Evaluation of microbial fuel cell Shewanella biocathodes for treatment of chromate contamination†
Lewis
Hsu
a,
Shelly A.
Masuda
a,
Kenneth H.
Nealson
b and
Massoud
Pirbazari
*a
aSonny Astani Department of Civil and Environmental Engineering, University of Southern California, Los Angeles, CA 90089. E-mail: pirbazar@usc.edu (M P); Tel: (213) 740 0592
bDepartment of Earth Sciences, University of Southern California, Los Angeles, CA 90089
First published on 13th April 2012
Abstract
This paper presents data comparing Shewanella strains acting as biocatalysts under fumarate and chromate reducing conditions in a microbial fuel cell. Catalyzing fumarate reduction, Shewanella strains show a maximum power generation of between 10.2 and 59.4 nW cm−2. Comparisons between product formation and current transfer indicate either incomplete oxidation of fumarate or utilization of a separate source of electrons. Similar comparisons under chromate reducing conditions indicate initial utilization of the electrode as the sole electron source followed by a use of an unknown reducing electron pool. Additionally, we show that fuel cell systems, with Shewanella acting as the sole biocatalysts at the cathode, are capable of achieving reduction of chromium concentrations to less than 5 ppb, well within acceptable guidelines established by regulatory agencies.
Introduction
Chromium is a metallic species widely used in industrial applications including metal plating, leather tanning, and dye manufacturing. Inadequate or inappropriate handling of waste often allows for the introduction of chromium into the environment. The two major forms found in the environment are the hexavalent, CrVI, and trivalent, CrIII, species. Exposure to CrVI has been linked to cancer, skin ulcers, and other diseases.1 The high mobility and solubility of CrVI frequently results in contamination of soils, surface waters, and ground water. In contrast, the trivalent species is generally considered innocuous in the environment due to its lack of mobility as an insoluble species.2,3 Thus, reduction of CrVI to CrIII has been proposed as an effective mechanism for limiting exposure and movement in the natural environment.An emerging technology for exploiting bacterial reduction-oxidation systems is that of microbial fuel cells (MFCs).4–6 This technology has recently been proposed for pollution remediation, capitalizing on the reducing power supplied by the anode compartment and the catalytic abilities of biocathodes.7,8 In particular, several studies utilizing enrichment communities as biocathode communities have been reported for CrVI contamination.9–11 While these studies show reduction of chromium at ppm concentration levels, drinking water regulations require removal to low ppb concentrations, orders of magnitude below what was reported in these studies.12 To demonstrate the feasibility of MFC systems to reduce chromium to levels appropriate for drinking water treatment, we present data here examining the utilization of single strains of bacteria as the sole biocatalyst at the cathode of a two-chamber MFC.
Using a mixed community does not allow us to directly understand both the organisms that are active at the electrode surface and the mechanisms for electron transfer, essentially a “black box” approach. Knowledge of Shewanellae, on the other hand, allows us to use them as model organisms since their physiology and genomes have been studied extensively.13–16 By comparing the behavior between these Shewanellae, we can take a systems biology approach to understanding the mechanisms behind electron transfer at the cathode. Understanding these mechanisms is critical if these systems are to be optimized and mixed microbial consortia are to be understood. For these reasons, we have examined several Shewanella strains as single-strain cathode biocatalysts (Table 1).
| Species name | Discovery location |
|---|---|
| Shewanella oneidensis MR-1 | Lake Oneida, New York, USA |
| (freshwater sediment) | |
| Shewanella putrefaciens W3-18-1 | Pacific Ocean sediment, Washington, USA |
| (coastal sediment) | |
| Shewanella amazonensis SB2B | Amazon River delta, Brazil |
| (coastal sediment) | |
| Shewanella sp. ANA-3 | Woods Hole, Massachusetts, USA |
| (brackish estuary) | |
| Shewanella loihica PV-4 | Loihi seamount , Hawaii, USA |
| (hydrothermal sea vent) | |
| Shewanella sp. MR-4 | Black Sea, 5 m depth |
| (oxic salt water) |
The selected Shewanellae represent a variety of physiologies and genetic differences. All strains contain the metal-reducing gene locus (mtrABC) implicated in anode biocatalysis along with a variety of other multi-heme cytochromes, some unique to individual strains.17 Comparison of these strains will help us to identify those strains with the most favorable transfer mechanisms for organisms operating at the cathode. This allows future research to focus on determining the gene products and/or physiological responses responsible for rapid and efficient electron transfer at the cathode.
The Shewanella strains used here have not been reported in any biocathode studies. Therefore, we first show biocatalytic abilities of these strains at the cathode utilizing fumarate reduction. Fumarate was chosen as the initial electron acceptor based on earlier studies with MR-1 which show no interference by fumarate and its metabolic by-product (succinate) with CrVI reduction.18 Once fumarate reduction (to succinate) was completed and verified by power production and organic acid analysis, each strain was examined under chromate reducing conditions. The data shown here detail the first comparisons using Shewanella strains as biocathodes, under both non-toxic, fumarate-reducing, conditions and toxic, chromate-reducing, conditions.
Materials and methods
Fuel cell system construction
The fuel cell systems utilized in this research were similar to those described by Rhoads et al. with two 500 mL compartments which serve as anode and cathode chambers (Fig. 1).19 In these fuel cells, the electrodes were prepared from reticulated vitreous carbon (80 ppi; ERG Aerospace; Oakland, CA) cut to a size of 5 cm × 10 cm × 0.5 cm. The electrode material was pretreated by soaking with an ethanol/water (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) mixture followed by an acid wash with 1 M HCl. Electrodes were then rinsed with ultrapure H2O and dried at 105 °C. Graphite rod leads were attached to pretreated electrodes with conductive epoxy. Stranded stainless steel wire was attached at the free end of the rods with vinyl tape to serve as connections from the electrode to the exterior of the fuel cell. Care was taken to ensure that this connection was above the working liquid level in the fuel cell chamber to avoid interaction of the stainless steel with bacterial cultures and electrolyte.
5) mixture followed by an acid wash with 1 M HCl. Electrodes were then rinsed with ultrapure H2O and dried at 105 °C. Graphite rod leads were attached to pretreated electrodes with conductive epoxy. Stranded stainless steel wire was attached at the free end of the rods with vinyl tape to serve as connections from the electrode to the exterior of the fuel cell. Care was taken to ensure that this connection was above the working liquid level in the fuel cell chamber to avoid interaction of the stainless steel with bacterial cultures and electrolyte.
