ZrO2-incorporated Bi6O6(OH)3(NO3)3·1.5H2O with superior photocatalytic activity for degradation of malachite green†
Liyan
Xie
,
Jixin
Wang
,
Yanhua
Hu
,
Shuying
Zhu
,
Zuyang
Zheng
,
Sunxian
Weng
and
Ping
Liu
*
State Key Laboratory Breeding Base of Photocatalysis, Research Institute of Photocatalysis, Fuzhou University, Fuzhou, 350002, P. R. China. E-mail: liuping@fzu.edu.cn (P. Liu); Fax: +86-591-8377-9105; Tel: +86-591-8377-9239
First published on 20th August 2012
Abstract
Bi6O6(OH)3(NO3)3·1.5H2O and ZrO2-modified Bi6O6(OH)3(NO3)3·1.5H2O as novel photocatalysts were successfully synthesized via a facile hydrothermal method and utilized in aqueous solution for the photocatalytic degradation of malachite green. The prepared samples were characterized by X-ray diffraction, BET surface area analysis and UV-vis reflectance spectrum. Comparing with the samples synthesized via bare Bi6O6(OH)3(NO3)3·1.5H2O, the ZrO2-modified sample exhibits higher catalytic activity and more durable stability in the photodegradation of malachite green. The electronic band structure was determined by the combination of optical absorption spectra and Mott–Schottky plots. Base on the research of structural characterization, band gap structure and electrochemical tests, the possible reasons for enhance photocatalytic performance by ZrO2-incorporation were proposed. It has been considered that the large specific surface area, more positive position of valance band and higher migration rate of photogenerated carriers have strong influence on the improvement of photocatalytic activity. Moreover, the ZrO2-modification delays photo corrosion behavior resulting in higher stability under irradiation.
1. Introduction
Nowadays, heterogeneous photocatalysis has become one of the most promising technologies for environment remediation because it is environmentally friendly, capable of performing at room temperature and it can treat organic contaminants at very low concentration.1,2 The effective photocatalytic treatment of all kinds of organic contaminants requires that the photocatalyst should have high efficiency and long life. Therefore, a tremendous effort was made in developing new photocatalysts during the past decade,3 including metal or non-metal ions doped TiO2, metal oxides or metal sulfide semiconductors. However, some photocatalysts show low activity, while others lack the catalytic stability, or require rigorous synthetic conditions. Developing a new efficient photocatalyst is still a great challenge in the photocatalysis field so far.Among the numerous semiconductors, bismuth compounds have received a lot of attention in environmental remediation over the past few years. For example the photocatalysts Bi2O3, either in the single phase 4,5 or in combination with other materials into heterojunctions,6,7 exhibits high efficiency in the degradation of organic pollutants. Bismuth-based binary metal salts, such as BiVO4,8–11 Bi2WO6,12–15 Bi2SiO5,16 and Bi4Si3O1217 were also investigated considerably. An important driving force for the growing interest in these environmentally relevant applications of bismuth is the relatively low toxicity of bismuth compounds as compared to related species containing heavy metals such as Hg, In, Cd, Sn or Pb. For the merits of simple, low-cost and low toxicity, bismuth-containing materials seem to be promising candidates in environmental remediation. While in the large family of bismuth compounds, rare reports are published on the photocatalytic performance about basic bismuth nitrates. The basic bismuth nitrates is a big family with more than 15 different structures.18,19 And in our previous work, it is found that one of the basic bismuth nitrates Bi6O6(OH)3(NO3)3·1.5H2O synthesized under conventional hydrothermal conditions could act as a novel photocatalyst in the degradation of dyes but was still unsatisfactory. As all we know, the modification or incorporation strategy has been employed in semiconductors to achieve the goal of improving photocatalytic activity.20–23
Among the varieties of modifiers, ZrO2 is one of the promising candidates adopted for the fabrication of catalysts. It has been successfully used in the modification and lifetime improvement of catalysts due to its high chemical stability.24 Moreover, the modification of zirconia has been shown to produce a better photocatalyst for the oxidation of ethylene.25 Similarly it is reported as a potential promoter in enhancing a visible-light photocatalyst TiO2−xNx for the oxidation of gaseous organic compounds.26 Recently ZrO2-modified TaON with improved photocatalytic hydrogen evolution activity was investigated under visible light.27
In this manuscript, we synthesized one of the bismuth basic nitrates Bi6O6(OH)3(NO3)3·1.5H2O via hydrothermal process and adopted ZrO2 to incorporate with Bi6O6(OH)3(NO3)3·1.5H2O. The photocatalytic performance for the degradation of a typical cationic dye malachite green (MG) over the Bi6O6(OH)3(NO3)3·1.5H2O and the ZrO2-modified sample is investigated. Furthermore, the explanation for the enhanced photocatalytic performance of ZrO2-modified sample is provided based on the results of XRD, BET, photocurrent test amongst others.
