Di-nuclear nonionic magnetic resonance contrast agents using pyrazinyl linking centers†
Guiyan
zhao
a,
Haolong
Li
b,
Cuige
Lu
a,
Yanmeng
Xiao
a,
Xinxiu
Fang
a,
Pixin
Wang
a,
Xuexun
Fang
b,
Kun
Zhao
c,
Xinlong
Li
c,
Shengguo
Yin
c,
Jingwei
Xu
*a and
Wei
Yang
*a
aThe State Key laboratory of Electroanalytic Chemistry, Changchun Institute of Applied Chemistry, Changchun, Jilin, China 130022. E-mail: yangwei@ciac.jl.cn; jwxu@ciac.jl.cn; Fax: (+)86-431-8526-2349
bKey Laboratory of Molecular Enzymology and Enzyme Engineering of the Ministry of Education, Jilin University, Changchun 130023, PR China
cDepartment of Radiology, Jilin Province Academy of Chinese Medicine, Changchun 130012, China
First published on 18th April 2012
Abstract
Two di-nuclear non-ionic MRI contrast agents, (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP, have been designed and synthesized by fusing the DO3A units together with a quinoxalinyl or pyrazinyl moiety. They have improved longitudinal relaxivities of 5.23 and 5.16 mM Gd−1 s−1, respectively, at 20 MHz magnet, 37 °C and pH 7.0. In addition, they possess high thermodynamic stability and kinetic inertness. The cytotoxicity studies indicated that the toxicity of these two agents were low. All these properties satisfy the requirements for clinical applications.
Magnetic resonance imaging (MRI) techniques have become a routinely used noninvasive clinical diagnostic modality for various diseases.1 The high spatial resolution and capability of distinguishing soft tissues give it distinct advantages over radio-diagnostic methods.2 Contrast agents greatly improve the resolutions of the images and thus, the development of contrast agents accompanies the development of MRI techniques.2,3
With the applications of higher-field magnets and new MRI methods, the development of contrast agents with high relaxivity is necessary.4 The relaxivity of a contrast agent is determined by several parameters that are summed up in the well-known Solomon–Bloembergen–Morgan equations, such as residence time (τM), number of first-shell water molecules (q), rotational correlation time (τR), and electron spin relaxation time (T1e) of the paramagnetic cations.3–5
A longer rotational correlation time is expected to improve the relaxivity for small-molecule contrast agents. To slow down the rotational movement of the molecules, contrast agents have been conjugated to macromolecules such as proteins, dendrimers, or liposomes.6–8 Construction of multiple-nuclear agents by fusing several small-molecule agents together is another approach to elongate the rotational correlation time. At present, several multiple-nuclear contrast agents based on Gd-DTTA or Gd-DO3A have been reported with greatly improved relaxivities.9–18
For the purpose of clinical applications, high thermodynamic stability and kinetic inertness for avoiding in vivo transmetallation and low toxicity are the basic requirements.19 Unfortunately, the improvement of the relaxivity for these multiple-nuclear agents often affects the thermodynamic stability when one of the acetic chelating arms for Gd3+ has been removed, i.e. from DTPA to DTTA or DOTA to DO3A in resulted agents.
To enhance the relaxivity of the contrast agent without impairing thermodynamic stability, we designed two novel dimeric nonionic contrast agents, (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP (Scheme 1), in which two DO3A (1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid) units that functioned as Gd3+ chelators, were affixed onto pyrazine or quinoxaline, respectively. The nitrogen atoms on the pyrazine ring were expected to provide an additional donor for the chelated Gd3+, compensating for the missing acetic chelating arm from DOTA to DO3A during the di-nuclear agent construction. Furthermore, the Gd3+ binding would neutralize the charges of three acetic groups of DO3A, resulting in nonionic contrast agents, which is beneficial for low osmotic pressures.
