Amino/quaternary ammonium groups bifunctionalized large pore mesoporous silica for pH-responsive large drug delivery†
Haoquan
Zheng
and
Shunai
Che
*
School of Chemistry and Chemical Engineering, State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China. E-mail: chesa@sjtu.edu.cn; Fax: +86–21–5474–1297; Tel: +86–21–5474–2852
First published on 2nd March 2012
Abstract
Mesoporous nanoparticles functionalized with amino groups on the pore surface and quaternary ammonium groups on the particle surface with particle sizes of 500–800 nm in length and 300–500 nm in diameter and a pore size of 7.2–7.4 nm, have been obtained through a post-synthesis and co-condensation method. Bleomycin (BLM) has been chosen as a model anti-cancer drug with a large molecular size, and the iron essential for organisms has been utilized for constructing NH2–Fe–BLM coordination bond architecture in the pore surface. The BLM was released under mildly acidic pH conditions by cleavage of the Fe–BLM coordination bond triggered by pH reduction. Cell assays show that mesoporous nanoparticles have good dispersity and good cell penetrating properties due to the positively charged quaternary ammonium groups on the outer surface of the nanoparticles. These organic functionalized large pore mesoporous materials can be utilized as carriers in the pH-responsive delivery of an anti-cancer drug with a large molecular size, opening up new opportunities for their further application in controlled release of biomacromolecules.
1. Introduction
Stimuli-responsive systems have attracted the interest of a broad range of researchers in recent years because of the promising applications of these “smart” systems in biomedical fields.1,2 These systems take up or release guest molecules in response to external stimuli such as pH,3–24 temperature,3,25 light irradiation,3,26–28 ionic strength,13,14 chemicals,29 enzymes,30etc. The acidic tumor microenviroment in solid tumors make the pH-sensitive drug delivery system of special interest due to their potential application in anti-cancer therapy.31 Therefore, the pH targeting approach is regarded as a general strategy to target acidic extracellular pH environment of solid tumors. Many diverse physical forms, including hydrogels,3,4 micelles,5,7 liposomes,6,8–11 inorganic solids,12–24etc. can be utilized as carriers in the pH-sensitive system.Recently, mesoporous silica based materials have been well recognized as potential carriers for drug delivery systems,32–45 especially pH-sensitive drug delivery systems,12–23 due to the large mesopore size, high surface area, large pore volume and the nontoxic nature of the amorphous silica framework. Moreover, it provides space to host guest molecules and prevents them from chemical or biological attack by external substances before their intended release. Typically, inorganic solid-based pH-responsive systems usually involve on/off capping or gating (by functional groups,12,13 polyelectrolyte14–17 and ring-shaped compound18,19,22) or host–guest interactions (electrostatic23 and covalent bonding24). Recently, we have developed a novel organic group functionalized mesoporous pH-responsive drug delivery system based on the coordination-bonding of a “host–metal–guest” architecture, where “host”, “metal” and “guest” represent amino groups on the mesopore surface, metal ions and drug molecules, respectively.46,47 The loading and release of the drug at a designed pH are achieved through the formation and cleavage of the coordination bonds, which are sensitive to the variation of external pH. However, their effective applications have been limited because of the small pore size.
It is well known that SBA-15 derived from triblock copolymers possess highly ordered two dimensional (2D)-hexagonal p6mm mesostructure with large pore size of 5–12 nm,48 which would offer new possibilities for large drug pH-responsive delivery systems based on coordination bonds.49 However, for pure SBA-15, only silanol groups are present on the mesopore surface and are unsuitable coordination bonding architecture to act as the “host” part because of their weak bonding strength.46 Hence, in order to choose suitable carriers with specific interactions, amino groups were introduced into the mesopore surface of SBA-15; for dispersity and good cell penetrating properties, the outer surfaces of SBA-15 nanoparticles have been functionalized with quaternary ammonium groups (Scheme 1). 3-aminopropyltrimethoxysilane (APS) and N-trimethoxylsilylpropyl-N,N,N-trimethyl ammonium chloride (TMAPS) have been used for the functionalization of the amino and quaternary ammonium groups, respectively. Herein, amino group functionalization has been achieved via two routes: (i) the subsequent modification of the mesopore surface of a purely inorganic silica material with organic groups in post-synthesis route (denoted as P);50,51 (ii) the simultaneous condensation of organosiloxane and the silica source in a co-condensation process (denoted as C).51–53 (i) In the post-synthesis method, the surface of the as-synthesized SBA-15 nanoparticles (denoted as P1) has first been functionalized with quaternary ammonium groups (denoted as P2), in which the nonionic surfactant filled pore can ensure the selective functionalization onto the outer surface of particle. The surfactant was then extracted by an ethanolic solution of HCl (denoted as P3); which was finally further functionalized with amino groups (denoted as P4) through post-synthesis route . (ii) In the co-condensation method, amino group functionalized SBA-15 nanoparticles (denoted as C1) were synthesized by introducing APS into the synthesis gel; which was functionalized with quaternary ammonium groups on the particle surface (denoted as C2). The surfactant was then extracted and amino/quaternary ammonium group bifunctionalized SBA-15 nanoparticles (denoted as C3) were obtained.
