Cationic quaternary chalcohalide nanobelts: Hg4In2Q3Cl8 (Q = S, Se, Te)†
Yi
Liu
a,
Pushkar D.
Kanhere
a,
Yuan Shyuan
Hoo
a,
Kaiqi
Ye
a,
Qingyu
Yan
a,
Rajdeep Singh
Rawat
b,
Zhong
Chen
a,
Jan
Ma
a and
Qichun
Zhang
*a
aSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore. E-mail: qczhang@ntu.edu.sg
bNatural Sciences and Science Education, National Institute of Education, Nanyang Technological University, Singapore 637616, Singapore
First published on 29th June 2012
Abstract
Novel cationic quaternary chalcohalide nanobelts were found in Hg4In2Q3Cl8 (Q = S, Se, Te), obtained by solid-state reaction. Due to the effects of dimensional reduction, both theoretical and experimental results demonstrate that their bandgaps are remarkably increased compared to those of the zinc-blende structure HgQ (Q = S, Se, Te).
Crystalline chalcohalides and chalcogenides have received great interest in advanced materials research because of their diverse structural features and interesting physical properties, which could have potential applications in non-linear optics, phase-transitions, thermoelectricity generation, X-ray detection and so on.1–8 In particular, low dimensional chalcogenide and chalcohalide materials are very attractive, because these complexes usually display novel and enhanced physical properties.
It is well-known that low-dimensional structures could be obtained via the “dimensional reduction” (DR) method.9–11 In the metal chalcogenide system, alkali sulfide and metal halides are widely used to act as “scissors” to reduce the dimensionality of the parent metal chalcogenide structures. In addition, theoretical calculations show that orbital overlap is reduced in certain space directions and the bandgaps (the difference between the valence band and the conduction band) are increased. Thus, the bandgaps of the dimension-reduced compounds could be tuned by changing the amounts of metal halides and alkali sulfides.
Some interesting illustrations of dimensional reduction in multiple chalcohalides and chalcogenides have been demonstrated. For example, the family of binary HgQ (Q = S, Se, Te) chalcogenides, which crystallize in the 3D zinc-blende structure, have a zero energy gap owing to the inverted bands near the Fermi level through the electronic band structure calculations.12 After the introduction of the dimensional reduction agent A2Q (A = K, Rb, Cs, Tl; Q = S, Se, Te) into the parent compounds HgQ, a series of ternary A/Hg/Q compounds with reduced dimensions were produced. It is found that the choice of countercation has a significant effect on the derived framework.10c,d,13,14 Although A2Q compounds were successfully employed to tailor HgQ networks, relatively few of the new dimensional reduction agents have been reported. To the best of our knowledge, HgQ does not breed quaternary Hg–M–Q–X chalcohalides, where M represents main group metals. Four exceptions are two three-dimensional (3D) frameworks: Hg7InS6Cl5,15a Hg2PbI2S2,15b and two isostructural one-dimensional (1D) structures: 2HgS·SnBr2 and 2HgS·PbBr2.8d,16 Here, we report a new class of 1D chalcohalides, Hg4In2Q3Cl8 (Q = S, Se, Te), featuring novel cationic quaternary chalcohalide nanobelts. It is shown for the first time that 1D cationic quaternary chalcohalide nanobelts could be produced in chalcohalide systems.
The new compounds Hg4In2Q3Cl8 (Q = S, Se, and Te) were prepared via the solid state reaction from a mixture containing Hg2Cl2 (1.15 mmol, Warning: due to the toxicity of mercury, more attention should be paid and extreme care must be taken when handling the sample and products), In2Q3 (0.30 mmol) and Q (1.15 mmol). A detailed synthesis has been provided in the supporting information.† The resulting products were obtained as rod-shaped crystals, which can be separated manually. The colors of the as-prepared crystals are yellow, grey, and chocolate for the S, Se, and Te analogues, respectively, which are in agreement with the electronic absorption spectra discussed below. Note that the crystalline Hg4In2S3Cl8 can only be stable in air for about 4 days, and then becomes amorphous, while the other two samples (Q = Se and Te) do not change in air for months. The crystal structures of the as-prepared complexes were determined using a Bruker APEX II CCD diffractometer equipped with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). The purities of all samples are confirmed by X-ray diffraction (XRD) patterns (Fig. S1, ESI†). The calculated atomic Hg/In/Q/Cl ratios from the single-crystal structure analysis are in agreement with energy-dispersive X-ray spectroscopy analysis (EDS, see Fig. S2, ESI†), and thermal gravimetric analysis (TGA) shows that all materials are stable up to 300 °C (Fig. S3, ESI†).
