Rapid synthesis of biocompatible gold nanoflowers with tailored surface textures with the assistance of amino acid molecules†
Liang
Wang
a,
Chia-Hung
Liu
b,
Yoshihiro
Nemoto
a,
Naoki
Fukata
a,
Kevin C.-W.
Wu
*cd and
Yusuke
Yamauchi
*ae
aWorld Premier International (WPI) Research Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), Tsukuba 305-0044, Japan. E-mail: Yamauchi.Yusuke@nims.go.jp
bDepartment of Urology, Taipei Medical University-Shuang Ho Hospital, No. 291, Jhongjheng Rd., Jhonghe Dist., New Taipei City, 23561, Taiwan
cDepartment of Chemical Engineering, National Taiwan University, No. 1, Sec. 4, Roosevelt Rd., Taipei, 10617, Taiwan. E-mail: kevinwu@ntu.edu.tw
dDivision of Medical Engineering Research, National Health Research Institutes, 35 Keyan Road, Zhunan, Miaoli County 350, Taiwan
eFaculty of Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo 169-8555, Japan
First published on 23rd April 2012
Abstract
Au particles with several unique morphologies (e.g., flower-shaped and confieto-shaped) are successfully synthesized through a chemical reduction with the assistance of amino acid molecules (gum Arabic). The highly branched nanostructures of the obtained Au particles show an enhanced SERS effect. Furthermore, the Au nanoflowers exhibit excellent biocompatibility to human bladder cancer cells T-24, which shows their potential in biomedical applications.
In recent years, the tailored design of gold (Au) nanostructures is of great importance, primarily due to their superior optical and catalytic properties.1–5 Urchin-like Au nanoparticles exhibit enhanced surface-enhanced Raman scattering (SERS) activity, due to their huge local electric field derived from branched shapes.6 Planar Au dendrites show improved catalytic activity for methanol oxidation ascribed to their unique dendritic features.7 Thus, the physical and chemical properties of Au nanostructures are highly dependent on their shapes and sizes.8–12 Up to date, many types of Au nanoparticles with different shapes (e.g., dimpled Au nanoplate,13 Au lace nanocapsules,14 Au nanocorolla,15etc.16–20) have been reported. Tailoring structural characteristics of Au particles favors tuning their functionalities.
Highly branched Au nanostructures have attracted considerable interest.21 The presence of a large number of sharp edges and cavities endows integrated hierarchical architectures, which can drastically enhance the SERS and catalytic activities in comparison with simply bumped Au nanostructures.7,21 However, it is difficult to spontaneously form highly branched Au nanostructures in aqueous solution, mainly because Au itself has an inherently highly symmetric face-centered cubic (fcc) crystal nature.22 In the previous studies, a two-step seed-mediated growth approach has been successfully demonstrated to be a versatile route to the synthesis of branched gold nanostructures.9,23–25 Such two-step synthetic methods are strongly dependent on the faceted seeds to direct the formation of branched Au shapes. Without the preformed seeds, both the particle size and shape of the branched Au nanoparticles are uncontrollable. Therefore, the development of a direct and efficient method for one-step synthesis of branched Au nanostructures is highly desired and is a very important challenge.
In this communication, we newly develop a simple and efficient strategy for the direct synthesis of highly branched Au particles (named to be “Au nanoflowers”) with the assistance of amino acid molecules. The reaction proceeds in aqueous solution at room temperature and terminated within only 5 s. Furthermore, the surface morphology of the Au nanoflowers can be easily varied simply by selecting the Au precursors and tuning their concentrations. The highly branched structure of the Au nanoflowers is made by randomly assembling many Au nanoplates as building blocks.
In a typical synthesis, 5 mL of 20 mM KAuBr4 aqueous solution containing 0.01 g gum Arabic was placed in a beaker and then 5 mL of 0.1 M ascorbic acid (AA) with 0.01 g gum Arabic was quickly added. Gum Arabic, which is one of amino acid molecules, is a very complex mixture of polysaccharides and hydroxyproline-rich glycoproteins.26 The mixture solution was continuously sonicated for 5 s at room temperature. When the AA was added into reaction system, the color of the reaction solution immediately changed from transparent wine red to colorless, and then immediately changed to an opaque brown. The product was collected by consecutive washing/centrifugation cycles and washed with water and ethanol for the removal of the gum Arabic. The complete removal of organic substances was confirmed by elemental analysis (carbon, hydrogen, and nitrogen, CHN).