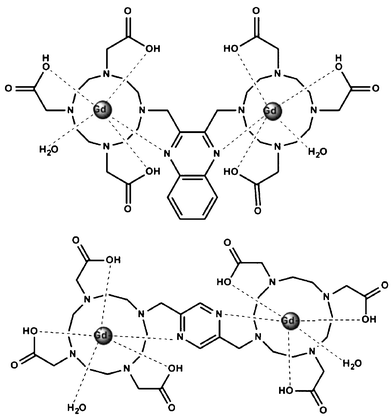 | ||
| Scheme 1 The structures of (Gd-DO3A)2-BMQX (top) and (Gd-DO3A)2-BMP (bottom). | ||
The synthesis of (DO3A)2-2,3-bismethyl-quinoxaline (BMQX) and (DO3A)2-2,3-bismethyl-pyrazine (BMP) followed the procedures reported in the ESI,† schemes 1 and 2. Ln3+ complexes were formed by mixing the cations and the agents with appropriate ratios. The products were characterized using NMR, mass spectra, and infrared (ESI,† Fig. 1–5).
The solvent proton longitudinal relaxivities (r1) of (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP were 5.2 ± 0.2 and 5.2 ± 0.3 mM Gd−1 s−1, respectively, at 20 MHz magnetic fields, 37 °C, and pH 7.0 (ESI,† Fig. 6). Both relaxivities are higher than that of [Gd(DOTA)(H2O)]− and comparable to that of Gd(DO3A)(H2O)2 with 2 attached hydrating water molecules.20,21 The increase in relaxivity with decreasing temperature in the range 10–60 °C indicates a fast chemical exchange (T1M≫τM) (ESI,† Fig. 6), similar to other clinical used contrast agents such as Dotarem, ProHance and Magnevist.15,22
The slightly greater molecular weight favors a slightly higher relaxivity of (Gd-DO3A)2-BMQX than (Gd-DO3A)2-BMP. On the other hand, the mutual influence of electronic relaxation of closely located Gd3+ lowers the relaxivity of (Gd-DO3A)2-BMQX. In the presence of 10-fold La3+ in solutions, the relaxivity of (Gd-DO3A)2-BMQX increases by 8.8% from 5.23 to 5.69 mMGd−1s−1 while that of (Gd-DO3A)2-BMP is almost unchanged (ESI,† Fig. 6), suggesting that an “electronic quenching” exists in (Gd-DO3A)2-BMQX but not in (Gd-DO3A)2-BMP.20,23
In addition, as expected, luminescence lifetime studies using (Tb-DO3A)2-BMP and (Eu-DO3A)2-BMQX possess typical Tb3+ and Eu3+ luminescence spectra (ESI,† Fig. 7), respectively. The luminescence lifetimes of (Tb-DO3A)2-BMP and (Eu-DO3A)2-BMQX (ESI,† Fig. 8) in H2O and D2O solutions gave hydration numbers of 0.81 for Tb3+ and 0.99 for Eu3+, respectively. The results suggest that the dominant form for both (Tb-DO3A)2-BMP and (Eu-DO3A)2-BMQX was monohydrate (q = 1), while part of the molecule was in a dehydrated (q = 0) form in solution. The hydration number difference between the two complexes should be due to the greater radius of the Eu3+ ion relative to the Tb3+ ion.
A hydration number of 1 suggests that the pyrazinyl nitrogen directly coordinates to the chelated paramagnetic ions. This coordination improves the contrast agent in two aspects. First, it further slows down the rotational movement of the paramagnetic cations. In addition, it increases the thermodynamic stability of the agents.
