Highly selective colorimetric and fluorescent sensors for the fluoride anion based on imidazo[4,5-f]-1,10-phenanthroline metal-complexes†
Sisi
Li
,
Chan
Zhang
,
Shuyuan
Huang
,
Fang
Hu
,
Jun
Yin
and
Sheng Hua
Liu
*
Key Laboratory of Pesticide and Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, Wuhan, 430079, P. R. China. E-mail: chshliu@mail.ccnu.edu.cn
First published on 27th February 2012
Abstract
A new imidazo[4,5-f]-1,10-phenanthroline ligand and its Pt(II), Ru(II) and Re(I) derivatives have been synthesized. It is shown that these metal complexes can be utilised as colorimetric and fluorescence chemosensors for anions. The interaction of these complexes with different anions has been characterised by UV/Vis absorption spectra, fluorescence spectra and titration studies. The results indicated that two of the three complexes showed selective recognition for F−, of which [Re(CO)3ClL1] showed an immediate change from yellow to pink when the fluoride anion was added in DMSO. The phenomenon could be observed with the naked eye. On the other hand, the emission intensities of [Ru(bpy)2L1](PF6)2 and [PtCl2L1] in DMSO were strongly enhanced upon the addition of F−.
Introduction
Over the last few years, the design and synthesis of chemosensors for binding anions selectively has attracted much attention because of the fundamental role of anions in a wide range of biological and chemical processes.1 In particular, research into anion sensing using electrochemical change,2 fluorescent change3 or colorimetric change4 has been actively studied. In colorimetric sensing, anion recognition requires changes in the absorption spectra along with corresponding colour changes which can be detected by the naked eye.5 Among various kinds of anions, the fluoride anion has attracted the interest of chemists because of its importance in many areas such as the human diet and dental care.6,7 Therefore, it is of considerable importance to develop an effective sensor to detect the fluoride anion with the naked eye.8A large number of different receptors, based on the introduction of electrostatic interactions, Lewis acid groups, hydrogen bond donor groups and hydrophobic interactions, have been designed that use metal complexes as synthetic receptors to recognize anions.9,10 A single ligand metal complex may provide sufficient binding ability to obtain stable aggregates with high affinities for anions.11 Metal complexes such as Pt(II), Ru(II), Re(I), Ir(III), Au(I), and Os(II) based complexes have been well developed as chemosensors, because of the advantageous photophysical properties of heavy-metal complexes, such as sensitivity of emission properties, significant single-photon excitation in the visible region and a relatively long lifetime compared with purely organic chemosensors.12
2,2′-Bipyridine and its derivatives, such as phenanthroline, have played an important role in coordination chemistry due to their excellent coordination ability with a range of transition metals. When hydrogen bond donors are introduced after coordination, metal complexes can sometimes serve as good candidates as anion receptors because of some of their novel properties.13 Usually, heterocyclic ring systems containing the NH group can be used to detect anions such as calixpyrroles,14 porphyrinoids,15 pyrrole derivatives,16 indoles,17 bisindoles,18 bisimidazoles, indolocarbazoles19 and benzimidazoles.20 For instance, Molina and coworkers21 have synthesised imidazo[4,5-f]-1,10-phenanthroline as the ligand to form ruthenium(II) complexes. This new chemosensor could effectively recognize hydrogen pyrophosphate and the organic anions ADP and ATP. Wang et al. have reported a Eu(III) complex containing imidazo[4,5-f]-1,10-phenanthroline, in which the addition of F−, HSO4− and AcO− causes significant changes in the UV/Vis absorption and emission spectra.22
Following the above, we have designed and synthesized a new ligand containing the imidazo[4,5-f]-1,10-phenanthroline block (L1), followed by reaction of the ligand L1 with three metal salts (Pt(II), Ru(II) and Re(I) salts) to give appropriate complexes (Scheme 1). Their structures have been fully confirmed by NMR spectroscopy and mass spectrometry. In this case, the introduction of the long alkoxyl chain can improve the solubility. The anion binding was investigated by UV-Vis and fluorescence titration. These complexes showed colorimetric or fluorescent properties for the selective detection of fluoride ions. In this, [Re(CO)3ClL1] showed an immediate change from yellow to pink, which could be observed by the naked eye, with the addition of F− in DMSO. On the other hand, the emission intensities of [Ru(bpy)2L1](PF6)2 and [PtCl2L1] in DMSO were strongly enhanced upon the addition of F−.