2. Experimental
2.1 Preparation
Bi(NO3)3·5H2O was used as starting material for the syntheses, which was AR grade and used without further purification. In the procedure of synthesizing Bi6O6(OH)3(NO3)3·1.5H2O, 1.225 g Bi(NO3)3·5H2O is dissolved in 65 mL de-ionized water under stirring. The pH value of the slurry is adjusted to different pH values by the slow dripping of 4 mol L−1 NaOH solution. After stirring for an hour, the solution was transferred into a Teflon lined (80 mL) stainless autoclave; and filled to about 70% volume of the Teflon line. Then the autoclave is sealed and heated under autogenous pressure at 180 °C for 24 h and cooled to room temperature naturally. The solid products are collected and washed repeatedly with de-ionized water and absolute ethanol and then dried at 60 °C for 12 h. White powder of Bi6O6(OH)3(NO3)3·1.5H2O (denoted as BHN) is obtained. The ZrO2-modified Bi6O6(OH)3(NO3)3·1.5H2O (denoted as Z-BHN) is prepared by the hydrothermal reaction of 1.225 g Bi(NO3)3·5H2O and 0.4 g Zr(OH)4 at 180 °C for 24 h at pH 5. The mechanical mixture was obtained by mixing Bi6O6(OH)3(NO3)3·1.5H2O and the hydrothermal of product Zr(OH)4 together.2.2 Characterization
X-Ray diffraction patterns were collected on a Bruker D8 Advance X-ray diffractometer with Cu-Kα radiation. The acceleration voltage and the applied current were 40 kV and 40 mA, respectively. UV-visible absorption spectra (UV-DRS) of the powders were obtained using a UV-visible spectrophotometer (Cary 500 Scan Spectrophotometers, Varian, USA). BaSO4 was used as a reflectance standard in the UV-visible diffuse reflectance experiment. The specific surface area of the samples was measured by nitrogen sorption at 77 K on ASAP 2020 instrument and calculated by the BET method. The electrochemical Mott–Schottky plots were obtained with a ZENNIUM electrochemical workstation (Zahner, Germany) equipped with an impedance analyzer. The photocurrent measurements were conducted on a BAS Epsilon workstation.2.3 Photocatalytic activity measurements
The photocatalytic degradations of dyes in aqueous solution are conducted in a quartz tube with 4 cm inner diameter and an 18 cm length. Three 4 W UV lamps with a wavelength centered at 254 nm (Philips, TUV 4W/G4T5) are used as the illuminating source. 160 mg of powdered photocatalyst is suspended in 160 mL of MG (20 mg L−1) solution. The suspensions are stirred in dark for 120 min to ensure adsorption–desorption equilibrium prior to irradiation. An aliquot (3 mL) is taken at a certain time interval during the experiment and centrifuged (TDL-5-A) to remove the powders. The filtrates are analyzed on a Varian UV-vis spectrophotometer (Cary-50, Varian Co.). The percentage of degradation is reported as C/C0. C is the absorption of MG at each irradiated time interval of the maximum peak of the absorption spectrum. C0 is the absorption of the starting concentration when adsorption–desorption equilibrium was achieved. Total organic carbon (TOC) assays of the degraded solution after 60 min of irradiation are carried out on a TOC analyzer (TOC-VCPH, Shimadzu).3. Results and discussion