In the present reaction, the Au species was rapidly reduced by AA as a reducing agent in the presence of gum Arabic as capping agent in aqueous solution. The reduction potential of AA is 0.17 V (vs SHE). AA readily reduces KAuBr4 species (AuBr4−/Au, +0.858 V vs. SHE). By the above remarkably simple and rapid procedure, flower-shaped Au particles with highly branched structures were successfully obtained. No other shaped Au structures were observed, demonstrating a high yield synthesis of Au nanoflowers (∼100%) (Fig. 1a). The particle sizes ranged from 1.1 to 1.3 μm and the average size is around 1.2 μm (Fig.S1, ESI†). From the highly magnified SEM image, it was revealed that the highly branched Au particles were made by assembling thin nanoplates (with 10 nm thickness) as building blocks. Dendritic fractals were randomly oriented to form mesoscale cavities on the exterior surface (Fig. 1b).
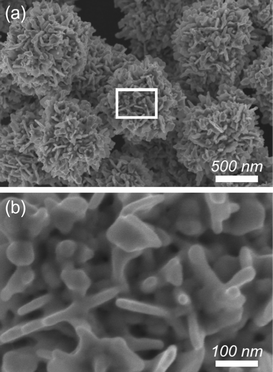 | ||
| Fig. 1 (a) Low- and (b) high-magnified SEM images of Au nanostructures prepared with 20 mM Au solution. | ||
In general, it is recognized that formation of branched Au structures prepared by a one-step protocol is based on a particle-mediated aggregation mechanism.21,27–29 Au species in solution are reduced to generate initial fine Au nuclei which aggregate into small particles. As the reduction proceeds, the particle directs the branched formation via continuous atomic additions with successive aggregation processes. Taking the above growth and aggregation processes into account, the concentration of the Au species is very critical for controlling the particle morphology.21
In the present system, the surface textures and the particle sizes can be effectively tuned by simply adjusting the concentration of the Au species in the initial reactive solution. With the decrease of the Au concentration to 10 mM and 5 mM, flower-shaped Au particles were obtained (Fig. S2, ESI†), but the nanocavities were slightly shallower and their particle sizes were dramatically reduced. As seen in Fig. S2c, ESI,† the particle sizes were distributed in the range of 400–600 nm and their average size was around 520 nm. When the Au concentration was further reduced from 5 mM to 1 mM, confeito-shaped Au nanostructures were obtained. The deepness and the amount of the nanocavities exposed on the particle surface were obviously decreased (Fig. S2d,e, ESI†). Thus, various Au nanostructures were readily produced by simply adjusting the Au concentrations. An insufficient Au source most likely resulted in the Au nanostructures with poorly branched surface, while a sufficient Au source was favorable for the extension of the bumps to form highly branched nanostructures.
In the proposed synthetic system, either halving or doubling the amount of gum Arabic could also produce branched Au nanoparticles. The typical gum Arabic amount used in this study was the most favorable for synthesis of Au nanoflowers in high quality. Thus, gum Arabic seems to play a critical role in directing the shape of the branched Au nanostructures in the present system. Like a polymeric capping agent, gum Arabic, with its rich functional hydroxyproline, is suspected to serve as a steric stabilizer, whose surface capping effect is desired for the kinetically controlled synthesis of branched Au nanostructures. The detailed mechanism for the shape control via gum Arabic in the studied system is still under further investigation. The branched Au nanoflowers prepared under the typical condition have a very good structural stability. Even after it was kept in the solution at room temperature for more than half a year, no shape variation was observed.
Wide-angle XRD profile for the obtained Au nanoflowers showed a fcc Au crystal nature (Fig. S3, ESI†) in which five peaks are assigned to (111), (200), (220), (311), and (222) facets of Au crystal, demonstrating well-crystallized frameworks. More detailed structural information is provided by a TEM study (Fig. 2). Each particle possessed a distinct three-dimensional (3D) structure with a dendritic surface (Fig. 2a-1). Elemental analysis attached with the TEM also confirmed that the Au nanoflowers consisted of pure metallic gold (Fig. 2a-2). Selected-area electron diffraction (ED) patterns taken from one particle exhibited typical fcc rings (Fig. 2b) with intense spots. From the highly magnified TEM image, it was observed that the lattice fringes were running in the same direction at the edge of the particle. The observed d-spacing (0.23 nm) for the adjacent fringes was assignable to the gold (111) facet (Fig. 2c), meaning that each nanoplate had single-crystalline nature.