The isostructural chalcohalides Hg4In2Q3Cl8 (Q = S, Se, Te) crystallize in an orthorhombic unit cell with the space group Pnma (No. 62). X-ray crystallography study reveals that the new quaternary phases feature the cationic nanobelts [Hg4Q3InCl4]+ with the charge-balanced isolated anions [InCl4]−, which are located in the channels constructed from the nanobelts (Fig. 1a). The nanobelts are made of infinite ribbons of [Hg4Q3]2+, which are decorated by coordinated [InCl4]− anions along the b-axis (Fig. 1b). Furthermore, the ribbons of [Hg4Q3]2+ are composed of 12-membered Hg6Q6 cyclohexane rings with a chair-like configuration (Fig. 1c). In the ribbons, every ring shares four edges with the neighboring rings, and the other two edges are forbidden to share due to the steric hindrance effect from coordinated [InCl4]− anions.
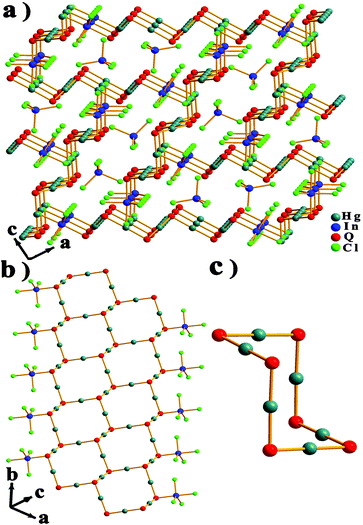 | ||
| Fig. 1 (a) Ball-and-stick model of the network of Hg4In2Q3Cl8 with atomic labels along the b direction. (b) Projection of the nanobelts in Hg4In2Q3Cl8. (c) The chair-like configuration of Hg6Q6. | ||
The nanobelts were interconnected with each other to form the pseudo-3D framework through weak Hg⋯Cl bonds, in which the apical Cl atoms coming from the coordinating [InCl4]− anions are situated at the center of the ring. With increasing chalcogenide radius, the width of the nanobelts also increases from 22.587 to 23.324 Å. In the nanobelts, all of the crystallographically independent Hg sites show a linear shape with two Q atoms. Meanwhile the In site adopts a slightly distorted trigonal bipyramidal geometry, in which the Cl and Q atoms are located on two vertices (Fig. 1b). All Q (Q = S, Se, Te) atoms acting as tridentate metal-linkers are trigonally coordinated by three Hg atoms or one In atom and two Hg atoms, while all Cl atoms coordinated with In atoms are unidentate terminal ligands. The crystallographically independent [InCl4]− ions adopt a moderately distorted tetrahedral coordination (Fig. 1a). In these structures, the Hg–Q bond lengths fall in the range 2.355(2) to 2.654(4) Å. The In–Q bond distances vary from 2.587(4) to 2.892(8) Å, which is similar to the reported results;18 the In–Cl bond distances range from 2.479(3) to 2.560(2) Å, which are comparable to the related compounds, such as Tl2Hg3Q4 (Q = S, Se, Te), [Hg3Te2][UCl6], Hg3ZnS2Cl4, [Ru(Te9)](InCl4)2, InSb2S4Cl, In5Se5Cl, and Hg5In2Te8.6c,17
The densities-of-state (DOS) and the electronic band structure for Hg4In2Se3Cl8 calculated by CASTEP,19 are shown in Fig. 2. The calculation results for Hg4In2S3Cl8 and Hg4In2Te3Cl8 are provided in the supporting information (Fig. S4 and Fig. S5, ESI†). As depicted in Fig. 2a, the valence bands (VBs) between the energy level −4.0 eV and the Fermi level (0.0 eV) contain significant contributions from the Hg 6s, Se 4p, and Cl 3p states mixed with a small amount of the In 5p and Se 4s states. The VBs ranging from −4.0 to −10.0 eV are mostly formed by Hg 5d states hybridized with a small portion of the In 5s and Cl 3p states. The VBs spreading from −10.0 to −15.0 eV are primarily the In 4d, Se 4s, and Cl 3s states. The conduction bands (CBs) above Fermi energy (EF) are mostly made up of the Hg 6s and Se 4p states, with minor contributions from the Se 4s states. Briefly, the non-bonding states of Se 4p and Cl 3p and the antibonding states of Hg 6s and Se 4p mainly influence the energy gaps of Hg4In2Se3Cl5. Since the CB minimum (2.25 eV) and VB maximum (0.00 eV) are not located at the same k-point, Hg4In2Se3Cl8 is an indirect semiconductor with a calculated band gap of 2.25 eV (Fig. 2b), which is in good agreement with the experimental value of 2.34 eV deduced from the solid state UV-visible absorption (Fig. 3). The other two S and Te analogues have similar DOS and electronic band structures to those of Hg4In2Se3Cl8 (Table 1, Fig. S4, and Fig. S5, ESI†). Clearly, these calculated results are much bigger than the theoretical bandgaps of HgQ20 (HgS, 0.30 eV; HgSe, −0.24 eV; HgTe −0.34 eV). Both theoretical studies and experimental measurements show that the band gap of Hg4In2Q3Cl8 increases remarkably, relative to HgQ, because of the effects of DR.