 | ||
| Scheme 1 Synthesis of complexes 1–4. | ||
Results and discussion
General information
As shown in Scheme 1, the metal complexes (1–4) were obtained by the reaction of the ligands with the corresponding metal salts, the compounds were characterized by 1H NMR spectroscopy and mass spectrometry. The photophysical properties of these metal complexes with several anions (F−, Cl−, Br−, I−, HSO4−, H2PO4−, CH3COO− and ClO4−) using their tetrabutylammonium salts in DMSO were investigated by UV-Vis, fluorescence measurements and titration studies.Colorimetric anion-sensing in DMSO solution
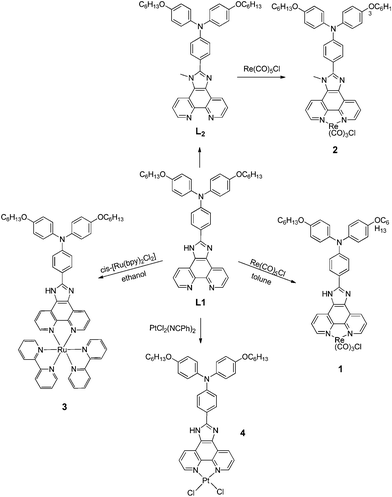
An initial test of complex 1 in DMSO with the addition of F− showed an immediate change from yellow to pink (Fig. 1). However, no obvious changes in colour were observed with the addition of other anions (Cl−, Br−, I−, HSO4−, H2PO4−, CH3COO− and ClO4−). These results suggested that the change in colour is caused only by fluoride and not by other anions. To further study the colorimetric sensing behaviour of receptor 1, UV-Vis absorption experiments were performed. The changes in UV-Vis absorption of complex 1 (2 × 10−5 mol L−1 in DMSO) upon addition of F−, Cl−, Br−, I−, HSO4−, H2PO4−, CH3COO− and ClO4− (10 equiv.) are shown in Fig. 2. The UV-Vis absorption spectrum of receptor 1 exhibits two strong absorption bands at λ = 284 nm (ε = 7.35 × 104 M−1 cm−1) and 368 nm (ε = 7.07 × 104 M−1 cm−1). The addition of the above anions (2 × 10−4 mol L−1 in DMSO) demonstrates that only F− promotes a significant response. According to Fig. 2, after addition of F−, the absorbance at 284 nm showed a 14 nm red shift while no obvious changes were observed for the 368 nm absorbance. In addition, a new low energy broad band appeared at around 512 nm, thereby inducing a promising color change. However, there were no significant changes in the absorption behaviour in the presence of Cl−, Br−, I−, HSO4−, H2PO4−, CH3COO− and ClO4−. | ||
| Fig. 1 Colour change of complex 1 (2 × 10−5 mol L−1 in DMSO) after addition of 10 equiv. of different anions. From left to right: F−, Cl−, Br−, I−, HSO4−, H2PO4−, CH3COO− and ClO4−. | ||
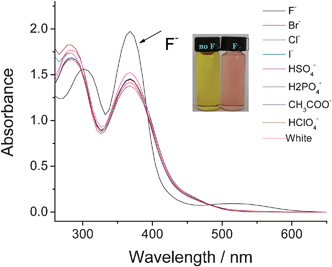 | ||
| Fig. 2 Changes in the absorption spectra of complex 1 (2 × 10−5 mol L−1 in DMSO) upon addition of 10 equiv. of several anions (2 × 10−4 mol L−1 in DMSO). | ||
In order to clarify the interaction of complex 1 with F−, we synthesized ligand L2 by methylation of the N–H on the imidazo-ring of L1 with CH3I in the presence of K2CO3. Subsequently, reaction of ligand L2 with Re(CO)5Cl gave the complex 2. However, complex 2 showed no change upon addition of F−, Cl−, Br−, I−, HSO4−, H2PO4−, CH3COO− and ClO4− (10 equiv.) in DMSO. This demonstrated that F− interacts with the N–H group of the imidazole ring. The absorption peak at 260 nm shifted to 284 nm when a protic solvent such as methanol or water was added, which leads to the suggestion that the interaction of complex 1 with F− depends on deprotonation or hydrogen bonding. As a result of the greater contribution of electron density for the conjugated system in the deprotonated complex 1, it is suggested that the interaction is deprotonation rather than hydrogen bonding because of the significant absorption shift.5 UV-Vis absorption titrations of complex 1 in the presence of different equivalents of F− were performed. As shown in Fig. 3, the intensity of the absorption band at 368 nm increased with the appearance of a new absorption band at 512 nm. On increasing the concentration of F− up to 20 equiv., there was an obvious red shift of the absorption band at 284 nm to 308 nm. Compounds 3 and 4 showed no obvious color change with the addition of different anions.