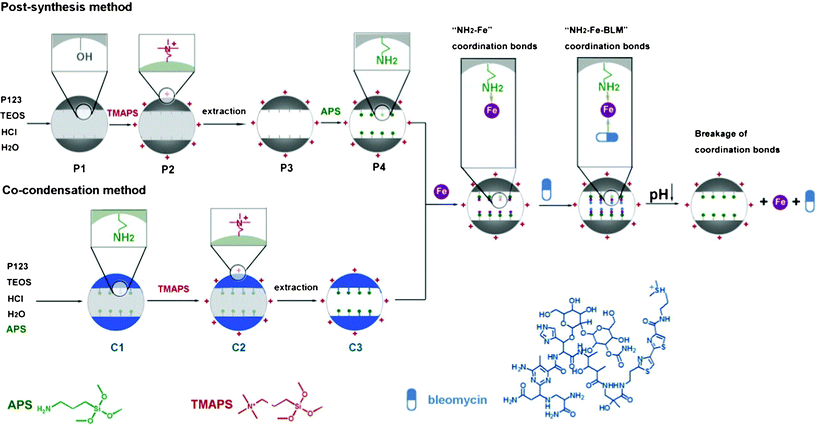 | ||
| Scheme 1 Schematic illustration of the two methods for the synthesis of large pore mesoporous silica nanoparticles and the coordinate bond based pH-responsive drug delivery system. The SBA-15 particles were selectively functionalized with amino groups on the mesopore surface and quaternary ammonium groups on the particle surface via the post-synthesis and co-condensation methods. The coordination bond based NH2–Fe–BLM architecture can be easily formed. BLM can be released under mildly acidic pH conditions by cleavage of either side of the NH2–Fe or Fe–BLM coordination bond by pH reduction. | ||
The amino groups on the mesopore surface of SBA-15 can act as the “host” part and form NH2–metal–drug coordination bond architecture by introducing metal ions and the drug in turn (Scheme 1). BLM has been chosen as a model anti-cancer drug with a large molecular size.54 Iron, which is essential for organisms, has been utilized for the NH2–Fe–BLM coordination bond architecture. The BLM can be released under mildly acidic pH conditions by cleavage of either side of the NH2–Fe or Fe–BLM coordination bond by pH reduction. It has been reported that the anti-cancer activity of BLM could be improved in the presence of Fe due to the more efficient DNA degradation caused by the Fe–BLM complex.54–56 Quaternary ammonium groups on the outer surface would make SBA-15 nanoparticles highly dispersive due to their electrostatic repulsion.57 Furthermore, the positively charged SBA-15 nanoparticles are preferably utilized to penetrate into living cells, because the cellular membranes possess an overall negative charge,58,59 which could give rise to the enhancement of cellular uptake efficiency and high anti-cancer activity in anti-cancer therapy. The cellular uptake efficiency and the low cytotoxicity of functionalized SBA-15 nanoparticles have been demonstrated by in vitro cell assays.
2. Experimental section
2.1 Material
The following reagents were purchased from Sinopharm and used without purification: TEOS, Fe(NO3)3·9H2O. Pluronic P123 (EO20PO70EO20, BASF), APS (TCI), TMAPS (TCI), BLM (Yinghuan Chempharm) were used as purchased.2.2 Synthesis of aminopropyl functionalized SBA-15 particles
Conventional mesoporous SBA-15 materials were synthesized using Pluronic P123 triblock copolymer as a template and TEOS as a silica source. In a typical synthesis, 1.0 g of P123 was added to a mixture of 7.50 g HCl (2 M) and 30 g H2O aqueous solution in a Teflon-lined container, which was stirred at 35 °C. Then 2.10 g of TEOS was added to this solution under vigorous stirring. The reactant composition was P123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEOS
TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl
HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 0.017
H2O 0.017![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 196. The mixture was keep static at 35 °C for 24 h, and then sealed in a polypropylene bottle at 100 °C under static conditions for another 24 h. The solid products were collected by filtration, washed with deionized water, and dried at 50 °C overnight.
196. The mixture was keep static at 35 °C for 24 h, and then sealed in a polypropylene bottle at 100 °C under static conditions for another 24 h. The solid products were collected by filtration, washed with deionized water, and dried at 50 °C overnight.
Synthesis of functionalized SBA-15 materials by the post-grafting route: In order to functionalize the outer surface of SBA-15 materials with quaternary ammonium groups, 1.2 g of as-synthesized SBA-15 was suspended in 100 mL of TMAPS solution (0.256% in H2O). The product was recovered by centrifugation and washed with ethanol. To remove the surfactant template, the quaternary ammonium group functionalized SBA-15 materials was dispersed in an ethanolic solution of 1 M HCl. The product was recovered by centrifugation and washed with ethanol. Then template free quaternary ammonium group functionalized SBA-15 materials were dispersed in 100 mL of toluene. Then 2 mmol of APS was added and the reaction mixture was refluxed for 6 h. The white solid was filtered, washed with deionized water, and dried at 50 °C overnight.
Synthesis of functionalized SBA-15 materials by the co-condensation route: the same synthesis process was carried out as that used in the synthesis of the as-synthesized SBA-15 except that APS was introduced with the TEOS. The reactant composition was P123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) APS
APS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEOS
TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl
HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 0.017
H2O 0.017![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 196. Then 1.2 g of the as-synthesized SBA-15 materials was suspended in 100 mL TMAPS solution (0.256% in H2O). The surfactant template was removed by an ethanolic solution of 1 M HCl.
196. Then 1.2 g of the as-synthesized SBA-15 materials was suspended in 100 mL TMAPS solution (0.256% in H2O). The surfactant template was removed by an ethanolic solution of 1 M HCl.
2.3 Loading of Fe ions and BLM into the aminopropyl functionalized SBA-15 nanoparticles to form the NH2–Fe–BLM coordinate bond
Stock solutions (0.1 M) of Fe3+ were prepared by dissolving Fe(NO3)3 in deionised water. In a typical charging process of Fe ions into the aminopropyl functionalised SBA-15 materials, 0.10 g of the SBA-15 material was dispersed in 8 mL of the stock solution, and the mixture was stirred at ambient temperature for 2 h. The SBA-15 materials were recovered by centrifugation, washed 10 times with ethanol and dried at 40 °C over-night. The complex of NH2–Fe was obtained in the mesopores.Typically, 5.0 mg of Fe ion loaded SBA-15 materials was dispersed in 8.0 mL of 2 mM solution of BLM in PBS pH 7.4 at ambient temperature, and further stirred for 4 h. After that, the solid product was recovered by centrifugation, washed with phosphate buffer solution (PBS, pH 7.4) 10 times and dried at 40 °C over night.