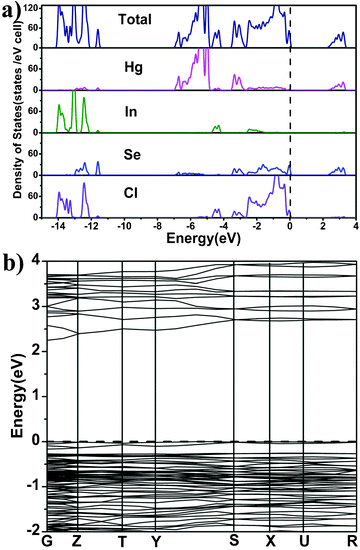 | ||
| Fig. 2 (a) Total and partial densities of state (DOS) and (b) band structure of Hg4In2Se3Cl8. | ||
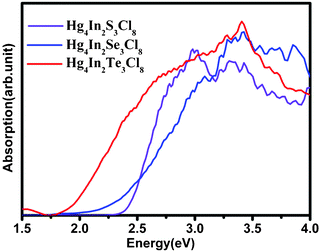 | ||
| Fig. 3 UV-visible absorbance of Hg4In2Q3Cl8. | ||
| Hg4In2S3Cl8 | Hg4In2Se3Cl8 | Hg4In2Te3Cl8 | |
|---|---|---|---|
| Calculated | 2.30 eV | 2.25 eV | 2.21 eV |
| Experimental | 2.43 eV | 2.34 eV | 1.89 eV |
In summary, three isostructural quaternary chalcohalides Hg4In2Q3Cl8 (Q = S, Se, Te) have been successfully synthesized through the solid state method. These compounds have a novel cationic nanobelt structure. Both experimental and theoretical studies confirm that they are semiconductors with narrow bandgaps. This research could offer us a new strategy to prepare other low-dimensional chalcohalides.
QZ thanks the support from the MOE Tier 1 fund and the Singapore National Research Foundation under CREATE programme: Nanomaterials for Energy and Water Management, and Singapore MPA 23/04.15.03 RDP 009/10/102 grant.
References
- (a) T. Nilges, S. Lange, M. Bawohl, J.-M. Deckwart, H.-D. Wiemhöfer, R. Decourt, B. Chevalier, J. Vannahme, H. Eckert and R. Weihrich, Nat. Mater., 2009, 8, 101 CrossRef CAS; (b) A. Günther, M. Heise, F. R. Wagner and M. Ruck, Angew. Chem., Int. Ed., 2011, 50, 9987 CrossRef; (c) Q. Zhan, X. Bu, J. Zhang, T. Wu and P. Feng, J. Am. Chem. Soc., 2007, 129, 8412 CrossRef; (d) T. Nilges, O. Osters, M. Bawohl, J. L. Bobet, B. Chevalier, R. Decourt and R. Weihrich, Chem. Mater., 2010, 22, 2946 CrossRef CAS; (e) P. Yu, L. J. Zhou and L. Chen, J. Am. Chem. Soc., 2012, 134, 2227 CrossRef CAS.
- (a) Q. Zhang, I. Chung, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, J. Am. Chem. Soc., 2009, 131, 9896 CrossRef CAS; (b) K. Biswas, Q. Zhang, I. Chung, J.-H. Song, J. Androulakis, A. J. Freeman and M. G. Kanatzidis, J. Am. Chem. Soc., 2010, 132, 14760 CrossRef CAS; (c) Q. Zhang, Y. Liu, X. Bu, T. Wu and P. Feng, Angew. Chem., Int. Ed., 2008, 47, 113 CrossRef CAS; (d) S. Johnsen, Z. F. Liu, J. A. Peters, J.-H. Song, S. Nguyen, C. D. Malliakas, H. Jin, A. J. Freeman, B. W. Wessels and M. G. Kanatzidis, J. Am. Chem. Soc., 2011, 133, 10030 CrossRef CAS.