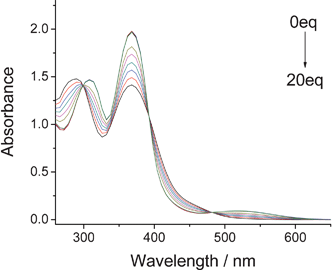 | ||
| Fig. 3 Changes in the absorption spectra complex 1 (2 × 10−5 mol L−1 in DMSO) upon addition of F− (equiv. = 0, 0.2, 0.4, 0.6, 0.8, 1, 5, 10, 15, 20.). | ||
Fluorescence anion-sensing in DMSO solution
The Ru(bpy)32+ moiety has been widely used in the development of fluorescence anion sensors bearing metal-based fluorescent units. The photochemistry of the Ru(bpy)32+ group is very well known.23 The recognition ability of the ruthenium(II) complex for F− was investigated by emission spectrophotometric studies. The emission intensity of the complex in DMSO was strongly enhanced by the addition of F− with a small red shift of emission maxima possibly due to deprotonation of NH in the presence of the fluoride anion, which was in agreement with a previous report.21As is shown in Fig. 4, complex 3 exhibits a very weak fluorescence in DMSO (2 × 10−5 mol L−1). The emission spectrum showed a structureless band at 489 nm typical of the imidazo-phenanthroline ring3 with a low quantum yield of 0.022. Upon addition of other anions as their tetrabutylammonium salts, no significant changes in the emission spectrum could be observed for Cl−, Br−, I−, HSO4−, H2PO4−, CH3COO− and ClO4−. However, the emission band showed a strong enhanced fluorescence in the presence of 10 equiv. of F− with a quantum yield Φ = 0.070.
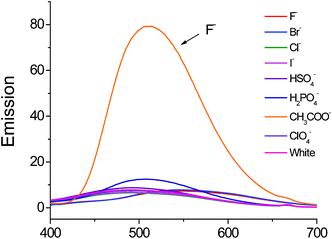 | ||
| Fig. 4 Fluorescence spectra of complex 3 (2 × 10−5 mol L−1 in DMSO) upon addition of 10 equiv. of several different anions (2 × 10−4 mol L−1 in DMSO) excited at 364 nm. | ||
Quantitative investigations of the binding affinity of complex 3 with F− were carried out in DMSO by fluorescence titration (Fig. 5). The emission intensity of 3 was gradually enhanced with increasing F− concentration, and reached saturation when 10 equiv. of F− was added. The Job’s plot for the binding between complex 3 and F− showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. 6). From the fluorescence titration, the apparent association constant was calculated to be 1.2 × 105 M−1.
1 stoichiometry (Fig. 6). From the fluorescence titration, the apparent association constant was calculated to be 1.2 × 105 M−1.
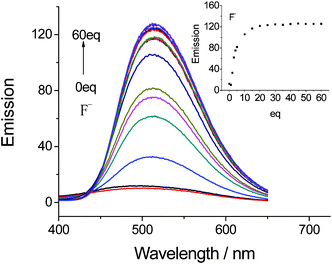 | ||
| Fig. 5 Changes in the fluorescence emission of complex 3 (2 × 10−5 mol L−1 in DMSO) upon addition of F− (equiv. = 0, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60) excited at 364 nm. | ||
2 with F− in DMSO.](/image/article/2012/RA/c2ra20331f/c2ra20331f-f6.gif) | ||
| Fig. 6 Job's plot for the reaction of [Ru(bpy)2L1](PF6)2 with F− in DMSO. | ||
Subsequently, the recognition ability of the platinum(II) complex 4 for anions was also investigated by emission spectrophotometric studies, and analogous fluorescence titrations of complex 4 with different anions were performed (Fig. S2†). Similar results were obtained for the solution of the platinum(II) complex 4 in DMSO, since the emission intensity of complex 4 was strongly enhanced upon addition of F− and reached saturation when 10 equiv. of F− was added. The Job's plot for the complexation of complex 4 with F− also showed that the overall anion-to-receptor binding stoichiometry is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the same as the ruthenium(II) complex 3. In addition, the apparent association constant was observed to be 1.4 × 104 M−1 (Fig. S4†). Such strong enhancement of the emission intensities for [Ru(bpy)2L1](PF6)2 and [PtCl2L1] could possibly be explained by the high sensitivity of N–H in the imidazo-phenanthroline in these complexes. Finally, complex 1 exhibited a very weak fluorescence in DMSO, and there was no significant change with the addition of different anions.