As reported in some papers, ZrO2 can be synthesized by a hydrothermal method.28,29 The XRD pattern (seen in Fig. S1†) confirms the ZrO2 obtained by hydrothermal reaction of Zr(OH)4 matches with the tetragonal ZrO2 with JCPDS card number 01-079-1771. With respect to Bi6O6(OH)3(NO3)3·1.5H2O, the pH value as an important reaction condition is investigated in order to carry out controlled synthesis. It is observed that pH value plays an important role in controlling the composition of final products. As shown in Fig. S2,† Bi6O6(OH)3(NO3)3·1.5H2O (JCPDS no.53-1038) can be obtained for the pH value in the region of 3 to 7 which indicates that Bi6O6(OH)3(NO3)3·1.5H2O can be synthesized under acidic and neutral pH conditions. Moreover the distinct peaks at 10.34°, 25.56°, 31.38° match with the (002), (102), (006) crystal planes of Bi6O6(OH)3(NO3)3·1.5H2O, respectively. When the ZrO2-modification is carried out on Bi6O6(OH)3(NO3)3·1.5H2O, as shown in Fig. 1 (a), XRD patterns of the ZrO2-modified sample (Z-BHN) are almost the same as pure phase Bi6O6(OH)3(NO3)3·1.5H2O (BHN) and no peaks for ZrO2 were observed. This phenomenon may be attributed to the following points. First, under the same detecting conditions the diffraction intensity of ZrO2 is much lower than that of BHN as shown in the Fig. S1.† And, the main peak of ZrO2 is closer to the peak of BHN. Therefore, no peak for ZrO2 can be observed in Fig. 1(a). Since the patterns of Z-BHN turn out to be overlying on the spectra for ZrO2 and BHN. For further comparison, the XRD patterns of the mechanical mixture of BHN and ZrO2 are investigated. As seen in Fig. 1 (b) and (c), comparing with the pure BHN, the peaks of the mechanical mixture did not show any shift. Therefore it can be deduced that the as-prepared material Z-BHN synthesized from the hydrothermal method is not the simple mechanical mixture of BHN and ZrO2.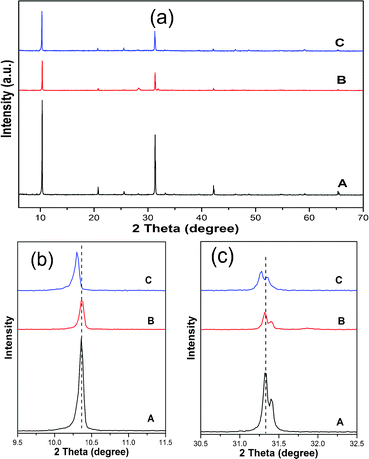 | ||
| Fig. 1 XRD patterns of the samples: (a) pure BHN at 180 °C for 24 h with pH = 5; (b) mechanical mixture of ZrO2 and BHN; (c) Z-BHN with pH = 5. | ||
While as the patterns were enlarged, as seen in Fig. 1 (b) and (c), the diffraction peaks of Z-BHN shift to low angles with the addition of zirconia. Considering that the ionic radius of Zr4+ (0.84 Å) is smaller than Bi3+ (0.96 Å), if Zr4+ is doped in BHN by substituting Bi3+ in the lattice, the diffraction peaks should shift to higher angle. Based on this results, the possibility of Zr4+ doping can be excluded. Therefore it is speculated that the ZrO2 is incorporated with Bi6O6(OH)3(NO3)3·1.5H2O in the way of lattice mismatch growth instead of Zr4+ doping. According to Christensen,30 Bi6O6(OH)3(NO3)3·1.5H2O has a tetragonal structure with lattice constants of a = b = 3.8175, c = 17.1490. While the lattice constants of ZrO2 (JCPDS no. 01-079-1771) are of a tetragonal crystal structure with a = b = 3.5916, c = 5.1790. As both materials have a tetragonal structure with similar lattice parameters, the incorporation of these two materials by lattice mismatch growth becomes a favorable process. The lattice misfit rate on the interface of the two different phases calculated from eqn (1) is 6%. In short, due to the similar lattice parameters and small lattice misfit rate, it is possible that ZrO2 and BHN are grown epitaxially along with each other. So both ZrO2 and Bi6O6(OH)3(NO3)3·1.5H2O keep their crystal structure individuality. Simultaneously there may be some defects in the junction or interface between these two substances, which may be conductive to the enhancement of photocatalytic activity.