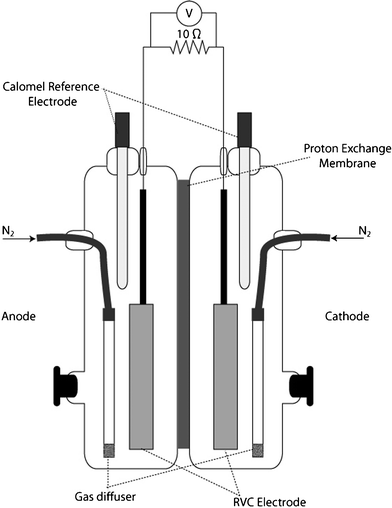 | ||
| Fig. 1 Side-view schematic of fuel cell reactor used in fumarate and chromate biocathode studies. | ||
A cation exchange membrane (CMI-7000; Membranes International, Inc.; Glen Rock, NJ) was utilized to separate the anode and cathode chambers. The membrane was prepared by hydration in 50 mM PIPES for 24 h prior to use.
Preparation of bacterial cultures
Cultures of Shewanella species (Table 1) were prepared by inoculating 10 mL of LB Broth, Miller (BD; Franklin Lakes, MD) from −80 °C glycerol stocks obtained from a collection of Shewanella maintained by the Nealson Lab at the University of Southern California. This culture was allowed to incubate for 16 h at 30 °C at 100 rpm. This overnight culture was used to inoculate a bacterial minimal media containing 50 mM piperazine-N,N′-bis(2-ethanesulfonic) acid (PIPES) buffer, 90 mM NaOH, 28 mM NH4Cl, 1.3 mM KCl, 4.3 mM NaHPO4, trace minerals, trace vitamins, and 15 mM sodium lactate at pH 7. The culture was allowed to incubate for 18–24 h at 30 °C and 100 rpm, followed by dilution with 50 mM PIPES buffer to reach a cell density of approximately 107 CFU/mL (OD600 = 0.2). The absence of lactate and other organic acid metabolites in these cell dilutions was confirmed by HPLC analysis.250 mL of MR-1 was added to the anode compartment of the each fuel cell to serve as the anodic biocatalyst. To evaluate various Shewanella as the sole cathodic biocatalyst, 250 mL of a single Shewanella strain to be tested was added to the cathode compartment in separate fuel cells. No carbon source for growth/maintenance of microorganisms was added to the cathode to encourage the electrode as the major source of electrons in the cathode. The fuel cell apparatus was then connected to a prepurified nitrogen gas line. Nitrogen gas was fed through sterile gas filters (0.2 μm) to each chamber of the fuel cell at a rate of 40 mL per minute. Fine frit glass gas dispersers (Chemglass, Inc.; Vineland, NJ) were utilized for gas sparging to ensure adequate mass transfer.
Operation and characterization of fuel cell systems
Anode and cathode electrodes were connected to a 10 ohm resistor and the system was allowed to equilibrate for 2–4 h. After achieving a baseline voltage, aliquots of lactate and fumarate were injected into the anode and cathode compartments, respectively, to reach final concentrations of 4 mM each (a stoichiometric excess of 2 mM lactate).The electrodes were disconnected from the resistor at this point and the system was allowed to equilibrate for several hours until a stable open circuit potential was reached. Calomel reference electrodes were used to determine electrode potentials and a linear polarization resistance curve was generated by employing a potentiostat (Reference 600; Gamry Instruments; Warminster, PA) using the anode as the working electrode and cathode as the reference and counter electrodes.
Following the completion of the polarization tests, the electrodes were connected to a 10 ohm resistor and generated voltage was monitored using a digital multimeter and data acquisition system (Model 2700; Keithley Instruments, Inc.; Cleveland, OH). Once voltage decreased to initial levels (indicating exhaustion of fumarate, about 2–3 days), a 1040 ppm (20 mM) CrVI solution was injected into the cathode compartment to increase the CrVI concentration level to 2.5 ppm. This injection was repeated every three days for a total of three semi-batch operation cycles. Samples were taken for chromium and organic acid analysis during both fumarate and chromium reduction.
Abiotic, or sterile, cathodes were tested under similar conditions as stated above except that MR-1 was added to the anode chamber and no biocatalyst was added to the cathode chamber. Instead, the cathode chamber was filled with a buffered fumarate solution containing 50 mM PIPES and 2 mM fumarate. Both anode and cathode chambers were purged continuously with nitrogen. Samples were taken periodically to determine any reduction in fumarate concentration. A set of similar experiments utilizing buffered chromate solution (50 mM PIPES, 2.5 ppm CrVI) at the cathode was used to determine if chromium reduction would occur with a sterile cathode. These MFCs were operated in semi-batch mode as stated for the biocathodes.
Additional control experiments were performed by operating the MFC under open circuit conditions to assess any effects in the absence of a solid electron donor. To examine metabolic activity under fumarate reducing conditions, each strain was cultured in the minimal media described previously. Once late log phase was achieved, the cells were diluted to approximately 107 CFU mL−1 before addition to the cathode compartment. Fumarate was added to the cathode chamber to bring the concentration to 2 mM. Samples were taken every 24 h for 4 days and analyzed by HPLC.
In order to assess chromate reduction in the presence of residual succinate, each of the strains was prepared for the MFC cathode. The initial concentrations of succinate and chromate for these experiments were prepared to be 2 mM for succinate and 0.05 mM (2.5 ppm) for chromate. Samples were taken every 24 h for 3 days and analyzed for organic acids and chromate concentrations.
HPLC analysis
In order to monitor the levels of organics, anolyte and catholyte samples were taken during three time periods: (1) immediately after feeding, (2) at peak voltage, (3) after reaching baseline. These samples were processed as described by Kan et al.20 Briefly, the samples were centrifuged for 5 min at 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 × g and the supernatants were collected and stored at −20 °C. Samples were then acidified by addition of sulfuric acid to bring the final concentration of sulfuric acid to 12.5 mM. After overnight incubation at 4 °C to facilitate precipitation of PIPES buffer, samples were filtered through a 0.22 μm syringe filter. An Agilent HPLC system (1100 series; Agilent, Inc.; Santa Clara, CA) was employed, using a reverse phase C18 column (Synergi-Hydro 4 μm 250 mm/4.6 mm; Phenomenex; Torrance, CA, USA) for separation. A diode array detector (G1315B; Agilent, Inc.; Santa Clara, CA) set at 210 nm was used for detection. Chromatograms were generated using a mobile phase of 4.5 mM sulfuric acid with a flow rate of 0.5 mL min−1 at ambient temperature.