 | ||
| Fig. 2 (a) HAADF-STEM image and Au elemental mapping of Au nanoflowers prepared with 20 mM Au solution, (b) low-magnified TEM image and the corresponding ED patterns of Au nanoflowers prepared with 5 mM Au solution, and (c) highly-magnified TEM image of the edge part of Au plate. | ||
Selection of the Au precursor is also critical for the formation of Au nanostructures. Replacing KAuBr4 with HAuCl4 under the typical synthetic condition resulted in spherical Au particles with highly bumped surfaces (Fig. S4, ESI†). Compared with KAuBr4 (AuBr4−/Au, +0.858 V vs. SHE), HAuCl4 (AuCl4−/Au, +1.002 V vs. SHE) has a stronger oxidation property. The process of reduction of HAuCl4 precursor by AA should be faster in comparison with that of the KAuBr4. During growth and aggregation, successive aggregation plays the dominant role over the continuous atomic additions, leading to the formation of highly bumped Au spherical particles.
In contrast, the ideal reaction with KAuBr4 can spontaneously realize spatially-controlled assembly for thin Au nanoplates as building units to form a functional integrated assembles with hierarchical architectures. Such nanostructures exposed on the particle exterior are quite important for biomedical applications. Three kinds of Au sample prepared with KAuBr4 species were used as active SERS substrates. As shown in Fig. S5, ESI†, a significant signal enhancement of the Au architectures using R6G as a typical probe molecule was clearly observed in comparison with that flat Au substrate. The enhanced signals at 1185, 1314, 1364, 1510 and 1576 cm−1 were characteristic Raman peaks of R6G.30–32 The enhanced SERS signals of the Au particles with a flower-like shape was caused by the their nanocreviced exteriors, which not only enable electromagnetic (EM) enhancement, but also provide more active sites.33 Furthermore, the junction of adjacent staggered nanoplates can act as a hot-spot, which greatly facilities Raman scattering. Therefore, the highly branched Au particles with richer junctions prepared from 20 mM Au solution (as shown in Fig. 1) exhibited superior enhancement ability than the poorly branched Au particles prepared from 10 mM and 5 mM Au solutions (as shown in Fig. S2b,c, ESI†).
By using synthesized Au nanoflowers with sensitive SERS property and highly nanoporous structure as drug carriers, we can perform a new intracellular drug delivery system in future. In general, the confirmation of loading drugs into drug carriers is through UV-Vis or photoluminescence measurements. For example, mesoporous silica nanoparticles (MSNs) have been widely used to load and deliver fluorescent anticancer drugs (e.g., Doxorubicin) in order to demonstrate a successful delivery.34–37 However, for drugs exhibiting weak UV-Vis or PL features (e.g., cisplatin) or for those drug carriers exhibiting strong UV-Vis or PL features, the confirmation of drug loading becomes difficult and complicated.38,39 Cisplatin is a more effective chemotherapeutic drug than Doxorubicin in treating bladder cancer T-24 cells. Therefore, using our Au nanoflowers with high SERS activity to load cisplatin can easily confirm whether cisplatin is successfully loaded or not, which is another advantageous point that not be realized by other drug carriers such as MSNs or liposomes.
It is always important to measure the safety and toxicological issues of a new nanomaterial before applying the material in intracellular applications. Here, the cytotoxicity of our Au nanoflowers was examined by MTT assays of bladder cancer cells T-24 treated with various concentrations of the samples (Fig. 3). The results in Fig. 3b showed that the cell viability was more than 90%, even when the dosage of the Au NPs is as high as 500 μg mL−1. In fact, the IC50 (50% Inhibitory Concentration) value of Au nanoflowers was estimated to be around 1000 μg mL−1, indicating an excellent biocompatibility. The successful uptake was further confirmed by the observation of T-24 cells treated with/without the Au samples. To avoid the influence of organic dyes used for staining the cell organisms on the differentiation of Au particles, we did not stain the cells and directly observed the cells using differential interference contrast microscopy. In contrast to the control sample (i.e., the cells not treated with Au samples) showing a smooth surface (Fig. 3c-1), the cells treated with Au samples clearly showed a lot of black spots surrounding the nucleus, indicating the appearance of Au nanoflowers. The cells treated with Au samples for 8 h still remained in a regular elongated morphology, which indicates that there was little cytotoxicity of Au nanoflowers towards cells (Fig. 3c-2).