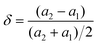 | (1) |
The UV-DRS spectra of BHN, Z-BHN, ZrO2 and the mechanical mixture are shown in Fig. 2. The onset of the adsorption edge for ZrO2 is 246 nm, which is consistent with the band gap of 5.0 eV. The absorption edge of the pure BHN sample and the ZrO2-modified sample at ca. 375 nm and ca. 342 nm, correspond to band gap energy of about 3.38 eV and 3.52 eV, respectively. This indicates that the modification of ZrO2 lead to the small blue-shift of absorption edge of BHN. It is also found that the mechanical mixture has two absorption peaks, the sharp one attributed to the ZrO2 and the broad one attributed to BHN. As the mechanical mixing is equivalent to the dilution of pure BHN, the absorption strength of the mechanical mixture is lower than the pure BHN sample. The optical absorption of the ZrO2-modified sample was quite different from the mechanical mixture, which would be further proof that the ZrO2-modified BHN sample synthesized by hydrothermal is different from the mechanical mixture of ZrO2 and BHN.
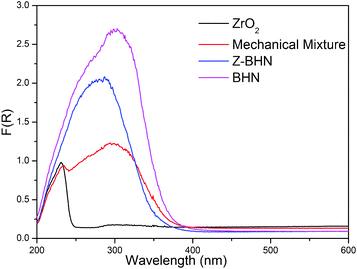 | ||
| Fig. 2 UV-DRS spectra of BHN, Z-BHN, ZrO2 and the mechanical mixture. | ||
The photocatalytic activity of as-prepared BHN and Z-BHN was evaluated by the degradation of a typical cationic dye malachite green (MG) under UV light irradiation. The original concentration of MG was 20 mg L−1. Temporal changes in the concentration of MG as monitored by the maximal absorption in the UV-Vis spectra at 618 nm are shown in Fig. 3 (A). MG is completely discolored in the presence of Z-BHN in 60 min, while 27% remains over BHN in the same time. The temporal evolution of the spectral changes taking place during the photodegradation of MG over Z-BHN are shown in Fig. 3 (B). Furthermore, the extent of mineralization is evaluated by measuring the total organic carbon (TOC). After UV irradiation for 60 min, the TOC value of the final solution for Z-BHN is 4.511 mg L−1, while it is 8.955 mg L−1 for BHN. The corresponding mineralization efficiency is evaluated to be ca. 70% for Z-BHN and 40% for BHN. Moreover, for the purpose of practical application, the catalytic stability of the samples BHN and Z-BHN are examined by recycling. The results show that the performance of Z-BHN is almost the same as that of the first time even at the fifth run of photo-decomposition (Fig. 4 (A)), while BHN lost its photocatalytic activity from the second run (Fig. 4(B)). This comparison demonstrates that the ZrO2-modification can improve the photocatalytic stability of BHN. Therefore, ZrO2-modification is a good strategy for BHN derivatization to enhance the photocatalytic efficiency and improve stability of the photocatalyst as well.
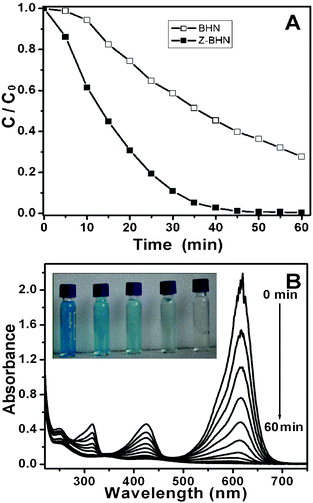 | ||
| Fig. 3 (A) Temporal changes of MG concentration as monitored by the UV-vis absorption spectra at 618 nm over illuminated BHN and illuminated Z-BHN; (B) temporal absorption spectral patterns of MG during the photodegradation process over the as-prepared Z-BHN; and the inset in B is the photo of MG at different irradiation times in the presence of Z-BHN (from left to right, 0 min, 10 min, 20 min, 30 min, 40min, respectively). | ||
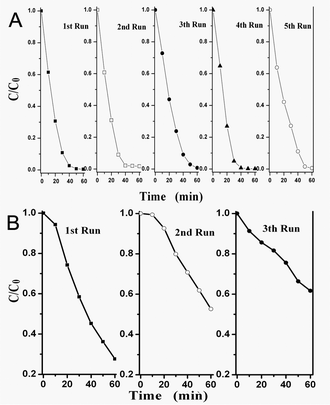 | ||
| Fig. 4 Cycling runs in the photocatalytic degradation of MG over illuminated Z-BHN (A) and BHN (B). | ||
According to the above information, the ZrO2-modified sample not only improves photocatalytic activity but also prolongs the catalyst life in the photodegradation of MG. While as we know, ZrO2 is inactive under UV light for its intrinsic light absorption. So why does the ZrO2-modified sample exhibit much higher photocatalytic efficiency and longer catalyst life time for MG than the pure BHN sample?