900 × g and the supernatants were collected and stored at −20 °C. Samples were then acidified by addition of sulfuric acid to bring the final concentration of sulfuric acid to 12.5 mM. After overnight incubation at 4 °C to facilitate precipitation of PIPES buffer, samples were filtered through a 0.22 μm syringe filter. An Agilent HPLC system (1100 series; Agilent, Inc.; Santa Clara, CA) was employed, using a reverse phase C18 column (Synergi-Hydro 4 μm 250 mm/4.6 mm; Phenomenex; Torrance, CA, USA) for separation. A diode array detector (G1315B; Agilent, Inc.; Santa Clara, CA) set at 210 nm was used for detection. Chromatograms were generated using a mobile phase of 4.5 mM sulfuric acid with a flow rate of 0.5 mL min−1 at ambient temperature.
Ion chromatography analysis
The presence of CrVI was measured by taking samples before, during, and after each injection period. These samples were adjusted to pH 10 by addition of a 0.25 M (NH4)2SO4/1 M NH4OH buffer solution and incubated at 4 °C until analysis.21 Samples were then filtered through a 0.22 μm syringe filter prior to injection to the ion chromatography (IC) system. An IC system (850 Professional; Metrohm AG; Switzerland) with a post-column derivatization coil and UV-Vis absorbance detector (Lambda 1010; Metohm AG; Switzerland) set at 530 nm was used for CrVI analysis. For separation, a 1000 μL sample injection loop was used with an anion exchange column (Metrosep A Supp 10 250/4.0; Metrohm AG; Switzerland). Chromatograms were generated with an eluent (10 mM LiOH, 78 mM Li2SO4) flow rate of 0.8 mL min−1 and a post-column derivatization reagent (0.5 g L−1 diphenylcarbazide, 0.5 M H2SO4, 10% methanol) flow rate of 0.4 mL min−1.Electron microscopy
Electron microscopy was employed to visualize attached biomass and to determine the probable locations of reduced chromium species on the electrode surface. Following each experiment, sections of electrodes were extracted and fixed in 2.5% gluteraldehyde. Following fixation, samples were prepared by gradual ethanol dehydration and hexamethyldisilizane substitution.22,23 The samples were then mounted on aluminum stubs and carbon coated. Electron microscopy and elemental analysis were performed using a scanning electron microscope (JSM-6610LV; JEOL USA, Inc.; Peabody, MA) operated with a 10 kV accelerating voltage and equipped with an energy dispersive spectroscopy (EDS) detector (JSM 6490; EDAX Inc.; Mahwah, NJ).Theoretical electrode potential and electron transfer comparisons
Reactions and standard potentials for lactate oxidation and fumarate reduction from literature were compared with observed open circuit cell potentials.24 The chromate reduction standard potential was calculated from a published redox equilibria constant and adjusted.2 Redox potentials for conditions found in the fuel cell reactor were also calculated for all by the Nernst equation (ESI†) from standard potentials.The potential at the cathode under open circuit conditions is given by the following equation:
| Ecathode = Ereduction − (ηactivation + ηconcentration) |
where Ecathode is the cathode potential and Ereduction is the theoretical potential of the reduction half reaction. The overpotentials (ηactivation + ηconcentration) refer to losses due to activation potential and concentration polarization. Ohmic losses are not expected to be significant during open circuit measurements since no current is being transferred. Mass transfer losses due to concentration polarization are also expected to be minimal since measurements are being done at peak fumarate concentrations. Therefore, the differences in cathode potentials that we observe between Shewanella strains will be associated with activation overpotentials and are governed by how these strains perform electron transfer within the electrode-biofilm-oxidant system.
As a measure of efficiency, comparisons were made between the measured electron transfer from the anode compartment (e−charge) and the electron transfer required to explain the observed reduction/oxidation change (e−chem). When the ratio of e−chem to e−charge is 1, then the charge transfer likely proceeds through the reactions presented in Table 2. Values less than one indicate more charge was accepted by the cathode system than was necessary for the reduction reaction to occur. This condition implies that additional electron sinks were present in the reactor system in the cathode chamber. On the other hand, values greater than one indicate that an insufficient amount of electrons were transferred to yield the observed chemistry, suggesting that 1) an additional source of electrons was present to facilitate the reduction at the cathode, 2) CrVI was removed by other means, such as physical adsorption, and/or 3) unstable CrV and CrIV species were formed from 1 or 2 electron transfer reaction.
| Reaction | Standard potential @ 298 K (E°) vs. SHE2 | Standard potential @ 298 K and pH 7.0 (E°') vs. SHE24 | Calculated potential @ reactor conditions |
|---|---|---|---|
| a E° calculated from K. b Initial conditions: pH 7; [C3H5O3−] = 4 mM; [C3H3O3−] = 1 μM. c Initial conditions: pH 7; [C4H2O42−] = 4 mM; [C4H4O42−] = 1 μM. d Initial conditions: pH 7; [HCrO4−] = 48 μM; [Cr3+] = 1 nM. | |||
| Lactate oxidation to pyruvate | N/A | +0.185 V | +0.291 Vb |
| C3H5O3− → C3H3O3− +2H+ + 2e− | |||
| Fumarate reduction to succinate | N/A | +0.031 V | +0.137 V c |
| C4H2O42− +2H+ +2e− → C4H4O42− | |||
| Chromate reduction | +1.348 Va | +0.382 V | +0.474 V d |
| HCrO4− +7H+ + 3e− → Cr3+ + 4 H2O | |||
Results and discussion
Fumarate reduction
While all the selected strains of Shewanella are known to be capable of electron transfer to the anodic electrode,17,25 previous reports have not determined whether these strains of Shewanella are capable of catalyzing the cathodic reduction reaction in a bioelectrochemical system. Thus, the first set of experiments was aimed at verifying activity and evaluating performance at the cathode. For this purpose, fumarate reduction at the cathode was evaluated based on both the known physiology of Shewanella species14,16,26 and previously reported work with Geobacter species.27–29Anodic and cathodic potentials were measured versus a standard calomel electrode (Table 3). These potentials can be compared with calculated theoretical potentials under conditions experienced in the fuel cell reactor. The anode potentials were all similar in magnitude, an expected result since the conditions and species were the same at the anode for all experiments. The cathode potentials show wide and unexplained variability, probably due to the different species, specifically their interaction with the cathode electrode and differences in physiology. As an estimation of losses, these potentials can be compared to the calculated potential (E) of the fumarate half reaction under the given cathode conditions of +0.137 V vs. Standard Hydrogen Electrode (SHE). Because more negative values indicate losses due to the species and bioelectrochemical system,4 those species with more positive values should be capable of more effective electron transfer (lower activation losses), and consequently more power generation.