Compared to other di- or tri-nuclear contrast agents at present (Table 1), the relaxivities of (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP are greater than those with one hydration number also. This improvement arises from the slower rotational movement of these two molecules. The studies on the contrast agents conjugated to macromolecules such as liposomes, dendrimers, or proteins have shown that the local rotational correlation time of the paramagnetic unit (τr-local) instead of the rotational time of the whole molecule (τr-global) contributed to the relaxivity of the agents.24–26 Thus, the flexibility of the linkers between the paramagnetic units and the host macromolecules impairs the efforts of such construction.
| Contrast agent | r1 (mM Gd−1 s−1) | q | Ref. |
|---|---|---|---|
| a 37 °C, 20 MHz. b 35 °C, 24 MHz. | |||
| (Gd-DO3A)2-BMQX | 5.23 a | 1 | This work |
| (Gd-DO3A)2-BMP | 5.16 a | 1 | |
| p-Xylene{Gd(DO3A)(H2O)2}2 | 8.34 a | 2 | 29 |
| m-Xylene{Gd(DO3A)(H2O)2}2 | 7.09 a | 2 | |
| m-Xylene-p-COOH{Gd(DO3A)(H2O)2}2 | 9.20 a | 2 | |
| Mes{Gd(DO3A)(H2O)2}3 | 10.5 a | 2 | 12 |
| Mes{H2DO3A} {Gd(DO3A)(H2O)}2 | 6.1 a | 2 | |
| BO{Gd(DO3A)(H2O)}2 | 4.61 a | 1 | 30 |
| en{Gd(DO3A)(H2O)}2 | 3.60 a | 1 | 15 |
| pip{Gd(DO3A)(H2O)}2 | 5.79 a | 1 | 20 |
| bisoxa{Gd(DO3A)(H2O)}2 | 4.94 a | 1 | |
| pentaerythrityl-{Gd(DO3A)(H2O)2}4 | 4.93 b | 2 | 17 |
The flexibility of the center linker groups for di- or multiple-nuclear contrast agent construction also influences the τr-local of the paramagnetic units. For example, a di-nuclear construction using the rigid carbonyl group resulted in a more than 50% increase of the relaxivity compared to the flexible alkane linkers (our work, submitted). In (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP, the coordination bond between the pyrazinyl nitrogen and the Gd3+ inhibits the free rotation of methylene, elongating the τr-local of the Gd-DO3A units.
There are several di- and tri-nuclear contrast agents with higher relaxivity than (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP (Table 1). However, it is noteworthy that they all have 2 hydration numbers, which might lower the thermodynamic stability of the agents. For example, Gd-DOTA has a log KML of 25.3 while Gd-DO3A, with one less acetic chelating arm, only has a log KML less than 20.22,27 The Gd3+ was depleted completely in 6 h by DTPA in another DO3A-based agent.28
For clinical applications of MRI contrast agents, a high thermodynamic stability or, indeed, a kinetic inertness is necessary to avoid transmetallation in vivo. In (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP, the pyrazine nitrogen directly enters into the coordination of the Ln3+ center acting as chelating-donors. This allows the molecules to gain increased relaxivity without the cost of thermodynamic stability or kinetic inertness. Competition of (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP with DTPA confirmed the high stability of these two compounds.
(DO3A)2-BMQX shows two peaks at 239 and 321 nm in UV-vis spectra while the chelating of Gd3+ red-shifts the peaks to 247 and 333 nm, respectively (Fig. 1). Similarly, (DO3A)2-BMP shows one distinctive peak at 274 nm while the Gd3+ binding results in one peak at 296 nm. Neither DTPA nor Gd-DTPA has any interference peak at this region.