For the improvement of photocatalytic performance, there may be several factors listed below. Firstly, the specific surface area is always an important influencing factor in photocatalysis. Test results show that the BET specific surface areas for the as-prepared BHN and Z-BHN are ca. 1 m2 g−1 and ca. 108 m2 g−1 respectively. In order to describe the photocatalytic performance more accurately, the initial reaction rate of photocatalytic degradation is investigated. For the low concentration of the original solution, the photocatalytic reactions are supposed to accord with a first-order reaction kinetics model and the results are shown in Fig. S3.† However, the degradation constant of Z-BHN (k'Z-BHN = 9.42 × 10−4) is much lower than that of BHN (k'BHN = 0.022) after normalization with surface areas. It is implied that the specific surface area plays a key role in the improvement of the photocatalytic performance for the ZrO2-modified sample and the specific surface area of the photocatalyst will be enhanced by the ZrO2 modifier. The increment of BET specific surface area is in favor of the absorption of a substrate and the incident light as well, sequentially contributing to the forward photo oxidative reaction.
Secondly, the ZrO2-modification may lead to some changes in the electronic band structure. According to the UV-vis spectra, the band gap energy of BHN and Z-BHN is about 3.38 eV and 3.52 eV, respectively. For bismuth(III)-based semiconductors, it was also found that the Bi 6s and O 2p levels can form a preferable hybridized valence band.4,31,32 According to the depiction, it is assumed that the valence band of BHN is composed of hybridized Bi 6s and O 2p orbitals, whereas the conduction band is composed of Bi 6p orbitals. In order to determine the band gap structure of BHN, electrochemical investigations were carried out. The Mott–Schottky plots in Fig. 5 reveals typical n-type characteristics for BHN. Obviously the flat band potential of BHN is identical with that of Z-BHN at −1.10 eV (vs Ag/AgCl at pH 6.6), which means that Z-BHN has the same conduction band as the BHN sample. Combining with the UV-DRS spectra, the valance band is determined to be 2.48 eV (vs NHE) and 2.62 eV (vs NHE) for BHN and Z-BHN as depicted in Fig. 6, respectively. The valence band of ZrO2-incorporation is 0.14 V more positive than BHN, which implies much stronger oxidizing ability for degrading organic pollutants more effectively. In other words, the photogenerated holes with stronger oxidizability will give rise to higher photocatalytic activity and more complete mineralization.
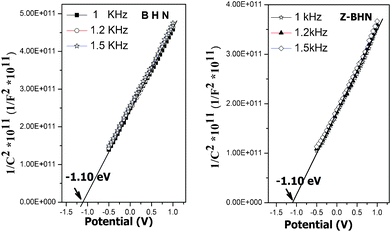 | ||
| Fig. 5 Electrochemical Mott–Schottky plots of BHN and Z-BHN. | ||
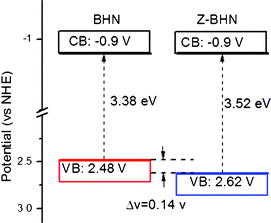 | ||
| Fig. 6 Electronic band structure of BHN and Z-BHN. | ||
Finally, it is speculated that when ZrO2 is grown epitaxially along with the BHN crystal, the incorporation of ZrO2 and BHN may generate some kind of defects on the incorporated interface or aggravate the distortion of the Bi–O polyhedron. According to other reports,33,34 crystal defects are in favor of photocatalytic performance. Therefore, the existence of these defects produced by distortion or the epitaxial growth interface may be propitious to the migration of photon-generated carriers when the catalysts were illuminated. Photocurrent measurements were carried out in order to verify our viewpoint. As can be seen in Fig. 7, when the light is on, the photocurrent generated by Z-BHN is 3-fold higher than that by BHN, which indicates that the modification of zirconia into BHN is in favor of the migration of photo-generated carriers. And when the light is off, the decline of photocurrent of Z-BHN is slower than BHN, which implies that the recombination of photo-generated electrons and holes is slower in the Z-BHN sample. Although the nature of defects in Z-BHN is not clear at present, the defects are definitely an important issue in exploiting a high efficient semiconductor photocatalyst for environmental purification. To sum up, the junctions induced by ZrO2-modification facilitates the effective separation of photo-generated carriers, thus enhances the photocatalytic activity.