As shown in Fig. 2, linear polarization results show maximum power densities ranging from 10.2 to 59.4 nW cm−2 (26.5 to 154 nW cm−3) for MR-1 and W3-18-1, respectively. Current measurements during this determination show maximum current densities between 0.5 A m−3 for MR-1 and 2.4 A m−3 for W3-18-1. These values are lower, but on the same order of magnitude as current densities reported for fumarate-reducing systems utilizing Geobacter species and poised electrodes.28,29
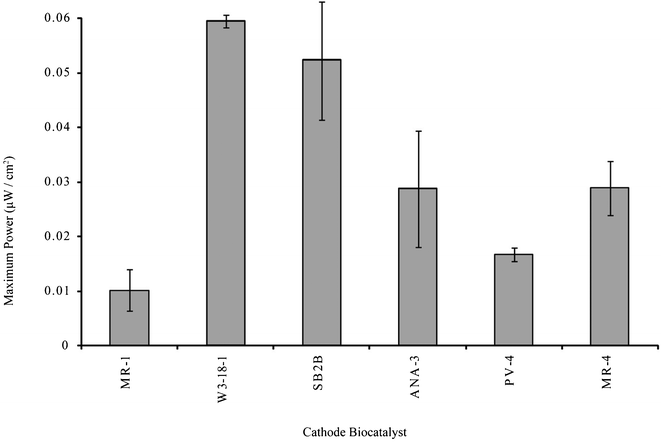 | ||
| Fig. 2 Maximum power determination from linear polarization sweeps of fumarate-reducing bioelectrochemical systems. | ||
Reduction of fumarate to succinate requires a 2 electron transfer reaction (Table 2). Previous studies with Geobacter have shown that incomplete oxidation of fumarate may occur, leading to a lower amount of charge transfer from the electrode to fumarate than would be predicted for full reduction to succinate. For example Gregory et al. showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electron consumption to succinate production ratio, implying that only half of the necessary electrons required for fumarate reduction were transferred from the electrode.27 On the other hand, Strycharz et al. reported an exact stoichiometric match (2
1 electron consumption to succinate production ratio, implying that only half of the necessary electrons required for fumarate reduction were transferred from the electrode.27 On the other hand, Strycharz et al. reported an exact stoichiometric match (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of electrons transferred to succinate production.29 Comparisons between the results reported in this paper and the results of previous studies may be difficult to make due to the differences in reactor architecture and its components.
1) of electrons transferred to succinate production.29 Comparisons between the results reported in this paper and the results of previous studies may be difficult to make due to the differences in reactor architecture and its components.
The ratio of e−chem to e−charge is shown in Fig. 3. The values here also show an imbalance, where electrons for succinate production are not completely accounted for by electrons transferred from the anode compartment. The ratios reported here represent a deficiency greater than the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 succinate to electron ratio (corresponding to an e−chem/e−charge of 2) reported by Gregory et al., which they attributed to incomplete fumarate oxidation.27 We show here that between 10.6 (MR-4) and 57.4 (SB2B) times the observed electron transfer would be required to account for the observed succinate production.
1 succinate to electron ratio (corresponding to an e−chem/e−charge of 2) reported by Gregory et al., which they attributed to incomplete fumarate oxidation.27 We show here that between 10.6 (MR-4) and 57.4 (SB2B) times the observed electron transfer would be required to account for the observed succinate production.
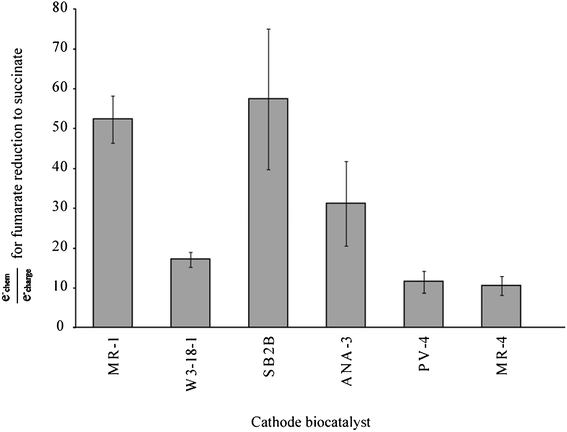 | ||
| Fig. 3 Efficiency ratio of hypothetical charge transfer requirements to observed transfers for a 2 electron reduction of fumarate to succinate. | ||
The extra electron pool could be explained through a number of possibilities. For example, substances in the extracellular biofilm matrix could be metabolized or inactive biomass could be degraded by extant bacteria. Freguia et al. demonstrated the use of a storage polymer as a source of electrons for the anodic catalysis, and the use of a similar storage compound by Shewanella for cathodic catalysis.30 This supports the open circuit control results (Fig. 4) which show reduction of fumarate by cells harvested in late-log/stationary growth phase in the absence of an electron donor. The speed and extent of fumarate conversion by closed circuit MFC systems was greater than that observed in these controls. No fumarate was detectable by HPLC in the cathode compartment of closed circuit experiments after 3 days, while open circuit experiments show fumarate persisting at detectable levels through the 4th day of operation.
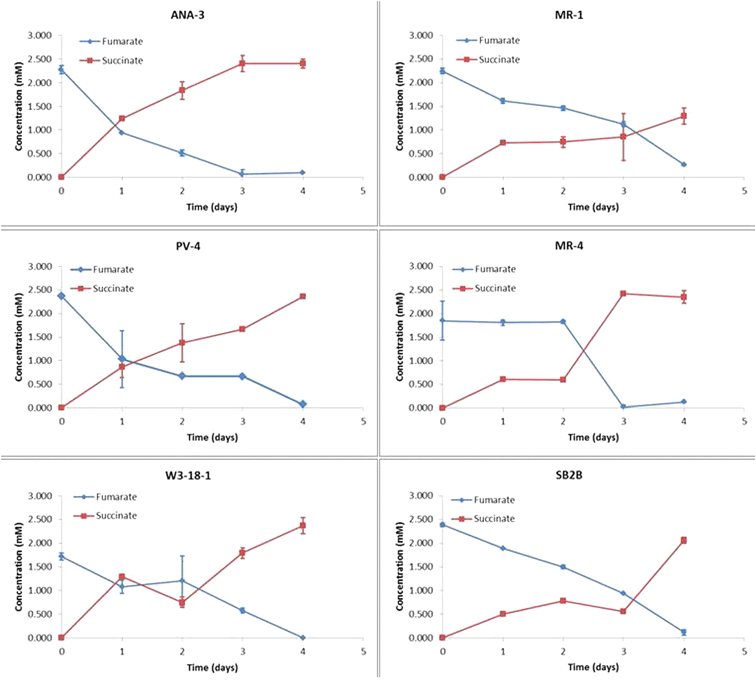 | ||
| Fig. 4 Fumarate reduction observed under open circuit conditions with each of the Shewanella strains. | ||
The cultures used in these experiments were harvested from minimal media batch cultures during late-log to stationary phase. Tang et al. reported that during aerobic and anaerobic growth on lactate, MR-1 will allocate a portion of carbon to be sent through the glyoxolate shunt, presumably for more complex carbon molecules used in biosynthesis and growth.31 Heidelberg et al. also present the possibility of glycogen synthesis and utilization as a possible energy storage strategy.13 Regardless of the source of these electrons, the use of this extra pool decreases the amount of current transferred from the anode side, adversely affecting overall fuel cell performance with fumarate as the electron acceptor.