1, the same as the ruthenium(II) complex 3. In addition, the apparent association constant was observed to be 1.4 × 104 M−1 (Fig. S4†). Such strong enhancement of the emission intensities for [Ru(bpy)2L1](PF6)2 and [PtCl2L1] could possibly be explained by the high sensitivity of N–H in the imidazo-phenanthroline in these complexes. Finally, complex 1 exhibited a very weak fluorescence in DMSO, and there was no significant change with the addition of different anions.
1H NMR titration experiment
To further investigate the anion binding properties of fluoride and the metal-complexes 1, 3 and 4, NMR titration experiments were carried out in the absence and presence of different equiv. of fluoride anion in d6-DMSO. Fig. S5† shows the changes in chemical shift when the fluoride anion was added to compound 1. As can be seen from the 1H NMR spectra, for the addition of TBAF, the resonance signals of protons on the unsymmetrical phenanthroline ring exhibited little change when the fluoride anion at 0.5 equiv. was added in d6-DMSO solution at room temperature. An obvious downfield shift was observed when the fluoride anion at 1.0 equiv. was added under the same conditions. Further additions of the fluoride anion showed almost no new shifts. At the same time, the hydrogen donor NH signal disappears along with the addition of the fluoride anion due to the formation of [FHF]−, a result in good agreement with our previous reports.24 When fluoride anions were added the NH signals completely disappeared. However, a new signal at around 16.00 ppm was observed when the fluoride anion reached 5.0 equiv. of complex 1 in concentration due to the formation of [FHF]− and a broad peak at 16.0 ppm was observed, a result in good agreement with previous reports.24 Similar results were observed in the titration experiments of complexes 2 and 3 (see ESI†, Fig. S5–7).Conclusions
We have synthesized and characterized a new imidazo[4,5-f]-1,10-phenanthroline ligand and three metal complexes, which can be utilised as colorimetric and fluorescent chemo-dosimeters for anions. The results showed that [Re(CO)3ClL1] revealed an immediate change in colour from yellow to pink, which could be observed by the naked eye, with the addition of F−, and the emission intensities of [Ru(bpy)2L1](PF6)2 and [PtCl2L1] in DMSO were strongly enhanced with the addition of F−. Furthermore, these metal complexes could be used as colorimetric and fluorescent chemo-dosimeters for F−.Experimental
General information
The compounds cis-[Ru(bpy)2Cl2]·2H2O, [PtCl2(NCPh)2] and 1,10-phenanthroline-5,6-dione were synthesized according to literature methods.21 All other starting materials were obtained commercially at analytical-grade and were used without further purification. All anions, in the form of tetrabutylammonium salts, were purchased from Alfa Aesar (USA), and they were used as received without further purification. Elemental analyses (C, H, N) were performed by Microanalytical Services, College of Chemistry, CCNU. 1H and 13C NMR spectra were collected on a American Varian Mercury Plus 400 spectrometer (400 MHz). 1H and 13C NMR chemical shifts are relative to TMS. UV-Vis spectra were obtained on a U-3310 UV spectrophotometer. Fluorescence spectra were taken on a Fluoromax-P luminescence spectrometer (Horiba Jobin Yvon inc.).Synthesis
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (No. 20931006, 21072070) and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT0953).References
- C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520–563 RSC.
- (a) C. Desemund, K. R. A. S. Sanyadanake and S. Shinkai, Chem. Commun., 1995, 333–334 RSC; (b) H. Yamamoto, A. Ori, K. Ueda, C. Desemund and S. Sinkai, Chem. Commun., 1996, 407–408 RSC; (c) F. Oton, A. Tarraga and P. Molina, Org. Lett., 2006, 8, 2107–2110 CrossRef CAS; (d) F. Oton, A. Espinosa, A. Tarraga, C. Ramirez de Arellano and P. Molina, Chem.–Eur. J., 2007, 13, 5742–5752 CrossRef CAS.