2.4 Release of BLM under different pH conditions
In a typical release experiment, 6.0 mg of SBA-15 materials with absorbed anti-cancer drugs were suspended by vibration in 20 mL of PBS with various pH, at 37 °C. When sampling, the release system was centrifuged, after which 1.0 mL of the supernatant solution was withdrawn and replaced by the same amount of fresh medium. The released amount of BLM was measured by UV-Vis spectrophotometry.2.5 Cell assay
Cellular uptake assays: SPCA-1 cancer cells were seeded into a 35 mm × 10 mm Petri dish (corning) at a concentration of 2 × 105 cells mL−1 in RPMI-1640 plus 10% FBS culture medium in 2 mL. Cells were allowed to adhere to the Petri dish for 24 h. Before adding particles, the culture media was replaced with pH 7.4 RPMI-1640 plus 10% FBS with 1% penicillin and streptomycin. To each Petri dish, 100 μL of calcein loaded C3 or P4 samples were added. Nanoparticles were incubated with cells for 2 h. After incubation, cells were washed with PBS 5 times and examined under confocal microscopy (Zeiss, LSM-510).MTT assays: Briefly, SPCA-1 cancer cells were seeded into a 35 mm × 10 mm Petri dish (corning) at the concentration of 1 × 104 cells ml−1 in RPMI-1640 plus 10% FBS culture medium in 2 mL. The cells were allowed to adhere to the Petri dish for 24 h. Before adding particles, the culture media was replaced with pH 7.4 RPMI-1640 plus 10% FBS with 1% penicillin and streptomycin. To each Petri dish, C3–NH2–Fe–BLM or P4–NH2–Fe–BLM was added in different concentrations (from 0.1 to 1 μl/well) to cells in triplicate wells for each sample. C3–NH2–Fe–BLM or P4–NH2–Fe–BLM was incubated with the cells for 24 h. Cells were incubated with MTT at 37 °C, 5% CO2 atmosphere for 2 h. To dissolve the resulting formazan crystals, 100 μl MTT solubilization solvent was added to each well.
3. Results and discussion
3.1. SBA-15 mesoporous nanoparticles functionalized with amino groups on the mesopore surface and quaternary ammonium groups on the outer surface of the particles
The SBA-15 particles have been synthesized using Pluronic P123 (EO20PO70EO20) as the template and TEOS as silica source. Fig. 1 shows the X-ray diffraction (XRD) patterns of SBA-15 particles. In the post-synthesis route, the as-synthesized P1 sample exhibits a very intense diffraction peak at 2θ ≈ 0.94° and two additional reflections in the region of 2θ close to 1.60 and 1.87 indexed as the 10, 11 and 20 reflections of 2D-hexagonal p6mm mesostructure. After functionalization of the quaternary ammonium groups onto the outer surface of SBA-15 nanoparticles,22 The P2 sample shows intense diffraction peaks similar to the P1 sample. The extracted P3 sample shows a higher XRD intensity than the P1 and P2 samples due to a higher density contrast between the mesopores and the walls of the mesostructure after removal of the surfactant. After further functionalization with APS, diffraction peaks were shifted from 2θ ≈ 0.94° to 1.00° and the intensity was decreased owing to the slight shrinking of the unit cell by thermal treatment. In the co-condensation route, the XRD pattern of the C1 sample shows very intense diffraction peaks, indicating a highly-ordered mesoporous structure. It can be inferred from the XRD patterns of the C2 and C3 samples that the highly-ordered mesoporous structures remained after the selective functionalization with quaternary ammonium groups on the outer surface of the particles and removal of the surfactant. Therefore, mesoporous nanoparticles functionalized with amino groups on the mesopore surface and quaternary ammonium groups on the outer surface have been obtained through both a post-synthesis route and a co-condensation route.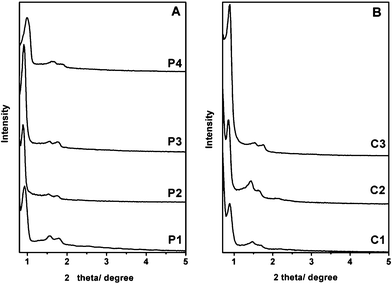 | ||
| Fig. 1 The XRD patterns of various SBA-15 mesoporous silica nanoparticles obtained during the two synthesis routes shown in Scheme 1. | ||
The SEM images of SBA-15 mesoporous silica nanoparticles are shown in Fig. 2. Upon bifunctionalization with the amino and quaternary ammonium groups, the P4 and C3 nanoparticles possess uniform rod-like morphology with a length and diameter in the range of 500–800 nm and 300–500 nm, respectively. Well separated particles were observed in both the P4 and C3 samples, indicating that a better dispersity is achieved due to the static electrical repulsion between positively charged particles from the introduced quaternary ammonium groups. Fig. 3 shows the TEM images of the P4 and C3 samples. Well ordered hexagonal arrays of mesopores and the straight lattice fringes can be seen along and perpendicular to the pore axis, confirming the existence of 2D-hexagonal p6mm symmetry. As expected, the corresponding Fourier diffractograms perpendicular to the pore axis show sharp reflections with hexagonal symmetry, suggesting excellent long-range order in the mesophase.