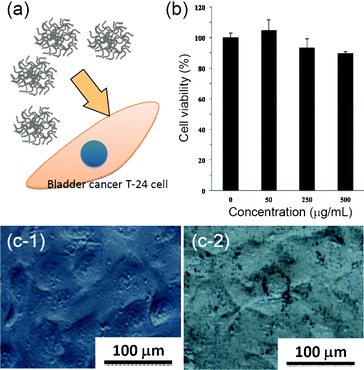 | ||
| Fig. 3 (a) Schematic illustration of Au nanoflowers for a biocompatiblity test. (b) MTT assays of T-24 cells treated with different concentrations of Au nanoflowers for 24 h. (c) Differential interference contrast microscopy images of T-24 cells treated (c-1) without and (c-2) with Au nanoflowers (100 μg mL−1) for 8 h. | ||
In conclusion, we reported the synthesis of novel Au nanoflowers with tailored surface textures by a remarkably simple one-step aqueous solution reaction at room temperature within 5 s. The excellent biocompatibility, unique surface configuration, and intrinsic property of the Au framework suggested that our new Au nanomaterials can act as a potentially useful molecular sensing and intracellular nanovehicle for biomedical applications.
References
- S. J. Guo and E. K. Wang, Acc. Chem. Res., 2011, 44, 491–500 CrossRef CAS.
- K. L. Ai, Y. L. Liu and L. H. Lu, J. Am. Chem. Soc., 2009, 131, 9496–9497 CrossRef CAS.
- C. J. Murphy, A. M. Gole, J. W. Stone, P. N. Sisco, A. M. Alkilany, E. C. Goldsmith and S. C. Baxter, Acc. Chem. Res., 2008, 41, 1721–1730 CrossRef CAS.
- M. Grzelczak, J. Perez-Juste, P. Mulvaney and L. M. Liz-Marzan, Chem. Soc. Rev., 2008, 37, 1783–1791 RSC.
- S. E. Skrabalak, J. Y. Chen, Y. G. Sun, X. M. Lu, L. Au, C. M. Cobley and Y. N. Xia, Acc. Chem. Res., 2008, 41, 1587–1595 CrossRef CAS.
- L. H. Lu, K. L. Ai and Y. Ozaki, Langmuir, 2008, 24, 1058–1063 CrossRef CAS.
- T. Huang, F. Meng and L. M. Qi, Langmuir, 2010, 26, 7582–7589 CrossRef CAS.
- X. Huang, X. Y. Qi, Y. Z. Huang, S. Z. Li, C. Xue, C. L. Gan, F. Boey and H. Zhang, ACS Nano, 2010, 4, 6196–6202 CrossRef CAS.
- H. L. Wu, C. H. Chen and M. H. Huang, Chem. Mater., 2009, 21, 110–114 CrossRef CAS.
- T. Ming, W. Feng, Q. Tang, F. Wang, L. D. Sun, J. F. Wang and C. H. Yan, J. Am. Chem. Soc., 2009, 131, 16350–16351 CrossRef CAS.
- J. Zhang, M. R. Langille, M. L. Personick, K. Zhang, S. Y. Li and C. A. Mirkin, J. Am. Chem. Soc., 2010, 132, 14012–14014 CrossRef CAS.
- T. H. Lin, C. W. Lin, H. H. Liu, J. T. Sheu and W. H. Hung, Chem. Commun., 2011, 47, 2044–2046 RSC.
- Y. Kuroda and K. Kuroda, Angew. Chem., Int. Ed., 2010, 49, 6993–6997 CrossRef CAS.
- M. Yang, R. Alvarez-Puebla, H. S. Kim, P. Aldeanueva-Potel, L. M. Liz-Marzan and N. A. Kotov, Nano Lett., 2010, 10, 4013–4019 CrossRef CAS.
- T. Soejima and N. Kimizuka, J. Am. Chem. Soc., 2009, 131, 14407–14412 CrossRef CAS.
- S. D. Perrault and W. C. W. Chan, J. Am. Chem. Soc., 2009, 131, 17042–17043 CrossRef CAS.