Chromate reduction
Once fumarate reduction (succinate production) was completed, as indicated by a drop in current production (and later verified by HPLC analysis) the cathode compartments were injected with 20 mM CrVI stock solution to achieve a concentration of 2500 ppb (48 μM). CrVI was measured periodically during this semi-batch operation over a three day period, at which point another spike of CrVI was injected into the system. The residual CrVI concentrations after 3 successive exposures are shown in Fig. 5.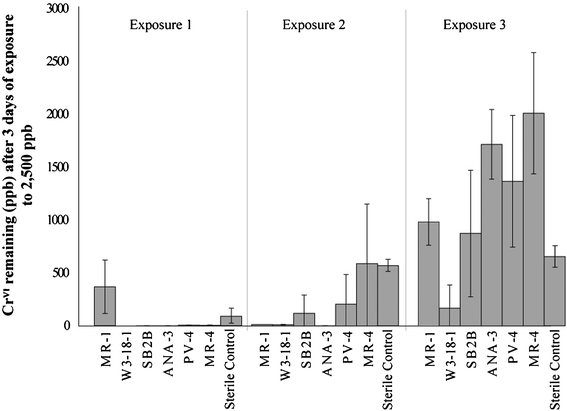 | ||
| Fig. 5 Residual chromium for each of the semi-batch chromium injection cycles. | ||
For the first exposure, CrVI levels were reduced to below 10 ppb for most species tested, and well below levels achieved with abiotic reduction. The exception was MR-1, where the remaining CrVI was about 4 times this amount (88 ppb). The second injections showed more variability between the species. Both MR-4 and the sterile cathode were able to bring CrVI concentrations to around 560 ppb. PV-4 and SB2B showed similar degrees of removal, with the final 3 day concentration between 100–200 ppb. MR-1, W3-18-1, and ANA-3 were all able to bring concentration to below 10 ppb. All species were able to reduce as well or better than the abiotic cathode for this CrVI exposure.
Final injections showed drastic differences from the first two CrVI exposures. For this injection, all species except for W3-18-1 showed an average residual chromium level between 700 and 2000 ppb, above that of the abiotic cathode (640 ppb). W3-18-1 catalyzed systems resulted in average residual chromium levels of 167 ppb. The relatively high concentrations of residual chromium indicate a finite tolerance limit to exposure of CrVI, consistent with previously reported batch studies.32 For W3-18-1, this tolerance appears to be much higher than the rest of the species tested.
Additionally, the inability to remove CrVI to concentrations comparable to the abiotic cathode indicates the possibility of fouling of the system by the biological species or reduced chromium species. Non-reducing biological material (such as cell debris, biofilm matrices, inactive/dead cells) may reduce the active surface area at the electrode surface. Reduced chromium species may also persist at the electrode surface after reduction, also resulting in a decrease in available surface area for reduction. The compounded effect of both biological fouling and chromium fouling serve to increase mass transfer limitations at the electrode surface, resulting in losses due to concentration polarization.
Fig. 6 shows that the e−chem/e−charge, the ratios for the initial cycle of chromium exposures are similar for all biocathodes. These values range from 0.87 (MR-1) to 1.64 (SB2B), indicating that most of the chromium underwent a 3 electron reduction to CrIII and that these electrons came from the cathode electrode. The slight increase of values greater than 1 indicates that either there is some incomplete oxidation of CrVI, or that minor adsorption to reactor surfaces and/or biomass may have occurred.
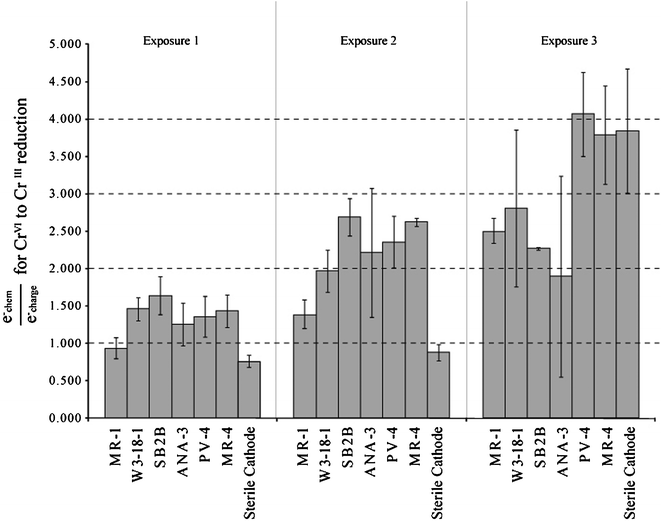 | ||
| Fig. 6 Ratio of hypothetical charge transfer requirements to observed transfers for a 3 electron reduction of CrVI to CrIII. | ||
The next exposure of chromium resulted in increased e−chem/e−charge values suggesting that electrons resulting in chromium reduction were probably sourced from other electron pools (as in fumarate reduction). Other reasons for this increase could be explained by adsorption of CrVI to fuel cell components or reduction of CrVI as single or double electron transfers (leading to the formation of unstable CrV and CrIV species). While MR-1 still exhibited ratios comparable to those observed in the first CrVI exposure, other strains showed values ranging from 1.97 (W3-18-1) to 2.69 (SB2B). These values show that only around half of the electrons necessary for a 3 electron reduction were passed from the anode side of the fuel cell. Studies have reported CrVI reduction by simple amino acids and natural organic matter which may explain the electrons unaccounted for by charge transfer from the anode side.2 Use of succinate as an electron source may also be possible, since MR-1 is reported to be able to oxidize succinate through TCA cycle enzymes.31,33
The amount of CrVI reduced during the third exposure suggest little to no activity for most species. Larger e−chem/e−charge values were observed that may indicate a higher dependence on the alternate electron sources. These sources likely include organic material resulting from cell inactivation or death due to chromium toxicity. High residual levels resulting for several of the Shewanella strains indicate that biological catalysis of chromate may have ceased and abiotic removal of chromate may be dominant during this period.