- (a) P. Anzenbacher, A. C. Try, H. Miyaji, K. Jursikova, V. M. Lynch, M. Marquez and J. L. Sessler, J. Am. Chem. Soc., 2000, 122, 10268–10272 CrossRef CAS; (b) T.-H. Kima and T. M. Swager, Angew. Chem., Int. Ed., 2003, 42, 4803–4806 CrossRef; (c) G. Xu and M. A. Tarr, Chem. Commun., 2004, 1050–1051 RSC; (d) J.-S. Wu, J.-H. Zhou, P.-F. Wang, X.-H. Zhang and S.-K. Wu, Org. Lett., 2005, 7, 2133–2136 CrossRef CAS; (e) M. Melaimi and F. P. Gabbai, J. Am. Chem. Soc., 2005, 127, 9680–9681 CrossRef CAS; (f) S. K. Kim, J. H. Bok, R. A. Bartsch, J. Y. Lee and J. S. Kim, Org. Lett., 2005, 7, 4839–4842 CrossRef CAS.
- (a) H. Miyaji, W. Sato and J. L. Sessler, Angew. Chem., Int. Ed., 2000, 39, 1777–1780 CrossRef CAS; (b) H. Miyaji, W. Sato, J. L. Sessler and M. Lynch, Tetrahedron Lett., 2000, 41, 1369–1373 CrossRef CAS; (c) H. Miyaji and J. L. Sessler, Angew. Chem., Int. Ed., 2001, 40, 154–157 CrossRef CAS; (d) S. Yamaguchi, S. Akiyama and K. Tamao, J. Am. Chem. Soc., 2001, 123, 11372–11375 CrossRef CAS; (e) M. Vazquez, L. Fabbrizzi, A. Taglietti, R. M. Pedrido, A. M. Gonzalez-Noya and M. R. Bermeji, Angew. Chem., Int. Ed., 2004, 43, 1962–1695 CrossRef CAS; (f) S. Watanabe, H. Seguchi, K. Yoshida, K. Kifune, T. Tadaki and H. Shiozaki, Tetrahedron Lett., 2005, 46, 8827–8829 CrossRef CAS; (g) Z.-H. Lin, S.-J. Ou, C.-Y. Duan, B.-G. Zhang and Z.-P. Bai, Chem. Commun., 2006, 624–626 RSC; (h) T. Ghosh, B. G. Maiya and A. Samanta, Dalton Trans., 2006, 795–801 RSC.
- T. Gunnlaugsson, H. D. P. Ali and P. E. Kruger, New J. Chem., 2008, 32, 1153–1161 RSC.
- D. Buckland, S. V. Bhosale and S. J. Langford, Tetrahedron Lett., 2011, 52, 1990–1992 CrossRef CAS.
- K. L. Kirk, Biochemistry of the Halogens and Inorganic Halides, Plenum Press: New York, 1991 Search PubMed.
- (a) G. Yang, B. A. Trofimov, Z. Yang, K. Zhang, F. Gong, S. Li, J. Chen and J. S. Ma, J. Photochem. Photobiol., A, 2011, 217, 29–34 CrossRef; (b) X. F. Yang, H. Qi, L. Wang, Z. Su and G. Wang, Talanta, 2009, 80, 92 CrossRef CAS; (c) R. Hu, J. Feng, D. Hu, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2010, 49, 1 CrossRef; (d) P. A. Gale and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3595 RSC; (e) M. Cametti and K. Rissanen, Chem. Commun., 2009, 2809 RSC.
- (a) B. Wu, J. Yang, X. Huang, S. Li, C. Jia, X. J. Yang, N. Tang and C. Janiak, Dalton Trans., 2011, 40, 5687 RSC; (b) P. A. Gale, Acc. Chem. Res., 2006, 39, 465–475 CrossRef CAS; (c) V. Amendola and L. Fabbrizzi, Chem. Commun., 2009, 513–531 RSC; (d) D. J. Mercer and S. J. Loeb, Chem. Soc. Rev., 2010, 39, 3612–3620 RSC; (e) P. Ballester, Chem. Soc. Rev., 2010, 39, 3810–3830 RSC; (f) A. D. Cort, P. De Bernardin, G. Forte and F. Y. Mihan, Chem. Soc. Rev., 2010, 39, 3863–3874 RSC; (g) V. Amendola, L. Fabbrizzi and L. Mosca, Chem. Soc. Rev., 2010, 39, 3889–3915 RSC.