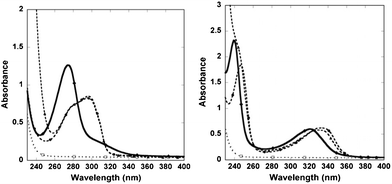 | ||
| Fig. 1 (left) The UV-vis spectra of (DO3A)2-BMP (solid), (Gd-DO3A)2-BMP (dashed, ●), and the mixture of (Gd-DO3A)2-BMP with 100-fold of DTPA after a storage at room temperatures for more than 42 days (dashed, ▲) are shown. (right) The spectra of (DO3A)2-BMQX (solid), (Gd-DO3A)2-BMQX (dashed, ●), and the mixture of (Gd-DO3A)2-BMQX with 100-fold of DTPA after 42 days at room temperatures (dashed, ▲). The results suggest that DTPA could not remove Gd3+ from the agents. DTPA and Gd-DTPA (dotted lines in both figures) have no absorption at this region. | ||
When (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP were mixed with different amounts of chelating agent DTPA (agent:DTPA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, or 1
10, or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) at neutral pHs and stored at room temperature, the UV-vis spectra overlapped with that of the Gd3+-chelated agents during the whole time period of measurements lasting 6 weeks (Fig. 1). In addition, the UV-vis spectra were unchanged when (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP were stored in 100 mM phosphate buffer for more than 6 weeks. The results demonstrated that the Gd3+ in the complexes could not been depleted by either DTPA or phosphate. This suggests that (DO3A)2-BMQX and (DO3A)2-BMP either have high binding affinity compared to DTPA, that has a log KML of 22.2,19,31 or have very slow off-rate for Gd3+. This thermodynamic stability or kinetic inertness is sufficient to allow in vivo applications of these agents.
100) at neutral pHs and stored at room temperature, the UV-vis spectra overlapped with that of the Gd3+-chelated agents during the whole time period of measurements lasting 6 weeks (Fig. 1). In addition, the UV-vis spectra were unchanged when (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP were stored in 100 mM phosphate buffer for more than 6 weeks. The results demonstrated that the Gd3+ in the complexes could not been depleted by either DTPA or phosphate. This suggests that (DO3A)2-BMQX and (DO3A)2-BMP either have high binding affinity compared to DTPA, that has a log KML of 22.2,19,31 or have very slow off-rate for Gd3+. This thermodynamic stability or kinetic inertness is sufficient to allow in vivo applications of these agents.
Low toxicity is another prerequisite for the clinical application of contrast agents. Cytotoxicity investigation using HeLa cells (Fig. 2) showed that the Ic50 values were 6.8 ± 0.3 and 9.9 ± 0.4 mM of Gd3+ for (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP, respectively. As a control, Magnevist (Gd-DTPA) shows an Ic50 of 30 ± 0.5 mM under the same experimental conditions. The Ic50 values for 6 h co-incubation of the agents and the cells were greater than 10 mM of Gd3+ for all agents. This indicates that both of our agents have insignificant cytotoxicity at potential clinical concentrations (e.g. < 1 mM)28 and are worthy for further in vivo toxicity investigation.
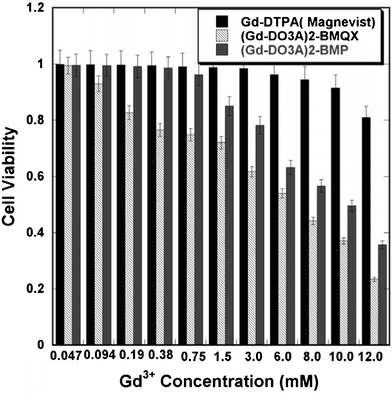 | ||
| Fig. 2 The viability of HeLa cells after 24 h incubations with different concentrations. | ||
The effects of the contrast agents were tested in rats (Fig. 3). The injection of the agents resulted in clearer images of kidneys and blood vessels. Furthermore, the images show that three hours after injection, the agents were mainly in the kidney, suggesting a renal excretion of the contrast agent.