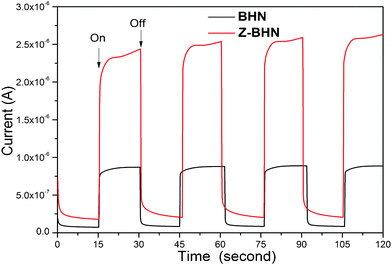 | ||
| Fig. 7 Photocurrent changes of BHN and Z-BHN sample with incident light on and off. | ||
As for the life time of the photocatalysts, the stability of the relevant catalysts after photodegradation is investigated by XRD characterization. As can be observed in Fig. 8, after 1 h of photocatalytic degradation for MG, both BHN (Fig. 8 (b)) and Z-BHN (Fig. 8 (d)) are found to be the same as the fresh sample. While extending the irradiation time to 5 h, it is surprisingly discovered that the XRD pattern of BHN presents some kind of impurity which is identified as Bi2(CO2)O3 (JCPDS no. 00-001-0884). Therefore, it is suggested that the main reason for BHN losing its photocatalytic activity in the second run is the phase transformation of its surface state. The BHN sample suffers from photo corrosion and the new substance of Bi2(CO2)O3 is generated on its surface. In contrast the XRD pattern of Z-BHN after 5 h irradiation is still identical to the fresh, which means that the stability of Z-BHN is much better than that of BHN, or at least the product of photo corrosion over Z-BHN is beyond XRD detection. Consequently, it is inferred that the ZrO2-modification to BHN delays the photo corrosion behavior and thus makes the photodegradation life time longer. The only mechanism we can surmise is that the large surface area caused by ZrO2-modification will provide a large platform where there are more active sites, meanwhile the chances for products (such as CO32−) to chelate with Bi species decrease.
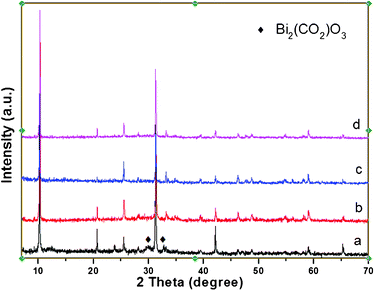 | ||
| Fig. 8 XRD pattern of photocatalysts with different illumination times, (a) BHN irradiated for 5 h, (b) BHN irradiated for 1 h; (c) Z-BHN irradiated for 5 h, (d) Z-BHN irradiation for 1 h. | ||
4. Conclusions
The basic bismuth nitrate, Bi6O6(OH)3(NO3)3·1.5H2O has been synthesized by a facile hydrothermal method. When ZrO2 is introduced into Bi6O6(OH)3(NO3)3·1.5H2O, the incorporation material Z-BHN gets higher catalytic activity and longer catalytic life in the photocatalytic degradation of malachite green. Meanwhile, the roles of ZrO2 modification during the photocatalysis are analyzed in detail. Results show that the enhanced photocatalytic performance of Z-BHN is attributed to the larger specific surface area, photo generated holes with stronger oxidative power, more effective separation of photo-induced carriers. And longer photocatalytic lifetime of Z-BHN lies in that Z-BHN is relatively more stable under irradiation.Acknowledgements
The work is supported by the National Natural Science Foundation of China (U1033603, 211730461, 21033003, 20977016), National Basic Research Program of China (973 Program: 2007CB613306 and 2010CB234604) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT0818).References
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1 CrossRef CAS.
- S. Ouyang and J. Ye, J. Am. Chem. Soc., 2011, 133, 7757 CrossRef CAS.
- L. Zhang, W. Wang, J. Yang, Z. Chen, W. Zhang, L. Zhou and S. Liu, Appl. Catal., A, 2006, 308, 105 CrossRef CAS.
- H. Lu, S. Wang, L. Zhao, B. Dong, Z. Xu and J. Li, RSC Adv., 2012, 2, 3374 RSC.