- (a) J. Perez and L. Riera, Chem. Soc. Rev., 2008, 37, 58–2667 Search PubMed; (b) X. F. Shang, J. Li, H. Lin, P. Jiang, Z. S. Cai and H. K. Lin, Dalton Trans., 2009, 2096–2102 RSC; (c) C. R. Bondy, P. A. Gale and S. J. Loeb, J. Am. Chem. Soc., 2004, 126, 5030–5031 CrossRef CAS; (d) M. G. Fisher, P. A. Gale, M. E. Light and S. J. Loeb, Chem. Commun., 2008, 5695–5697 RSC.
- (a) Y. Fan, Y.-M. Zhu, F.-R. Dai, L.-Y. Zhang and Z.-N. Chen, Dalton Trans., 2007, 3885–3892 RSC; (b) B. Konig and R. Reichenbach-Klinke, Dalton Trans., 2002, 121–130 Search PubMed.
- (a) Q. Zhao, F. Li and C. Huang, Chem. Soc. Rev., 2010, 39, 3007–3030 RSC; (b) K. R. J. Thomas, M. Velusamy, J. T. Lin, C. H. Chien, Y. T. Tao, Y. S. Wen, Y. H. Hu and P. T. Chou, Inorg. Chem., 2005, 44, 5677 CrossRef CAS; (c) W. J. Tan, Q. Zhang, J. J. Zhang and H. Tian, Org. Lett., 2009, 11, 161 CrossRef CAS; (d) W. Lu, B. X. Mi, M. C. W. Chan, Z. Hui, C. M. Che, N. Zhu and S. T. Lee, J. Am. Chem. Soc., 2004, 126, 4958 CrossRef CAS; (e) M. S. Lowry and S. Bernhard, Chem.–Eur. J., 2006, 12, 7970 CrossRef CAS.
- (a) A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85–277 CrossRef CAS; (b) C. Kaes, A. Katz and M. W. Hosseini, Chem. Rev., 2000, 100, 3553–3590 CrossRef CAS; (c) E. C. Constable and P. J. Steel, Coord. Chem. Rev., 1890, 93, 205–223 CrossRef.
- P. Anzenbacher, K. Jursikova and J. L. Sessler, Coord. Chem. Rev., 2000, 122 Search PubMed.
- (a) J. L. Sessler and J. M. Davis, Acc. Chem. Res., 2001, 34, 989–997 CrossRef CAS; (b) J. L. Sessler, S. Camiolo and P. A. Gale, Coord. Chem. Rev., 2003, 240, 17–55 CrossRef CAS.
- (a) C. I. Lin, S. Selvi, J. M. Fang, P. T. Chou, C. H. Kai and Y. Y. Cheng, J. Org. Chem., 2007, 72, 3537–3542 CrossRef CAS; (b) J. L. Sessler, G. Dan Pantos, P. A. Gale and M. E. Light, Org. Lett., 2006, 8, 1593–1596 CrossRef CAS; (c) P. A. Gale, Chem. Commun., 2005, 3761–3772 RSC.
- F. M. Pfeffer, K. F. Lim and K. J. Sedgwick, Org. Biomol. Chem., 2007, 5, 1795–1799 CAS.
- K. J. Chang, D. Moon, M. S. Lah and K. S. Jeong, Angew. Chem., Int. Ed., 2005, 44, 7926–7929 CrossRef CAS.
- D. Curiel, A. Cowley and P. D. Beer, Chem. Commun., 2005, 236–238 RSC.
- J. Kang, H. S. Kim and D. O. Jang, Tetrahedron Lett., 2005, 46, 6079–6082 CrossRef CAS.
- P. Molina, A. Tarraga, F. Zapata, A. Caballero and A. Espinosa, J. Org. Chem., 2008, 73, 4034–4044 CrossRef.
- Q. Wang, C. Tan, H. Tamiaki and H. Chen, Photochem. Photobiol. Sci., 2010, 9, 791–795 CAS.
- R. Martinez-Manez and F. Sancenon, Chem. Rev., 2003, 103, 4419–4476 CrossRef CAS.
- Z. Li, C. Zhang, Y. Ren, J. Yin and S. H. Liu, Org. Lett., 2011, 13, 6022–6025 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR and mass spectra. See DOI: 10.1039/c2ra20331f/ |
| This journal is © The Royal Society of Chemistry 2012 |