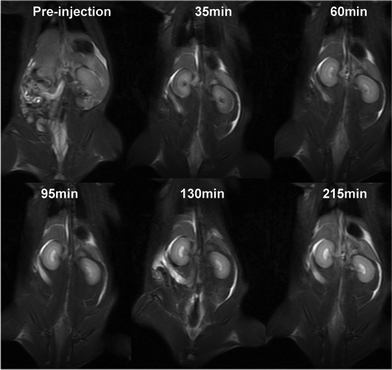 | ||
| Fig. 3 In vivo MR coronal images of a rat before and after intravenous injection of (Gd-DO3A)2-BMQX at a dose of 0.1 mmol Gd kg−1 weight. Animal care and handing procedures were in agreement with the guidelines of the Regional Ethics Committee for Animal Experiments. | ||
Conclusions
In conclusion, two nonionic di-nuclear MRI contrast agents, (Gd-DO3A)2-BMQX and (Gd-DO3A)2-BMP, have been designed and synthesized using quinoxaline or pyrazine rings as the linker centers. These two agents have longitudinal relaxivities of 5.23 and 5.16 mM Gd−1 s−1, respectively, at 20 MHz magnetic fields, at 37 °C, and pH 7.0, and are higher than that of Gd-DTPA or Gd-DOTA. More importantly, this relaxivity improvement does not affect the thermodynamic stability or the toxicity of the agents. This is due to the coordination bonds formed between the nitrogen atoms in the pyrazine center and the chelated Gd3+, which (1) prohibits the free rotation of the methylene bonds linking the DO3A and pyrazine rings, elongating the τr-local of the paramagnetic units and (2) enhances the binding affinity of the compounds to the Gd3+. The improved relaxivity, high thermodynamic stability or very slow off-rate of the Gd3+, and low cytotoxicity make the two contrast agents potentially suitable for clinical applications.For the financial support of this work we thank the NSFC grant 30870491 to WY.
References
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293 CrossRef CAS.
- S. Aime, E. Terreno, D. D. Castelli and A. Viale, Chem. Rev., 2010, 110, 3019 CrossRef.
- R. B. Lauffer, Chem. Rev., 1987, 87, 901 CrossRef CAS.
- P. Caravan, Acc. Chem. Res., 2009, 42, 851 CrossRef CAS.
- P. Hermann, J. Kotek, V. Kubicek and I. Lukes, Dalton Trans., 2008, 3027 RSC.
- J. J. Yang, J. H. Yang, L. X. Wei, O. Zurkiya, W. Yang, S. Y. Li, J. Zou, Y. B. Zhou, A. L. W. Maniccia, H. Mao, F. Q. Zhao, R. Malchow, S. M. Zhao, J. Johnson, X. P. Hu, E. Krogstad and Z. R. Liu, J. Am. Chem. Soc., 2008, 130, 9260 CrossRef CAS.
- S. Langereis, Q. G. de Lussanet, M. H. P. van Genderen, E. W. Meijer, R. G. H. Beets-Tan, A. W. Griffioen, J. M. A. van Engelshoven and W. H. Backes, NMR Biomed., 2006, 19, 133 CrossRef CAS.
- K. Nwe, L. H. Bryant and M. W. Brechbiel, Bioconjugate Chem., 2010, 21, 1014 CrossRef CAS.
- J. Paris, C. Gameiro, V. Humblet, P. K. Mohapatra, V. Jacques and J. F. Desreux, Inorg. Chem., 2006, 45, 5092 CrossRef CAS.
- T. N. Parac-Vogt, L. V. Elst, K. Kimpe, S. Laurent, C. Burtea, F. Chen, R. Van Deun, Y. C. Ni, R. N. Muller and K. Binnemans, Contrast Media Mol. Imaging, 2006, 1, 267 CrossRef CAS.
- J. B. Livramento, É. Tóth, A. Sour, A. Borel, A. E. Merbach and R. Ruloff, Angew. Chem., Int. Ed., 2005, 44, 1480 CrossRef CAS.
- P. Mieville, H. Jaccard, F. Reviriego, R. Tripier and L. Helm, Dalton Trans., 2011, 40, 4260 RSC.
- Y. Song, E. K. Kohlmeir and T. J. Meade, J. Am. Chem. Soc., 2008, 130, 6662 CrossRef CAS.