- C. Y. Song, G. P. Zhao, P. J. Zhang and N. L. Rosi, J. Am. Chem. Soc., 2010, 132, 14033–14035 CrossRef CAS.
- Z. D. Wang, J. Q. Zhang, J. M. Ekman, P. J. A. Kenis and Y. Lu, Nano Lett., 2010, 10, 1886–1891 CrossRef CAS.
- G. H. Jeong, M. Kim, Y. W. Lee, W. Choi, W. T. Oh, Q. Park and S. W. Han, J. Am. Chem. Soc., 2009, 131, 1672–1673 CrossRef CAS.
- W. Xie, L. Su, P. Donfack, A. G. Shen, X. D. Zhou, M. Sackmann, A. Materny and J. M. Hu, Chem. Commun., 2009, 5263–5265 RSC.
- L. Zhong, X. D. Zhai, X. F. Zhu, P. P. Yao and M. H. Liu, Langmuir, 2010, 26, 5876–5881 CrossRef CAS.
- Z. Q. Li, W. Y. Li, P. H. C. Camargo and Y. N. Xia, Angew. Chem., Int. Ed., 2008, 47, 9653–9656 CrossRef CAS.
- C. L. Nehl, H. W. Liao and J. H. Hafner, Nano Lett., 2006, 6, 683–688 CrossRef CAS.
- J. Li, J. Wu, X. Zhang, Y. Liu, D. Zhou, H. Z. Sun, H. Zhang and B. Yang, J. Phys. Chem. C, 2011, 115, 3630–3637 CAS.
- C. H. Kuo and M. H. Huang, Langmuir, 2005, 21, 2012–2016 CrossRef CAS.
- D. Renard, L. Lavenant-Gourgeon, M. C. Ralet and C. Sanchez, Biomacromolecules, 2006, 7, 2637–2649 CrossRef CAS.
- Z. H. Li, V. Ravaine, S. Ravaine, P. Garrigue and A. Kuhn, Adv. Funct. Mater., 2007, 17, 618–622 CrossRef CAS.
- H. Wang and N. J. Halas, Adv. Mater., 2008, 20, 820–825 CrossRef CAS.
- J. X. Fang, S. Y. Du, S. Lebedkin, Z. Y. Li, R. Kruk, M. Kappes and H. Hahn, Nano Lett., 2010, 10, 5006–5013 CrossRef CAS.
- W. C. Ye, J. F. Yan, Q. Ye and F. Zhou, J. Phys. Chem. C, 2010, 114, 15617–15624 CAS.
- X. T. Bai and L. Q. Zheng, Cryst. Growth Des., 2010, 10, 4701–4705 CAS.
- J. Sharma, Y. Tai and T. Imae, J. Phys. Chem. C, 2008, 112, 17033–17037 CAS.
- M. Bechelany, P. Brodard, J. Elias, A. Brioude, J. Michler and L. Philippe, Langmuir, 2010, 26, 14364–14371 CrossRef CAS.
- H. Zheng, Y. Wang and S. Che, J. Phys. Chem. C, 2011, 115, 16803–16813 CAS.
- F. Muharnmad, M. Guo, W. Qi, F. Sun, A. Wang, Y. Guo and G. Zhu, J. Am. Chem. Soc., 2011, 133, 8778–8781 CrossRef.
- C. H. Lee, S. H. Cheng, I. P. Huang, J. S. Souris, C. S. Yang, C. Y. Mou and L. W. Lo, Angew. Chem., Int. Ed., 2010, 49, 8214–8219 CrossRef CAS.
- H. Meng, M. Xue, T. Xia, Y. L. Zhao, F. Tamanoi, F. F. Stoddart, J. I. Zink and A. E. Nel, J. Am. Chem. Soc., 2010, 132, 12690–12697 CrossRef CAS.
- W. J. Rieter, K. M. Pott, K. M. L. Taylor and W. Lin, J. Am. Chem. Soc., 2008, 130, 11584–11585 CrossRef CAS.
- R. Vathyam, E. Wondimu, S. Das, C. Zhang, S. Hayes, Z. Tao and T. Asefa, J. Phys. Chem. C, 2011, 115, 13135–13150 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure and characterization data. See DOI: 10.1039/c2ra20348k |
| This journal is © The Royal Society of Chemistry 2012 |