- (a) A. Pfitzner, S. Reiser and T. Nilges, Angew. Chem., Int. Ed., 2000, 39, 4160 CrossRef CAS; (b) J. Beck, S. Schlueter and M. Dolg, Angew. Chem., Int. Ed., 2001, 40, 2287 CrossRef CAS; (c) Y. Cai, Y. Wang, Y. Li, X. Wang, X. Xing, C. Liu and H. Zeng, Inorg. Chem., 2005, 44, 9128 CrossRef CAS; (d) Q. Zhang, T. Wu, X. Bu, T. Tran and P. Feng, Chem. Mater., 2008, 20, 4170 CrossRef CAS; (e) S. P. Guo, G. C. Guo, M. S. Wang, J. P. Zou, H. Y. Zeng, L. Z. Cai and J. S. Huang, Chem. Commun., 2009, 4366 RSC.
- (a) H. Kabbour and L. Cario, Inorg. Chem., 2006, 45, 2713 CrossRef CAS; (b) J. P. Gabriel, K. Boubekeur, S. Uriel and P. Batail, Chem. Rev., 2001, 101, 2037 CrossRef CAS; (c) Q. Zhang, X. Bu, Z. Lin, T. Wu and P. Feng, Inorg. Chem., 2008, 47, 9724 CrossRef CAS; (d) Q. Zhang, Z. Lin, X. Bu, T. Wu and P. Feng, Chem. Mater., 2008, 20, 3239 CrossRef CAS; (e) M. D. Smith and G. J. Miller, J. Am. Chem. Soc., 1996, 118, 12238 CrossRef CAS.
- (a) S. T. Kong, H. J. Deiseroth, C. Reiner, O. Guen, E. Neumann, C. Ritter and D. Zahn, Chem.–Eur. J., 2010, 16, 2198 CrossRef CAS; (b) H. J. Deiseroth, S. T. Kong, H. Eckert, J. Vannahme, C. Reiner, T. Zaiss and M. Schlosser, Angew. Chem., Int. Ed., 2008, 47, 755 CrossRef CAS; (c) M. F. Braeu and A. Pfitzner, Angew. Chem., Int. Ed., 2006, 45, 4464 CrossRef CAS.
- (a) J. Beck and S. Hedderich, J. Solid State Chem., 2003, 172, 12 CrossRef CAS; (b) D. E. Bugaris and J. A. Ibers, J. Solid State Chem., 2008, 181, 3189 CrossRef CAS; (c) W. T. Chen, H. M. Kuang and H. L. Chen, J. Solid State Chem., 2010, 183, 2411 CrossRef CAS; (d) J. Beck, S. Hedderich and K. Koellisch, Inorg. Chem., 2000, 39, 5847 CrossRef CAS.
- (a) M. N. Sokolov, A. L. Gushchin, P. A. Abramov, A. V. Virovets, E. V. Peresypkina and V. P. Fedin, Inorg. Chem., 2007, 46, 4677 CrossRef CAS; (b) D. Hoppe, D. Schemmel, M. Schuetz and A. Pfitzner, Chem.–Eur. J., 2009, 15, 7129 CrossRef CAS; (c) A. Biegerl, E. Brunner, C. Groeger, M. Scheer, J. Wachter and M. Zabel, Chem.–Eur. J., 2007, 13, 9270 CrossRef CAS.
- (a) H. J. Deiseroth, C. Reiner, M. Schlosser, X. W. Wang, H. Ajaz and L. Kienle, Inorg. Chem., 2007, 46, 8418 CrossRef CAS; (b) D. L. Gray, G. J. Long, F. Grandjean, R. P. Hermann and J. A. Ibers, Inorg. Chem., 2008, 47, 94 CrossRef CAS; (c) J. Nieboer, J. P. Mack, H. P. A. Mercier and M. Gerken, Inorg. Chem., 2010, 49, 6153 CrossRef CAS; (d) R. Blachnik, K. Lytze and H. Reuter, J. Solid State Chem., 1996, 126, 95 CrossRef CAS.
- (a) J. R. Long, A. S. Williamson and R. H. Holm, Angew. Chem., Int. Ed. Engl., 1995, 34, 226 CrossRef CAS; (b) J. R. Long, L. S. McCarty and R. H. Holm, J. Am. Chem. Soc., 1996, 118, 4603 CrossRef CAS; (c) E. G. Tulsky and J. R. Long, Chem. Mater., 2001, 13, 1149 CrossRef CAS.