The sterile cathode results indicate that the anode biocatalysts clearly provide enough reducing power to achieve chromate reduction, but not to levels seen with the Shewanella biocathodes. The e−chem/e−charge values for the sterile cathode also showed differences from the biological cathodes. The initial exposure of the sterile cathode resulted in an e−chem/e−charge value of 0.80. For the second exposure, the e−chem/e−charge value rose to 0.88. Both of these values are very close to the expected value for 3 electron reduction of CrVI. The third injection shows a relatively large e−chem/e−charge. This may be explained removal of CrVI by other means such as adsorption to fuel cell surfaces or interactions with CrIII species. The abiotic cathode also maintained a lower average CrVI residual for the third exposure in comparison to the Shewanella biocathodes. This would suggest that biological material (either biofilm components or cellular debris) may prevent any significant adsorption/precipitation interactions with fuel cell components from taking place during this third exposure of CrVI.
The open circuit control experiment shows little to no removal of CrVI without an external electron source (Fig. 7). This is in contrast to observations of open circuit fumarate reduction where a significant fraction of the fumarate was reduced without an external electron source. The removal of a small amount of chromate by MR-1 and MR-4 begins after 24 h and could be attributed to reduction by cell lysate/debris or utilization of these materials by living cells as an electron donor in biocatalytic reduction mechanisms. This experiment also shows that adsorption of CrVI to cells and MFC materials is likely negligible in the presence of cellular material.
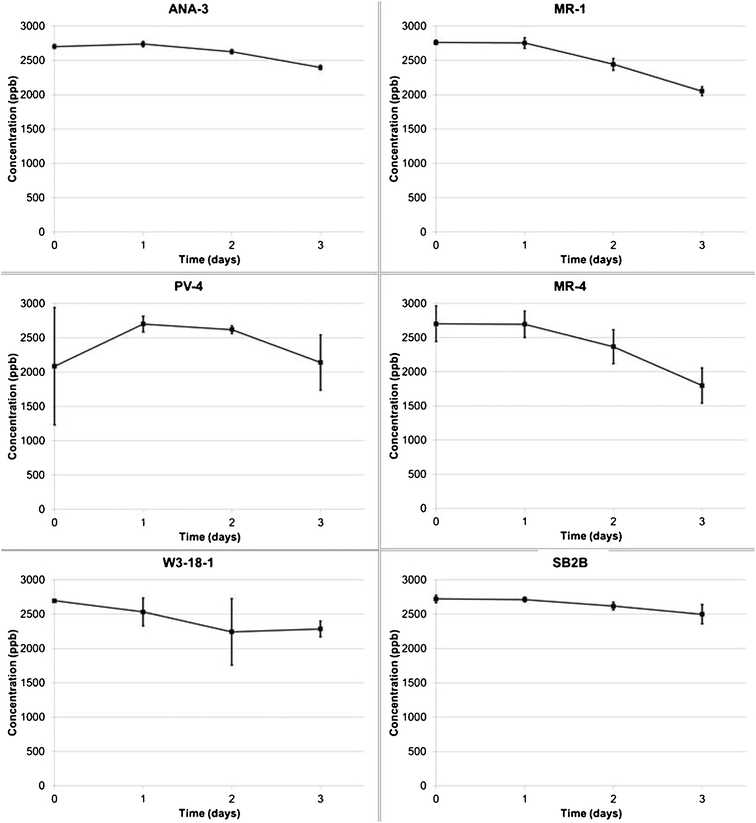 | ||
| Fig. 7 Chromium concentrations over 3 days under open circuit conditions for each of the Shewanella strains. | ||
To investigate the different possibilities of fouling (chemical species, biomass, etc.) further, electron microscopy and elemental analysis were employed to observe the electrode surface and determine the location of chromium at the electrode-biomass environment and to examine the extent of biofilm coverage. The results are shown in Fig. 8 and summarized in Table 4. The results show a variety of biofilm coverage, with W3-18-1 showing the greatest amount of bacteria attached to the electrode surface and PV-4 exhibiting the least. Furthermore, nodules of precipitated chromium were observed at the surface of several of the electrodes and associated with the biomass of certain species (MR-1, W3-18-1, SB2B, and ANA-3). It can be speculated from these observations that the mechanism of chromium reduction may be different for each Shewanella species and that factors such as biofilm attachment will influence CrVI reduction as well.
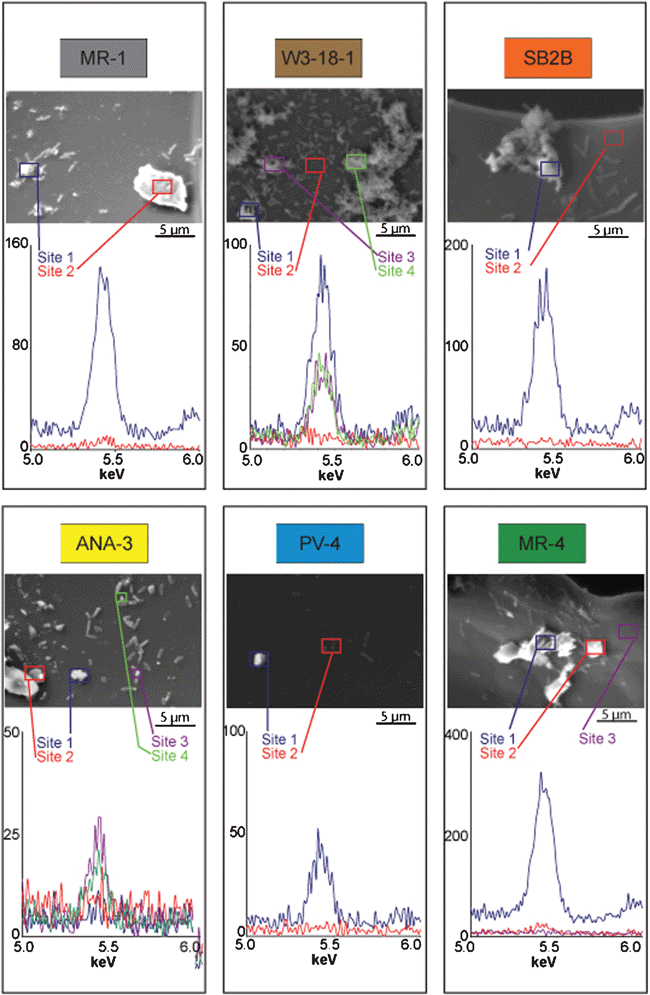 | ||
| Fig. 8 Scanning electron micrographs and elemental dispersive spectroscopy peaks for biocathode samples. | ||
| Species | Biomass attachment | Chromium seen as separate precipitates | Chromium associated with biomass |
|---|---|---|---|
| MR-1 | Moderate | Yes | Yes |
| W3-18-1 | High | No | Yes |
| SB2B | Moderate | No | Yes |
| ANA-3 | Moderate | Yes | Yes |
| PV-4 | Low | Yes | No |
| MR-4 | Moderate | Yes | No |
It should be noted that attempts at quantifying attached biomass by electron microscopy proved unreliable due to non-uniform coverage and attachment. Enumeration of planktonic biomass in the MFC, attempted through a serial dilution method, also gave variable results. Continuous transition between attached and planktonic states for biomass may also have affected quantification of any biomass changes. Thus, the contribution of planktonic or attached biomass to catalytic activity is not clear in these systems.