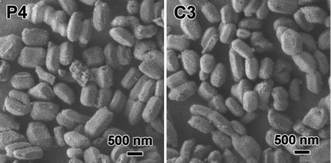 | ||
| Fig. 2 The SEM images of P4 and C3 samples. | ||
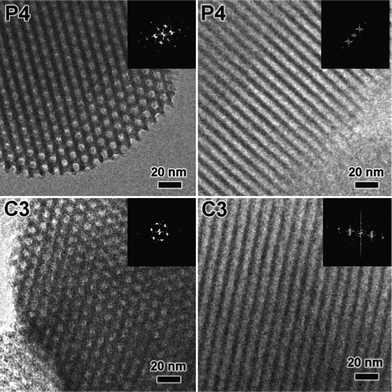 | ||
| Fig. 3 The TEM images of the P4 and C3 samples taken along the channel direction and perpendicular to the channel direction of 2D-p6mm structure. | ||
Fig. 4 shows the N2 adsorption/desorption isotherms and pore size distributions of the P4 and C3 samples. All samples show the typical IV with H1-type hysteresis loops, indicating rod-like pores in these mesoporous nanoparticles. The sharp capillary condensations in the range of relative pressure of 0.5–0.8 suggest a uniform pore size distribution. The BET surface areas of the P4 and C3 samples are 444 and 417 m2 g−1 and the pore diameters are 7.4 and 7.2 nm, respectively (Table 1). These materials have potential applications in the pH-responsive delivery system for biomedicines due to their large pore size.
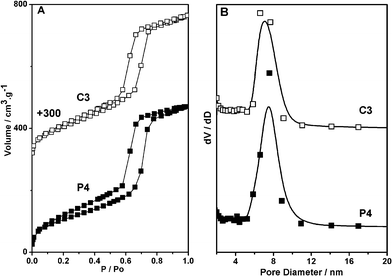 | ||
| Fig. 4 The Nitrogen adsorption/desorption isotherms and pore size distributions of the P4 and C3 samples. | ||
| Surface area (m2 g−1) a | Pore volume (cm3 g−1) b | Pore diameter (nm) c | N amount (mmol g−1) d | |
|---|---|---|---|---|
| a Calculated from N2 adsorption/desorption data. b Calculated from N2 adsorption/desorption data. c Determined by the BJH pore size distribution (based on adsorption branch of isotherms). d Determined by elemental analysis. | ||||
| P4 | 444 | 0.72 | 7.4 | 1.69 |
| C3 | 417 | 0.72 | 7.2 | 2.04 |
The functionalization of the amino and quaternary ammonium groups in the SBA-15 nanoparticles was confirmed by the solid-state 13C MAS NMR spectrum of the P4 and C3 samples (Fig. 5). No strong resonance peaks around 27 ppm, assignable to the –CH2– of the surfactant, were detected, indicating that the surfactant was completely removed. The NMR spectra of the P4 and C3 samples show three resonance signals at 9.1, 20.9 and 42.4 ppm which could be assigned to CI, CII and CIII of APS, respectively (Fig. 5). This result demonstrates that the amino groups were modified successfully in the mesoporous materials. Moreover, three additional peaks, assigned to CV, CIV, CVII and CVI of TMAPS, were observed at 8.5, 16.7, 53.3 and 68.4 ppm in the spectra of P4 and C3 samples, respectively. Therefore, it can be considered that the quaternary ammonium groups were also modified successfully in the mesoporous materials. Furthermore, the FTIR spectra and CHN elemental analysis of these samples could support these results (Fig. 6 and Table 1). The bands at 2925 and 2852 cm−1 of all samples assigned to –CH2– of APS and TMAPS were observed, and the band corresponding to quaternary ammonium groups appeared at 1550 cm−1, which overlapped with the vibration of the band N–H amino groups. The CHN elemental analysis of the P4 and C3 is shown in Table 1. For P4 and C3 samples, it can be seen that the samples were functionalized by amino groups and quaternary ammonium groups with high loadings (1.69 and 2.04 mmol g−1) via the post-synthesis method and co-condensation method, respectively.
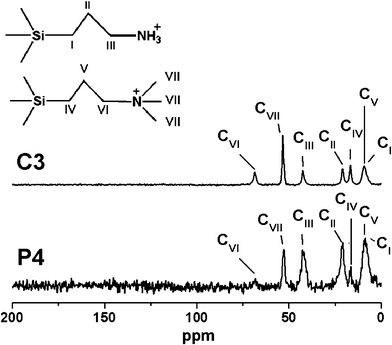 | ||
| Fig. 5 The Solid state 13C CP MAS NMR spectra of P4 and C3 samples. | ||
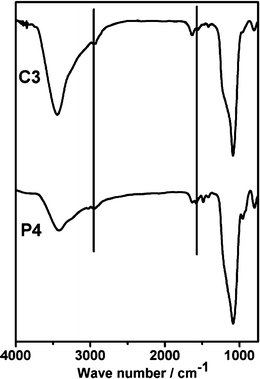 | ||
| Fig. 6 The FTIR spectra of P4 and C3 samples. | ||
3.2. pH-responsive release of anti-cancer drugs
As shown above, the P4 and C3 particles functionalized with amino groups on the mesopore surface can be considered strong candidates for carriers of this pH-responsive delivery system. BLM was used a model large molecule anti-cancer drug with amino, carbonyl and hydroxyl groups via a NH2–Fe–BLM architecture in mesopore. Iron has been utilized as a coordination center. The Fe ions were loaded into the mesopores and the coordination bonds between amino groups on the mesopore and Fe ions formed. The Fe ions of NH2–Fe complex are coordinately unsaturated due to the large mesopores and the isolated distribution of the aminopropyl groups on the pore surface, and the vacant binding sites were further filled by introducing drug molecules to form an NH2–Fe–BLM coordination bonding architecture. Fig. 7 shows a Fe (2p3/2) XPS spectra of the P4 and C3 samples after adsorption of metal ions and further adsorption of BLM. After loading of Fe3+ into the mesoporous nanoparticles, a peak centred at around 710.5 eV was observed, indicating that the Fe3+ ions were in the form of a low coordination state bonded to NH2 groups on the carrier.46 After further adsorption of BLM, the XPS peak was centered at a lower binding energy of 708 eV, which reveals an increase in the coordination number of Fe3+ and demonstrates the formation of NH2–Fe–BLM coordination bonding architecture. The P4 and C3 samples with NH2–Fe–BLM coordination bonding architecture were denoted as P4–NH2–Fe–BLM and C3–NH2–Fe–BLM.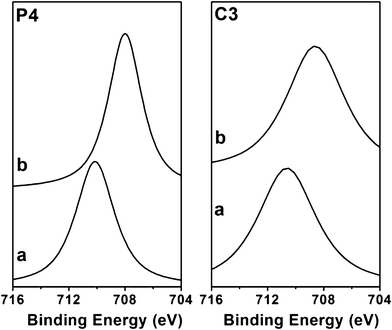 | ||
| Fig. 7 Fe (2p3/2) XPS spectra of P4 and C3 samples after adsorption of metal ions (a) and further adsorption of BLM (b). | ||
The in vitro releases of BLM from various mesoporous carriers in PBS pH 7.4, 6 and 5 have been investigated (Fig. 8). As expected, on the basis of functionalized SBA-15 particles, pH-responsive releases of BLM from all samples have been successfully achieved (Fig. 8). The drug-loading capacities of P4 and C3 particles reach as high as 93.4 and 101.5 mg g−1, respectively. Under physiological conditions (pH 7.4), a release of BLM below 10% has been detected in PBS within 12 h from the P4 and C3 samples (Fig. 8). The release percentages of BLM were 25 and 45% at PBS pH 6.0 and 43 and 71% at PBS pH 5.0 from P4–NH2–Fe–BLM and C3–NH2–Fe–BLM samples, respectively. As described above, the lower pH value triggers a release of a larger amount of drugs due to the less competitive formation of coordination bonds. The good dispersity and pH-responsive properties of the P4 and C3 samples imply the direct applicability of this pH-responsive system.