- (a) M. G. Kanatzidis, Chem. Mater., 1990, 2, 353 CrossRef CAS; (b) E. A. Axtell, J. H. Liao, Z. Pikramenou and M. G. Kanatzidis, Chem.–Eur. J., 1996, 2, 656 CrossRef CAS; (c) E. A. Axtell, Y. Park, K. Chondroudis and M. G. Kanatzidis, J. Am. Chem. Soc., 1998, 120, 124 CrossRef CAS; (d) S. Johnsen, S. C. Peter, S. L. Nguyen, J.-H. Song, H. Jin, A. J. Freeman and M. G. Kanatzidis, Chem. Mater., 2011, 23, 4375 CrossRef CAS.
- (a) C. R. Evenson and P. K. Dorhout, Inorg. Chem., 2001, 40, 2884 CrossRef CAS; (b) J. W. Lekse, M. A. Moreau, K. L. McNerny, J. Yeon, P. S. Halasyamani and J. A. Aitken, Inorg. Chem., 2009, 48, 7516 CrossRef CAS.
- O. Madelung, Semiconductors: DataHandbook, Springer, Berlin, 3rd edn, 2004 Search PubMed.
- (a) H. Sommer and R. Hoppe, Z. Anorg. Allg. Chem., 1978, 443, 201 CrossRef CAS; (b) M. G. Kanatzidis and Y. Park, Chem. Mater., 1990, 2, 99 CrossRef CAS; (c) R. M. Herath Banda, D. Craig, I. G. Dance and M. Scudder, Polyhedron, 1991, 10, 41 CrossRef.
- (a) K. O. Klepp and K. Prager, Z. Naturforsch., 1992, 47B, 491 Search PubMed; (b) K. O. Klepp, J. Alloys Compd., 1992, 182, 281 CrossRef CAS; (c) J. Li, Z. Chen, K.-C. Lam, S. Mulley and D. M. Proserpio, Inorg. Chem., 1997, 36, 684 CrossRef CAS.
- (a) Y. Liu, F. X. Wei, S. N. Yeo, F. M. Lee, C. Kloc, Q. Y. Yan, H. H. Hng, J. Ma and Q. C. Zhang, Inorg. Chem., 2012, 51, 4414 CrossRef CAS; (b) R. Blachnik, W. Buchmeier and H. A. Dreisbach, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1986, 42, 515 CrossRef.
- E. Ruiz and M. C. Payne, Chem.–Eur. J., 1998, 4, 2485 CrossRef CAS.
- (a) L. Wang and S.-J. Hwu, Chem. Mater., 2007, 19, 6212 CrossRef CAS; (b) V. Nickel, H. J. Deiseroth, L. Kienle, V. Duppel and C. Reiner, Z. Anorg. Allg. Chem., 2010, 636, 79 CrossRef CAS; (c) A. Günther, A. Isaeva, A. I. Baranov and M. Ruck, Chem.–Eur. J., 2011, 17, 6382 CrossRef; (d) V. R. Kozer, A. O. Fedorchuk, I. D. Olekseyuk and O. V. Parasyuk, J. Alloys Compd., 2010, 503, 40 CrossRef CAS.
- (a) T. Wu, X. Bu, P. Liao, L. Wang, S.-T. Zheng, R. Ma and O. Feng, J. Am. Chem. Soc., 2012, 134, 3619 CrossRef CAS; (b) Q. Zhang, I. Chung, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, Chem. Mater., 2009, 21, 12 CrossRef CAS; (c) Q. Zhang, X. Bu, L. Han and P. Feng, Inorg. Chem., 2006, 45, 6684 CrossRef CAS.
- M. D. Segall, P. J. D. Lindan, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne, J. Phys.: Condens. Matter, 2002, 14, 2717 CrossRef CAS.
- C.-Y. Moon and S.-H. Wei, Phys. Rev., 2006, B74, 045205 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental methods, XRD patterns, EDS and TGA analysis, additional table for crystal data and structure refinements, and figures for total and partial densities of state (DOS) and band structure of Hg4In2S3Cl8 and Hg4In2Te3Cl8. Files CSD-424490, 424491 and 424492 for Hg4In2Te3Cl8, Hg4In2Se3Cl8 and Hg4In2S3Cl8. See DOI: 10.1039/c2ra20781h/ |
| This journal is © The Royal Society of Chemistry 2012 |