- J. M. Bryson, W. J. Chu, J. H. Lee and T. M. Reineke, Bioconjugate Chem., 2008, 19, 1505 CrossRef CAS.
- T. M. Lee, T. H. Cheng, M. H. Ou, C. A. Chang, G. C. Liu and Y. M. Wang, Magn. Reson. Chem., 2004, 42, 329 CrossRef CAS.
- A. Mishra, P. Fousková, G. Angelovski, E. Balogh, A. K. Mishra, N. K. Logothetis and É. Tóth, Inorg. Chem., 2008, 47, 1370 CrossRef CAS.
- B. Jebasingh and V. Alexander, Inorg. Chem., 2005, 44, 9434 CrossRef CAS.
- T. N. Parac-Vogt, K. Kimpe, S. Laurent, L. Vander Elst, C. Burtea, F. Chen, R. N. Muller, Y. C. Ni, A. Verbruggen and K. Binnemans, Chem.–Eur. J., 2005, 11, 3077 CrossRef CAS.
- W. P. Cacheris, S. C. Quay and S. M. Rocklage, Magn. Reson. Imaging, 1990, 8, 467 CrossRef CAS.
- D. H. Powell, O. M. NiDhubhghaill, D. Pubanz, L. Helm, Y. S. Lebedev, W. Schlaepfer and A. E. Merbach, J. Am. Chem. Soc., 1996, 118, 9333 CrossRef CAS.
- S. Aime, M. Botta, S. G. Crich, G. Giovenzana, R. Pagliarin, M. Sisti and E. Terreno, Magn. Reson. Chem., 1998, 36, S200 CrossRef CAS.
- S. Laurent, L. V. Elst and R. N. Muller, Contrast Media Mol. Imaging, 2006, 1, 128 CrossRef CAS.
- É. Tóth, L. Helm, A. E. Merbach, R. Hedinger, K. Hegetschweiler and A. Jánossy, Inorg. Chem., 1998, 37, 4104 CrossRef.
- É. Tóth, D. Pubanz, S. Vauthey, L. Helm and A. E. Merbach, Chem.–Eur. J., 1996, 2, 1607 CrossRef.
- J. Rudovsky, M. Botta, P. Hermann, K. I. Hardcastle, I. Lukes and S. Aime, Bioconjugate Chem., 2006, 17, 975 CrossRef CAS.
- G. M. Nicolle, É. Tóth, K.-P. Eisenwiener, H. R. Mäcke and A. E. Merbach, JBIC, J. Biol. Inorg. Chem., 2002, 7, 757 CrossRef CAS.
- M. F. Tweedle, J. J. Hagan, K. Kumar, S. Mantha and C. A. Chang, Magn. Reson. Imaging, 1991, 9, 409 CrossRef CAS.
- K. Kittigowittana, C. T. Yang, W. C. Cheah, K. H. Chuang, C. Y. Tuang, Y. T. Chang, X. Golay and R. W. Bates, ChemMedChem, 2011, 6, 781 CrossRef CAS.
- J. Costa, E. Balogh, V. Turcry, R. Tripier, M. Le Baccon, F. Chuburu, H. Handel, L. Helm, É. Tóth and A. E. Merbach, Chem.–Eur. J., 2006, 12, 6841 CrossRef CAS.
- É. Tóth, S. Vauthey, D. Pubanz and A. E. Merbach, Inorg. Chem., 1996, 35, 3375 CrossRef.
- K. Kumar, C. A. Chang, L. C. Francesconi, D. D. Dischino, M. F. Malley, J. Z. Gougoutas and M. F. Tweedle, Inorg. Chem., 1994, 33, 3567 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental details, mass spectra, infrared, NMR, relaxivity, and luminescence data. See DOI: 10.1039/c2ra20450a/ |
| This journal is © The Royal Society of Chemistry 2012 |