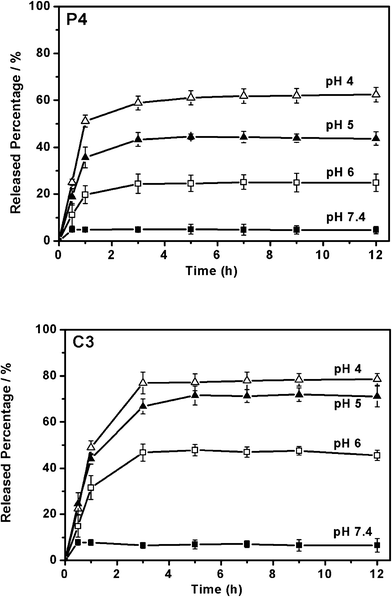 | ||
| Fig. 8 pH responsive release of BLM from P4–NH2–Fe–BLM and C3–NH2–Fe–BLM samples. | ||
The stabilities of SBA-15 nanoparticles during drug release process were confirmed (Fig. S1, Fig. S2 and Table S1, ESI†). After metal ions (Fe3+) or further BLM were loaded into the SBA-15 nanoparticles, the X-ray reflection intensity decreased due to a reduction in contrast between pores and walls. The nitrogen adsorption/desorption isotherms of the mesoporous silica carriers were close to that of the pristine one, confirming the intact mesopores. The pore volume and surface area slightly decreased, which could be reasonably attributed to the adsorption of metal ions and guest molecules. After the material was treated in PBS pH 7.4 for 6 h, the X-ray reflection intensity increased due to an increased reduction in contrast between pores and walls. The nitrogen adsorption/desorption isotherms still showed type IV features. The high porosity (surface area and pore volume) was detected by nitrogen adsorption, which confirmed that the mesopore system has been retained.
As mentioned in our previous work, the formation and cleavage of the coordination bond between metal ions and guest molecules in solution can be tested by UV-Vis spectra.46,47 However, the absorbance of the solution of Fe3+ was complex and significantly changed by the variation of the pH value due to strong interactions between Fe3+ and OH−. Therefore, it was difficult to detect the formation of the coordination bond between Fe3+ and the amino groups or Fe3+ and BLM based on the absorbance of Fe3+ by UV-Vis spectra in solution. On the basis of the solid-state UV-Vis spectra of BLM molecules, the states of the Fe–BLM coordination bonding at different stages were investigated due to differences between the free BLM molecules and the Fe–BLM complex. The solid state UV-Vis spectra of P4–NH2–Fe–BLM and C3–NH2–Fe–BLM samples treated under different pH condition are shown in Fig. 9. The band belonging to the free BLM molecules appears at 288 nm, while the band at 295 nm belonging to the complex of Fe–BLM shows no significant change, indicating the stability of the Fe–BLM complex even at PBS pH 4.0. On the other hand, as shown in Table 2, the constant loading amount of iron ions can be observed after treatment in PBS pH 7.4, indicating that NH2–Fe is highly stable under physiological conditions; however, after treatment in PBS pH 6, 5 and 4, the Fe3+ loading amounts show an obvious decrease, which could be attributed to a cleavage of the NH2–Fe3+ coordination bond. Therefore, both BLM and Fe3+ would be shed off under acidic condition. Therefore, BLM can be released from the NH2–Fe–BLM coordination bonding architecture under mildly acidic pH conditions due to the cleavage of the NH2–Fe3+ coordination bond and an improved anti-cancer activity could be achieved. It has been reported that the anti-cancer activity of BLM could be improved in the presence of Fe due to more efficient DNA degradation.55
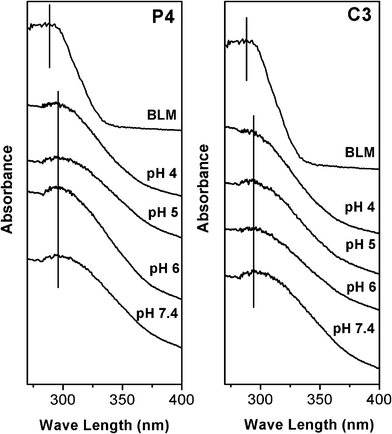 | ||
| Fig. 9 Solid state UV-Vis adsorption spectra of P4–NH2–Fe–BLM and C3–NH2–Fe–BLM samples treated in PBS solutions at different pH value. | ||
| P4–NH2–Fe–BLM sample | C3–NH2–Fe–BLM sample | |
|---|---|---|
| After adsorption of metal ions | 0.91 | 0.82 |
| Materials in pH 7.4 | 0.88 | 0.84 |
| Materials in pH 6 | 0.56 | 0.47 |
| Materials in pH 5 | 0.41 | 0.32 |
Control experiments using different carriers, with and without metal ions involved, have reconfirmed the coordination mechanism in this system (Fig. S3, ESI†). When the pure SBA-15 has been used as a carrier, a significant release of BLM molecules can be observed under physiological conditions (pH 7.4) due to the weak bonding strength of OH–Fe–BLM. Moreover, when a control experiment was conducted without any Fe3+ ions, no pH-responsibility was detected in vitro due to weak interactions between the amino group on the surface and the BLM molecules.