- A. Hameed, T. Montini, V. Gombac and P. Fornasiero, J. Am. Chem. Soc., 2008, 130, 9658 CrossRef CAS.
- S. Y. Chai, Y. J. Kim, M. H. Jung, A. K. Chakraborty, D. Jung and W. I. Lee, J. Catal., 2009, 262, 144 CrossRef CAS.
- D. Ke, T. Peng, L. Ma, P. Cai and K. Dai, Inorg. Chem., 2009, 48, 4685 CrossRef CAS.
- H. Jiang, H. Dai, X. Meng, K. Ji, L. Zhang and J. Deng, Appl. Catal., B, 2011, 105, 326 CrossRef CAS.
- A. Iwase and A. Kudo, J. Mater. Chem., 2010, 20, 7536 RSC.
- Z. Wang, W. Luo, S. Yan, J. Feng, Z. Zhao, Y. Zhu, Z. Li and Z. Zou, CrystEngComm, 2011, 13, 2500 RSC.
- L. Zhang, H. Wang, Z. Chen, P. K. Wong and J. Liu, Appl. Catal. B: Environ., 2011, 106, 1 CAS.
- M. Shang, W. Wang, S. Sun, L. Zhou and L. Zhang, J. Phys. Chem. C, 2008, 112, 10407 CAS.
- G. Colon, S. Murcia Lopez, M. C. Hidalgo and J. A. Navio, Chem. Commun., 2010, 46, 4809 RSC.
- Y. Li, J. Liu, X. Huang and J. Yu, Dalton Trans., 2010, 39, 3420 RSC.
- R. Chen, J. Bi, L. Wu, W. Wang, Z. Li and X. Fu, Inorg. Chem., 2009, 48, 9072 CrossRef CAS.
- P. Zhang, J. Hu and J. Li, RSC Adv., 2011, 1, 1072 RSC.
- N. Henry, M. Evain, P. Deniard, S. Jobic, O. Mentré and F. Abraham, J. Solid State Chem., 2003, 176, 127 CrossRef CAS.
- P. Pascal, Nouveau Traitéde Chimie Minérale, Masson, Paris, 1958, vol. XI, pp. 795 Search PubMed.
- G. Colón, M. C. Hidalgo and J. A. Navío, Appl. Catal., A, 2002, 231, 185 CrossRef.
- S. Yan, H. Yu, N. Wang, Z. Li and Z. Zou, Chem. Commun., 2012, 48, 1048 RSC.
- J. Bandara, C. P. K. Udawatta and C. S. K. Rajapakse, Photochem. Photobiol. Sci., 2005, 4 Search PubMed.
- F. Zhang, K. Maeda, T. Takata and K. Domen, Chem. Commun., 2010, 46, 7313 RSC.
- S. F. Wu and L. L. Wang, Int. J. Hydrogen Energy, 2010, 35, 6518 CrossRef CAS.
- X. Fu, L. A. Clark, Q. Yang and M. A. Anderson, Environ. Sci. Technol., 1996, 30, 647 CrossRef CAS.
- X. Wang, J. C. Yu, Y. Chen, L. Wu and X. Fu, Environ. Sci. Technol., 2006, 40, 2369 CrossRef CAS.
- S. S. K. Ma, K. Maeda and K. Domen, Catal. Sci. Technol., 2012, 2, 818 CAS.
- R. A. Kimel and J. H. Adair, J. Am. Ceram. Soc., 2005, 88, 1133 CrossRef CAS.
- R. P. Denkewicz Jr., K. S. TenHuisen and J. H. Adair, J. Mater. Res., 1990, 5, 2698 CrossRef.
- A. N. Christensen, M.-A. Chevallier, J. Skibsted and B. B. Iversen, J. Chem. Soc., Dalton Trans., 2000, 265 RSC.
- J. Tang, Z. Zou and J. Ye, Angew. Chem., Int. Ed., 2004, 43, 4463 CrossRef CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459 CrossRef CAS.
- L. Yang, G. Li, W. Hu, M. Zhao, L. Sun, J. Zheng, T. Yan and L. Li, Eur. J. Inorg. Chem., 2011, 14, 2211 CrossRef.
- L. Wang, F. Lu and F. Meng, Int. J. Photoenergy, 2012, 2012, 1 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20410j |
| This journal is © The Royal Society of Chemistry 2012 |
