. Wang, N. Jiang, C. Q. Xia, H. H. Lin, J. Wua and X. Q. Yu, Bioorg. Med. Chem., 2006, 14, 4151 CrossRef CAS; (d) H. Miyachi, H. Kiyota and M. Segawa, Bioorg. Med. Chem. Lett., 1999, 9, 3003 CrossRef CAS; (e) S. J. Dominianni and T. T. Yen, J. Med. Chem., 1989, 32, 2301 CrossRef CAS; (f) R. Lis, T. K. Morgan Jr., R. J. De Vita, D. D. Davey, W. C. Lumman Jr., R. A. Wohl, J. Diamond, S. S. Wong and M. E. Sullivan, J. Med. Chem., 1987, 30, 696 CrossRef CAS; (g) K. Iizuka, T. Kamijo, R. Yamamoto and H. Harada, US. Patent 4,461,905, 1984 Search PubMed; (h) R. D. Haugwitz and V. L. Narayanan, U.S. Patent 3,852,301, 1974 Search PubMed.
- (a) C. G. Fortuna, V. Barresi, G. Berellini and G. Musumarra, Bioorg. Med. Chem., 2008, 16, 4150 CrossRef CAS; (b) F. P. Ballistreri, V. Barresi, P. Benedetti, G. Caltabiano, C. G. Fortuna, M. L. Longo and G. Musumarraa, Bioorg. Med. Chem., 2004, 12, 1689 CrossRef CAS.
- B. Cui, B. L. Zheng, K. He and Q. Y. Zheng, J. Nat. Prod., 2003, 66, 1101 CrossRef CAS.
- (a) W. Chen, X. D. Yang, Y. Li, L. J. Yang, X. Q. Wang, G. L. Zhang and H. B. Zhang, Org. Biomol. Chem., 2011, 9, 4250 RSC; (b) X. H. Zeng, X. D. Yang, Y. L. Zhang, C. Qing and H. B. Zhang, Bioorg. Med. Chem. Lett., 2010, 20, 1844 CrossRef CAS; (c) X. D. Yang, X. H. Zeng, Y. L. Zhang, C. Qing, W. J. Song, L. Li and H. B. Zhang, Bioorg. Med. Chem. Lett., 2009, 19, 1892 CrossRef CAS.
- (a) E. M. Guantai, K. Ncokazi, T. J. Egan, J. Gut, P. J. Rosenthal, P. J. Smith and K. Chibale, Bioorg. Med. Chem., 2010, 18, 8243 CrossRef CAS; (b) C. Viegas Jnr, A. Danuello, V. S. Bolzani, E. J. Barreiro and C. A. M. Fraga, Curr. Med. Chem., 2007, 14, 1829 CrossRef; (c) J. J. Walsh and A. Bell, Curr. Pharm. Des., 2009, 15, 2970 CrossRef CAS.
- S. Pautus, S. W. Yee, M. Jayne, M. P. Coogan and C. Simons, Bioorg. Med. Chem., 2006, 14, 3643 CrossRef CAS.
- M. Ashram, J. Chem. Soc., Perkin Trans. 2, 2002, 1662 RSC.
- K. A. Korthals and W. D. Wulff, J. Am. Chem. Soc., 2008, 130, 2898 CrossRef CAS.
- General procedure for the preparation of 2-substituted benzofuran–imidazole hybrids 4–18. To a solution of benzofuran 2-methanol compound 3 (1 mmol) in dichloromethane (50 mL) was added methanesulfonyl chloride (1.2 mmol) and triethylamine (2 mmol) at 0 °C. The resulting mixture was stirred at room temperature for 2 h. After quenching the reaction with water (50 mL), the layers were separated. The organic phase was dried over anhydrous Na2SO4 and concentrated, and used for the next synthetic step. A mixture of the previous methanesulfonate and imidazole or substituted imidazole (3 mmol) was stirred in toluene (20 ml) at reflux for 24–48 h (monitored by TLC). After cooling to room temperature, the solvent was concentrated, and the residue was diluted with EtOAc (20 mL). The organic layer was washed with water (20 mL) and brine (20 mL), dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography (silica gel, petroleum ether 60–90 °C, ethyl acetate) to afford 4–18 in 54–83% yield (two steps). Compound 8: white powder, yield 78%, mp 128–130 °C (CHCl3). 1H NMR (300 MHz, CDCl3) δ 7.96 (1H, s), 7.84–7.81 (1H, m), 7.47–7.37 (3H, m), 7.28–7.17 (4H, m), 6.56 (1H, s), 5.32 (2H, s). 13C NMR (75 MHz, CDCl3) δ 155.08 (C), 151.07 (C), 143.82 (C), 143.02 (CH), 133.66 (C), 127.69 (C), 124.86 (CH), 123.24 (CH), 122.42 (CH), 121.20 (CH), 120.47 (CH), 111.32 (CH), 109.77 (CH), 105.51 (CH), 42.18 (CH2). HR-ESI-MS m/z Calcd for C16H12N2O 248.0950, Found 248.0944. Anal. Calcd for C16H12N2O: C, 77.40; H, 4.87; N, 11.28. Found: C, 77.33; H, 4.86; N 10.95.
- General procedure for the preparation of 2-substituted benzofuran imidazolium bromides 19–24. A mixture of 2-substituted benzofuran–imidazole hybrids 4–18 (1 mmol) and alkyl bromides (1.2 mmol) was stirred in toluene (10 ml) at reflux for 8–16 h. A white solid was formed. After completion of the reaction as indicated by TLC, the precipitate was filtered through a small pad of Celite, and washed with toluene (3 × 10 ml), then dried to afford 19–24 in 75–92% yield. Pure samples were obtained after recrystallization from an appropriate solvent (acetone or methanol). Compound 24: white powder, yield 89%, mp 218–220 °C (MeOH). 1H NMR (300 MHz, MeOD) δ 8.39 (1H, s), 6.60 (1H, d, J = 7.6 Hz), 6.37 (1H, d, J = 7.6 Hz), 6.19–6.10 (3H, m), 6.01–5.91 (6H, m), 5.80–5.73 (2H, m), 5.70 (s, 1H), 4.52 (s, 2H), 4.27 (s, 2H). 13C NMR (75 MHz, MeOD) δ 148.74 (C), 142.01 (C), 133.02 (C), 131.42 (C), 129.04 (CH), 128.91 (CH), 128.04 (CH), 127.57 (C), 127.20 (CH), 127.09 (CH), 125.19 (CH), 123.12 (CH), 121.37 (CH), 113.67 (CH), 113.48 (CH), 110.81 (CH), 107.80 (CH), 50.77 (CH2), 43.86 (CH2). HR-ESI-MS m/z Calcd for C23H19N2O [M-Br]+ 339.1492, Found 339.1483. Anal. Calcd for C23H19BrN2O: C, 65.88; H, 4.57; N, 6.68. Found: C, 66.29; H, 4.57; N 6.31.
- (a) D.-K. Kim, D. H. Ryu, J. Y. Lee, N. Lee, Y.-W. Kim, J.-S. Kim, K. Chang, G.-J. Im, T.-K. Kim and W.-S. Choi, J. Med. Chem., 2001, 44, 1594 CrossRef CAS; (b) R. Cao, Q. Chen, X. Hou, H. Chen, H. Guan, Y. Ma, W. Peng and A. Xu, Bioorg. Med. Chem., 2004, 12, 4613 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: scan spectral data of the novel hybrid compounds. See DOI: 10.1039/c2ra20376f. |
| This journal is © The Royal Society of Chemistry 2012 |