3.3. The dispersity of bifunctional SBA-15 nanoparticle
Normally, the dispersity of particles could determine factors in the endocytosis efficiency.43 Therefore, a good dispersity of particles plays an important role in applying these large pore mesoporous materials to the field of anti-cancer drug delivery, which needs unique efficiency of endocytosis. Fig. 10 shows the dispersions of the P4–NH2–Fe–BLM and C3–NH2–Fe–BLM samples. Both the samples remain a stable colloidal suspension in PBS at pH 7.4, indicating good dispersity of the multi-functionalized SBA-15 nanoparticles. The dispersity of particles was measured using a dynamic light scattering (DLS) technique. Specifically, both the samples show a peak ranging from 1000 to 1100 nm (Fig. 11). The average particle sizes determined with DLS (1000–1100 nm) were larger than those obtained with SEM (500–800 nm in length). This result could be attributed to the water layers attached to the surface of the mesoporous nanoparticles.43 The good dispersity of P4–NH2–Fe–BLM and C3–NH2–Fe–BLM particles could be due to the static electrical repulsion between positively charged particles introduced by TMAPS. To determine the charge present of various functionalized SBA-15 particles in PBS pH 7.4, zeta potential of functionalized SBA-15 particles in PBS pH 7.4 was measured. The zeta potentials of P4–NH2–Fe–BLM and C3–NH2–Fe–BLM samples were 33.5 and 32.4 mV at pH 7.4, respectively, indicating that both of the particles were positively charged. It is well known that the zeta value should be higher than 30 mV for maintaining the well dispersity.60 | ||
| Fig. 10 Photographs of the P4–NH2–Fe–BLM and C3–NH2–Fe–BLM samples in PBS pH 7.4. | ||
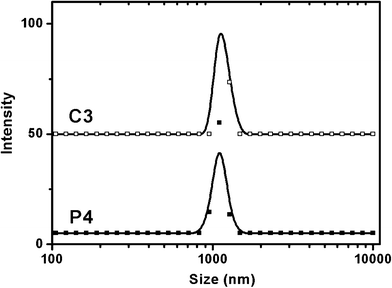 | ||
| Fig. 11 Dynamic light scattering data of P4–NH2–Fe–BLM and C3–NH2–Fe–BLM samples. | ||
3.4. Cell assays
To further investigate the cellular uptake and biocompatibility of mesoporous materials, cell assays have been conducted in Fig. 12. Calcein has been loaded into the functionalized SBA-15 particles to visually investigate the endocytosis under fluorescence confocal microscopy. SPCA-1 cells were incubated in PBS RPMI-1640 media with 10% FBS under pH 7.4 for 2 h in the presence of calcein loaded P4 and C3 particles. Distinct green fluorescence from the P4 and C3 particles were observed in the cell bodies, which is due to the high cellular uptake of the P4 and C3 particles into SPCA-1 cells. As it can be clearly observed from Fig. 12, a good dispersity of P4 and C3 particles in SPCA-1 cells indicates that the dispersities of the mesoporous particles were the determining factor in cellular uptake efficiency. On the other hand, without quaternary ammonium groups on the outer surface, the amino group functionalized mesoporous particles showed poor cellular uptake efficiency (Fig. S4, ESI†). Thus, the electrostatic interaction between positively charged SBA-15 particles and the negatively charged cellular membrane would be anticipated to enhance cellular uptake efficiency as well. Therefore, quaternary ammonium group functionalized SBA-15 particles of both P4 and C3 particles have been taken up into SPCA-1 cells to a great degree.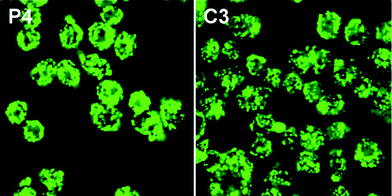 | ||
| Fig. 12 Fluorescence confocal images of SPCA-1 cells after incubation with calcein loaded P4 and C3 samples. | ||
The cytotoxic effects of P4 and C3 particles against SPCA-1 cells after 48 h incubation were studied using MTT assays (Fig. 13). The P4 and C3 particles appear slightly cytotoxic to SPCA-1 cells in concentrations of up to 1.0 mg mL−1, which could be reasonably attributed to the high cellular uptake of the particles into cells. The functionalized SBA-15 particles at various concentrations showed low cytotoxicities to SPCA-1 cells upon exposure for 24 h, demonstrating their safety as a pH-responsive delivery system. On the other hand, the inhibition effect has been observed when SPCA-1 cells were incubated with SBA-15 nanoparticles containing NH2–Fe–BLM, indicating the presence of high anti-cancer activities. Notably, C3–NH2–Fe–BLM sample present higher anti-cancer activity against SPCA-1 cells compared to that of free BLM at high concentration. This result can be attributed to an improved anti-cancer activity in the presence of Fe ions.55
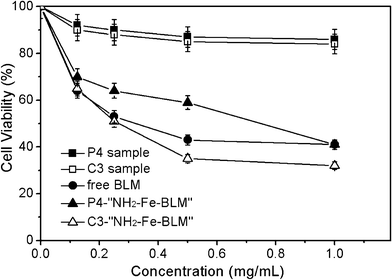 | ||
| Fig. 13 Effect of P4 and C3 samples, BLM, P4–NH2–Fe–BLM and C3–NH2–Fe–BLM on the inhibition ratio of SPCA-1 cells. | ||
4. Conclusions
In conclusion, SBA-15 nanoparticles with large pores and a small particles size have been functionalized with amino groups on the mesopore surface and quaternary ammonium groups on the outer surface by post-synthesis and co-condensation methods. The pH-responsive release of the large molecular sized BLM has been achieved from NH2–Fe–BLM coordination bonding architecture under acidic conditions (pH 4–6), while the drug delivery system was stable under physiological conditions (pH 7.4). Good dispersities could be achieved by the electrostatic repulsion of the positively charged particles. The efficient cellular uptake, low cytotoxicity of amino group/quaternary ammonium group bi-functionalized SBA-15 nanoparticles and high anti-cancer activity of the NH2–Fe–BLM loaded SBA-15 nanoparticles have been demonstrated by in vitro cell assays. This pH-responsive system could be utilized in broader applications, such as targeted delivery of the macromolecules, peptide, protein etc, due to the large pore size of the SBA-15 nanoparticles.Acknowledgements
We acknowledge the support of the 973 project (2009CB930403) of China and Grand New Drug Development Program (No. 2009ZX09310-007) of China.References
- C. d. l. H. Alarcon, S. Pennadam and C. Alexander, Chem. Soc. Rev., 2005, 34, 276–285 RSC.