Similar results were reported by Huang et al. indicating chromium reduction occurring at the cell surface and in the biofilm matrix.11 Their results also indicate that there was no significant abiotic effect at the concentrations used. While biocathodes were able to reduce chromium to much lower levels than sterile cathodes in this study, reduction at the sterile cathode did seem to play a significant role at the concentrations of chromium used in the experiments presented here. This is in contrast to previous studies.10,11 However, those studies used levels of chromium at least one order of magnitude above the concentrations used here, and as a result the abiotic effects of reduction may have been insignificant when compared to the overall reduction reported. Although oxidation states of chromium were not determined in our study, previous batch reactor studies with MR-1 have shown that biomass associated precipitates are predominantly CrIII with minor amounts of CrVI.34
Mechanisms for electron transfer to electron acceptors
In order to understand the abilities of Shewanellae performing reduction at the cathode, it is important to understand the fundamental electron transfer strategies leading from the cathode, through the bacteria, and finally, to the terminal electron acceptor. There are several proposed mechanisms for electron transfer within the electrode-bacteria-oxidant system. These include direct electron transfer, use of an external mediator, or a combination of both strategies.7,8 The results presented here suggest that the electron transfer mechanisms for one species will show different behaviors based on the electron acceptor. Such results should not be surprising when considering the electron transfer pathways of the different species with different electron acceptors.Similar electron transfer pathways might be expected for fumarate reduction at the cathode when considering that there is only a single fumarate reductase that has been reported. The terminal fumarate reductase in MR-1 is a soluble periplasmic flavoprotein (FccA).35 Homologous genes appear in all strains tested, suggesting the fumarate reduction enzyme is the same among all the selected Shewanella strains.36
On the other hand, no common CrVI reducing enzyme or pathway has been found. Batch studies have shown CrIII precipitation to occur primarily at the exterior and periplasm of cells.32 These observations suggest reduction occurring at both the cell surface and in the periplasm. Previous studies also indicate that multiple mechanisms of chromium removal may be significant for MR-1.37 Enzymes similar to the variety of reductases and multi-heme cytochromes found in MR-1 can also be found in all of the species tested, and imply that several different physiological mechanisms may be involved in both chromium reduction and interaction with the electrode surface.16 The built-in redundancy of this system may lead to the observed inefficiencies in electron transfer, but may also enable a greater chance for bacteria survival when toxic chemicals such as CrVI are encountered.
Variability exists not only in the electron transfer to the terminal electron acceptor, but also in the transfer of electrons between the bacteria and electrode surface. Important genes implicated at anode studies of MR-1 show that proteins required for metal reduction (MtrABC) appear to be necessary for transfer of electrons to a solid electrode surface.25 The numerous cytochromes harboured by Shewanella suggest that the electron transfer pathways between the cell surface and the interior of the cell are very complicated and not yet fully understood.16 For example, several paralogs of the mtrABC genes are seen in MR-1 and orthologs for these genes are present in all species tested.36 These genes are believed to form complex electron transfer pathways for transfer of electrons to the exterior of the cell13 and may be used for transfer of electrons from an electrode to the interior of the cell. The complexity and variability of these electron transfer pathways between species may explain the differences in power generation and coulombic efficiencies observed in the fuel cell reactor.
Conclusions
The studies here give evidence of a fumarate reducing biocathode by Shewanella strains and provide a comparison between these species using the same fuel cell architecture. The results show differences in efficiency and power output that are related to the strain catalyzing reduction at the cathode.The present studies also demonstrate the ability of these species to reduce low levels of chromium at the cathode of an MFC, and that chromium is associated with the electrodes, and even biomass for certain Shewanella strains. Previously reported studies have primarily utilized enriched consortia as biological catalysts to provide electrons for reduction at ppm levels.9–11 While such methods may be useful for treatment of waste streams with relatively high chromium content, the highest reported CrVI concentrations for contaminated groundwater used for drinking water have not exceeded 6 ppm.38 Our studies demonstrate the feasibility of these systems to repeatedly bring CrVI concentrations from low ppm concentrations to low ppb concentrations. Considering the US EPA's chromium drinking water standard of 100 ppb12 and the World Health Organization's guideline of 50 ppb,39 removal of CrVI to these levels will be necessary if such systems will be used as remediation tools for contaminated water sources.
We also show here that the variety of electron transfer pathways that may be available to Shewanella likely accounts for the interspecies variability in efficiency and reduction capacity. If only a single pathway was utilized for electron transfer, then one would expect these values to be similar. The data presented here show that this is likely not to be the case, and that understanding these mechanisms will be important in understanding mechanisms of electron transfer in biocathodes.
Future work will focus on determining the physiological mechanisms for electron transfer at the cathode (and for CrVI reduction in particular). Understanding the fundamentals of electron transfer at the cathode will enable the design of more effective electrode materials and selection of more efficient biological catalysts. From a practical standpoint, implementation of a single strain treatment system would not be useful for treating real-world systems due to competition from other organisms. Thus, enrichment of mixed communities for both enhanced resistance to CrVI toxicity as well as enhanced electron transfer between electrodes will also be pursued. Additional efforts will focus on implementing larger scale reactor architecture to accommodate larger scale remediation efforts while simultaneously decreasing losses within the system to increase electron transfer efficiency. Evaluation of systems implementing this future work will allow a more rigorous assessment of this technology in real applications.
Acknowledgements
Funding was provided by the National Science Foundation CBET Award 0826198 and the University of Southern California Women in Science and Engineering Program.References
- A. D. Dayan and A. J. Paine, Hum. Exp. Toxicol., 2001, 20, 439–451 CAS.
- F. C. Richard and A. C. M. Bourg, Water Res., 1991, 25, 807–816 CrossRef CAS.
- E. Niebor and A. A. Jusys, in Chromium in the Natural and Human Environments, ed. E. Niebor and J. O. Nriagu, John Wiley & Sons, New York, 1988 Search PubMed.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schroder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Environ. Sci. Technol., 2006, 40, 5181–5192 CrossRef CAS.
- K. Rabaey, J. Rodriguez, L. L. Blackall, J. Keller, P. Gross, D. Batstone, W. Verstraete and K. H. Nealson, ISME J., 2007, 1, 9–18 CrossRef CAS.