- E. S. Gil and S. M. Hudson, Prog. Polym. Sci., 2004, 29, 1173–1222 CrossRef CAS.
- Y. Qiu and K. Park, Adv. Drug Delivery Rev., 2001, 53, 321–339 CrossRef CAS.
- P. Gupta, K. Vermani and S. Garg, Drug Discovery Today, 2002, 7, 569–579 CrossRef CAS.
- J. Taillefer, M. C. Jones, N. Brasseur, J. E. Van Lier and J. C. Leroux, J. Pharm. Sci., 2000, 89, 52–62 CrossRef CAS.
- J.-C. Leroux, E. Roux, D. Le Garrec, K. Hong and D. C. Drummond, J. Controlled Release, 2001, 72, 71–84 CrossRef CAS.
- E. R. Gillies and J. M. J. Frechet, Chem. Commun., 2003, 1640–1641 RSC.
- M. B. Yatvin, W. Kreutz, B. A. Horwitz and M. Shinitzky, Science, 1980, 210, 1253–1255 CAS.
- D. C. Drummond, M. Zignani and J.-C. Leroux, Prog. Lipid Res., 2000, 39, 409–460 CrossRef CAS.
- X. Guo and F. C. Szoka, Acc. Chem. Res., 2003, 36, 335–341 CrossRef CAS.
- S.-M. Lee, H. Chen, C. M. Dettmer, T. V. O'Halloran and S. T. Nguyen, J. Am. Chem. Soc., 2007, 129, 15096–15097 CrossRef CAS.
- R. Casasús, M. D. Marcos, R. Martínez-Máñez, J. V. Ros-Lis, J. Soto, L. A. Villaescusa, P. Amorós, D. Beltrán, C. Guillem and J. Latorre, J. Am. Chem. Soc., 2004, 126, 8612–8613 CrossRef.
- R. Casasús, E. Climent, M. D. Marcos, R. Martínez-Máñez, F. Sancenón, J. Soto, P. Amorós, J. Cano and E. Ruiz, J. Am. Chem. Soc., 2008, 130, 1903–1917 CrossRef.
- Y. Zhu, J. Shi, W. Shen, X. Dong, J. Feng, M. Ruan and Y. Li, Angew. Chem., Int. Ed., 2005, 44, 5083–5087 CrossRef CAS.
- Q. Yang, S. Wang, P. Fan, L. Wang, Y. Di, K. Lin and F.-S. Xiao, Chem. Mater., 2005, 17, 5999–6003 CrossRef CAS.
- S. W. Song, K. Hidajat and S. Kawi, Chem. Commun., 2007, 4396–4398 RSC.
- W. Xu, Q. Gao, Y. Xu, D. Wu and Y. Sun, Mater. Res. Bull., 2009, 44, 606–612 CrossRef CAS.
- T. D. Nguyen, K. C. F. Leung, M. Liong, C. D. Pentecost, J. F. Stoddart and J. I. Zink, Org. Lett., 2006, 8, 3363–3366 CrossRef CAS.
- K. C. F. Leung, T. D. Nguyen, J. F. Stoddart and J. I. Zink, Chem. Mater., 2006, 18, 5919–5928 CrossRef CAS.
- C. R. Thomas, D. P. Ferris, J.-H. Lee, E. Choi, M. H. Cho, E. S. Kim, J. F. Stoddart, J.-S. Shin, J. Cheon and J. I. Zink, J. Am. Chem. Soc., 2010, 132, 10623–10625 CrossRef CAS.
- V. Cauda, C. Argyo and T. Bein, J. Mater. Chem., 2010, 20, 8693–8699 RSC.
- C. Park, K. Oh, S. C. Lee and C. Kim, Angew. Chem., Int. Ed., 2007, 46, 1455–1457 CrossRef CAS.
- C.-H. Lee, L.-W. Lo, C.-Y. Mou and C.-S. Yang, Adv. Funct. Mater., 2008, 18, 3283–3292 CrossRef CAS.
- B. Wang, C. Xu, J. Xie, Z. Yang and S. Sun, J. Am. Chem. Soc., 2008, 130, 14436–14437 CrossRef CAS.
- Z. Zhou, S. Zhu and D. Zhang, J. Mater. Chem., 2007, 17, 2428–2433 RSC.
- N. K. Mal, M. Fujiwara, Y. Tanaka, T. Taguchi and M. Matsukata, Chem. Mater., 2003, 15, 3385–3394 CrossRef CAS.
- T. D. Nguyen, K. C. F. Leung, M. Liong, Y. Liu, J. F. Stoddart and J. I. Zink, Adv. Funct. Mater., 2007, 17, 2101–2110 CrossRef CAS.
- C. Park, K. Lee and C. Kim, Angew. Chem., Int. Ed., 2009, 48, 1275–1278 CrossRef CAS.
- C.-Y. Lai, B. G. Trewyn, D. M. Jeftinija, K. Jeftinija, S. Xu, S. Jeftinija and V. S. Y. Lin, J. Am. Chem. Soc., 2003, 125, 4451–4459 CrossRef CAS.
- A. Schlossbauer, J. Kecht and T. Bein, Angew. Chem., Int. Ed., 2009, 48, 3092–3095 CrossRef CAS.
- K. Engin, D. B. Leeper, J. R. Cater, A. J. Thistlethwaite, L. Tupchong and J. D. McFarlane, Int. J. Hyperthermia, 1995, 11, 211–216 CrossRef CAS.
- M. Vallet-Regi, A. Rámila, R. P. del Real and J. Pérez-Pariente, Chem. Mater., 2001, 13, 308–311 CrossRef CAS.
- M. Vallet-Regí, Chem.–Eur. J., 2006, 12, 5934–5943 CrossRef.
- M. Vallet-Regí, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548–7558 CrossRef.
- I. I. Slowing, B. G. Trewyn, S. Giri and V. S. Y. Lin, Adv. Funct. Mater., 2007, 17, 1225–1236 CrossRef CAS.
- W. Xia and J. Chang, J. Controlled Release, 2006, 110, 522–530 CrossRef CAS.
- C.-P. Tsai, C.-Y. Chen, Y. Hung, F.-H. Chang and C.-Y. Mou, J. Mater. Chem., 2009, 19, 5737–5743 RSC.
- F. Qu, G. Zhu, S. Huang, S. Li, J. Sun, D. Zhang and S. Qiu, Microporous Mesoporous Mater., 2006, 92, 1–9 CrossRef CAS.
- H. H. P. Yiu and P. A. Wright, J. Mater. Chem., 2005, 15, 3690–3700 RSC.
- J. Andersson, J. Rosenholm, S. Areva and M. Lindén, Chem. Mater., 2004, 16, 4160–4167 CrossRef CAS.
- V. Cauda, L. Mühlstein, B. Onida and T. Bein, Microporous Mesoporous Mater., 2009, 118, 435–442 CrossRef CAS.
- V. Cauda, C. Argyo, A. Schlossbauer and T. Bein, J. Mater. Chem., 2010, 20, 4305–4311 RSC.
- B. G. Trewyn, J. A. Nieweg, Y. Zhao and V. S. Y. Lin, Chem. Eng. J., 2008, 137, 23–29 CrossRef CAS.
- C. Gao, I. Izquierdo-Barba, I. Nakase, S. Futaki, J. Ruan, K. Sakamoto, Y. Sakamoto, K. Kuroda, O. Terasaki and S. Che, Microporous Mesoporous Mater., 2009, 122, 201–207 CrossRef CAS.
- C. Charnay, S. Bégu, C. Tourné-Péteilh, L. Nicole, D. A. Lerner and J. M. Devoisselle, Eur. J. Pharm. Biopharm., 2004, 57, 533–540 CrossRef CAS.
- C. Gao, H. Zheng, L. Xing, M. Shu and S. Che, Chem. Mater., 2010, 22, 5437–5444 CrossRef CAS.
- H. Zheng, C. Gao, B. Peng, M. Shu and S. Che, J. Phys. Chem. C, 2011, 115, 7230–7237 CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- L. B. Fagundes, T. G. F. Sousa, A. Sousa, V. V. Silva and E. M. B. Sousa, J. Non-Cryst. Solids, 2006, 352, 3496–3501 CrossRef CAS.
- C.-m. Yang, Y. Wang, B. Zibrowius and F. Schuth, Phys. Chem. Chem. Phys., 2004, 6, 2461–2467 RSC.
- A. Stein, B. J. Melde and R. C. Schroden, Adv. Mater., 2000, 12, 1403–1419 CrossRef CAS.
- C.-m. Yang, B. Zibrowius and F. Schuth, Chem. Commun., 2003, 1772–1773 RSC.
- A. Sayari and S. Hamoudi, Chem. Mater., 2001, 13, 3151–3168 CrossRef CAS.
- G. M. Ehrenfeld, J. B. Shipley, D. C. Heimbrook, H. Sugiyama, E. C. Long, J. H. Van Boom, G. A. Van der Marel, N. J. Oppenheimer and S. M. Hecht, Biochemistry, 1987, 26, 931–942 CrossRef CAS.
- N. J. Oppenheimer, C. Chang, L. H. Chang, G. Ehrenfeld, L. O. Rodriguez and S. M. Hecht, J. Biol. Chem., 1982, 257, 1606–1609 CAS.
- M. Chien, A. P. Grollman and S. B. Horwitz, Biochemistry, 1977, 16, 3641–3647 CrossRef CAS.
- Q. Liu, Y. Li, S. Shen, Z. Zhou, B. Ou and S. Tang, J. Macromol. Sci., Part A: Pure Appl. Chem., 2011, 48, 518–525 CrossRef CAS.
- L. M. Bareford and P. W. Swaan, Adv. Drug Delivery Rev., 2007, 59, 748–758 CrossRef CAS.
- M. Nakanishi and A. Noguchi, Adv. Drug Delivery Rev., 2001, 52, 197–207 CrossRef CAS.
- C. Lei, Y. Shin, J. Liu and E. J. Ackerman, J. Am. Chem. Soc., 2002, 124, 11242–11243 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: characterizations (XRD, N2 adsorption–desorption) of the carrier material during drug release, porous and compositional properties and N loading amount, control release experiment, cell uptake assay of amino functionalized materials. See DOI: 10.1039/c2ra20380d/ |
| This journal is © The Royal Society of Chemistry 2012 |