- A. Rinaldi, B. Mecheri, V. Garavaglia, S. Licoccia, P. Di Nardo and E. Traversa, Energy Environ. Sci., 2008, 1, 417–429 CAS.
- L. Huang, J. M. Regan and X. Quan, Bioresour. Technol., 2011, 102, 316–323 CrossRef CAS.
- M. Rosenbaum, F. Aulenta, M. Villano and L. T. Angenent, Bioresour. Technol., 2011, 102, 324–333 CrossRef CAS.
- G. Wang, L. Huang and Y. Zhang, Biotechnol. Lett., 2008, 30, 1959–1966 CrossRef CAS.
- M. Tandukar, S. J. Huber, T. Onodera and S. G. Pavlostathis, Environ. Sci. Technol., 2009, 43, 8159–8165 CrossRef CAS.
- L. Huang, J. Chen, X. Quan and F. Yang, Bioprocess Biosyst. Eng., 2010, 33, 937–945 CrossRef CAS.
- 40 CFR §141.61(b), Code of Federal Regulations, 2010, vol. 40, part 141.61(b), p. 448.
- J. F. Heidelberg, I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson and C. M. Fraser, Nat. Biotechnol., 2002, 20, 1118–1123 CrossRef CAS.
- K. Nealson and J. Scott, in The Prokaryotes, SpringerNew York, 3 edn, 2006, vol. 6, pp. 1133–1151 Search PubMed.
- H. H. Hau and J. A. Gralnick, Annu. Rev. Microbiol., 2007, 61, 237–258 CrossRef CAS.
- J. K. Fredrickson, M. F. Romine, A. S. Beliaev, J. M. Auchtung, M. E. Driscoll, T. S. Gardner, K. H. Nealson, A. L. Osterman, G. Pinchuk, J. L. Reed, D. A. Rodionov, J. L. M. Rodrigues, D. A. Saffarini, M. H. Serres, A. M. Spormann, I. B. Zhulin and J. M. Tiedje, Nat. Rev. Microbiol., 2008, 6, 592–603 CrossRef CAS.
- O. Bretschger, A. C. M. Cheung, F. Mansfeld and K. H. Nealson, Electroanalysis, 2010, 22, 883–894 CrossRef CAS.
- S. Viamajala, B. M. Peyton, R. K. Sani, W. A. Apel and J. N. Petersen, Biotechnol. Prog., 2004, 20, 87–95 CrossRef CAS.
- A. Rhoads, H. Beyenal and Z. Lewandowski, Environ. Sci. Technol., 2005, 39, 4666–4671 CrossRef CAS.
- J. Kan, L. Hsu, A. C. M. Cheung, M. Pirbazari and K. H. Nealson, Environ. Sci. Technol., 2011, 45, 1139–1146 CrossRef CAS.
- Dionex Corporation, Determination of Hexavalent Chromium in Drinking Water Using Ion Chromatography, Application Update 144, 2003 Search PubMed.
- J. W. Weber, M. Pirbazari and G. Melson, Environ. Sci. Technol., 1978, 12, 817–819 CrossRef.
- D. F. Bray, J. Bagu and P. Koegler, Microsc. Res. Tech., 1993, 26, 489–495 CrossRef CAS.
- D. L. Nelson and M. M. Cox, Lehninger Principles of Biochemistry, Worth Publishers, New York, NY, 2000 Search PubMed.
- O. Bretschger, A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua, M. F. Romine, J. Zhou, A. S. Beliaev, R. Bouhenni, D. Saffarini, F. Mansfeld, B. H. Kim, J. K. Fredrickson and K. H. Nealson, Appl. Environ. Microbiol., 2007, 73, 7003–7012 CrossRef CAS.
- H. H. Hau and J. A. Gralnick, Annu. Rev. Microbiol., 2007, 61, 237–258 CrossRef CAS.
- K. B. Gregory, D. R. Bond and D. R. Lovley, Environ. Microbiol., 2004, 6, 596–604 CrossRef CAS.
- C. Dumas, R. Basseguy and A. Bergel, Electrochim. Acta, 2008, 53, 2494–2500 CrossRef CAS.
- S. M. Strycharz, T. L. Woodard, J. P. Johnson, K. P. Nevin, R. A. Sanford, F. E. Loffler and D. R. Lovley, Appl. Environ. Microbiol., 2008, 74, 5943–5947 CrossRef CAS.
- S. Freguia, K. Rabaey, Z. Yuan and J. r. Keller, Environ. Sci. Technol., 2007, 41, 2915–2921 CrossRef CAS.
- Y. J. Tang, H. G. Martin, P. S. Dehal, A. Deutschbauer, X. Llora, A. Meadows, A. Arkin and J. D. Keasling, Biotechnol. Bioeng., 2009, 102, 1161–1169 CrossRef CAS.
- R. Bencheikh-Latmani, A. Obraztsova, M. R. Mackey, M. H. Ellisman and B. M. Tebo, Environ. Sci. Technol., 2006, 41, 214–220 CrossRef.
- Y. J. Tang, A. L. Meadows, J. Kirby and J. D. Keasling, J. Bacteriol., 2007, 189, 894–901 CrossRef CAS.
- A. L. Neal, K. Lowe, T. L. Daulton, J. Jones-Meehan and B. J. Little, Appl. Surf. Sci., 2002, 202, 150–159 CrossRef CAS.
- C. R. Myers and J. M. Myers, FEMS Microbiol. Lett., 1992, 98, 13–19 CrossRef CAS.
- V. M. Markowitz, I. M. A. Chen, K. Palaniappan, K. Chu, E. Szeto, Y. Grechkin, A. Ratner, I. Anderson, A. Lykidis, K. Mavromatis, N. N. Ivanova and N. C. Kyrpides, Nucleic Acids Res., 2010, 38, D382–D390 CrossRef CAS.
- S. Viamajala, B. M. Peyton, W. A. Apel and J. N. Petersen, Biotechnol. Bioeng., 2002, 78, 770–778 CrossRef CAS.
- B. J. Collins, M. D. Stout, K. E. Levine, G. E. Kissling, R. L. Melnick, T. R. Fennell, R. Walden, K. Abdo, J. B. Pritchard, R. A. Fernando, L. T. Burka and M. J. Hooth, Toxicol. Sci., 2010, 118, 368–379 CrossRef CAS.
- World Health Organization, Guidelines for drinking-water quality, 3rd edition incorporating 1st and 2nd addenda, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Nernst equation calculations. See DOI: 10.1039/c2ra20478a |
| This journal is © The Royal Society of Chemistry 2012 |
