High compressibility of a flexible metal–organic framework†
Pablo
Serra-Crespo
a,
Eli
Stavitski
*b,
Freek
Kapteijn
a and
Jorge
Gascon
*a
aCatalysis Engineering–Chemical Engineering Dept, Delft University of Technology, Julianalaan 136, 2628, BL Delft, The Netherlands. E-mail: j.gascon@tudelft.nl
bNational Synchrotron Light Source, Brookhaven National Laboratory, Upton, NY 11973, US. E-mail: istavitski@bnl.gov
First published on 30th March 2012
Abstract
The metal–organic framework NH2-MIL-53(In) shows a very high amorphization resistance (>20 GPa) together with a large compressibility (K0 = 10.9 GPa).
Metal–Organic Frameworks (MOFs) are some of the most studied porous materials nowadays. Next to an exceptionally high surface area and pore volume, the chemical environment in MOFs can be fine-tuned by selecting the appropriate building blocks1 and/or by post-synthetic treatment,2 resulting in nearly infinite possibilities that might eventually allow the dream of rational design to be reached.
During the last few decades, extensive work has been published on the synthesis of new MOF structures as well as on promising applications, ranging from catalysis3 to gas separation,4 and from nanomedicine5 to optoelectronics.6 The high porosity of MOFs that, in general, is much higher than that of their inorganic counterparts, zeolites, is probably the main reason for their success. At the same time, this large porosity may restrict eventual applications: it is easy to envisage that very porous materials might collapse easily when submitted to relatively large pressures, i.e., during extrusion or pelletization (of the utmost importance for applications like catalysis or gas storage).
Despite the apparent importance of the research into the mechanical behaviour of this new class of materials, only a small number of studies on the MOF mechanical properties have been reported to date. Chapman et al. described the change in lattice parameters of CuBTC upon pressurization by different guest molecules.7 Later, Spencer et al., studied the phase transition of a zinc imidazolate in the range of 0.5–0.8 GPa.8 The compressibility and bulk moduli of single crystals and polycrystalline MOF powders have been mainly studied by high-pressure X-ray crystallography in a diamond anvil cell.9,10 Amorphization in most studied MOFs has been observed at pressures as low as 0.34 GPa for ZIF-86 and 3.5 MPa for MOF-5.11 More recently, the reversible pressure-induced amorphization of ZIF-4 was reported to occur at 6.5 GPa irrespective of pore occupancy and takes place via a high-pressure phase (formed at 3.7 GPa). On the other hand, based on the pressure-induced changes in lattice volume obtained from high pressure X-ray diffraction, the bulk moduli (K0) of MOFs were determined from the Birch–Murnaghan equation of state,12 with values varying from 3 to 30 GPa, mostly depending on the density of the studied MOF.
A special class of MOFs are those whose pore dimensions may change without breaking chemical bonds within the framework. This results in special properties like the breathing effect,13,14 where pores contract or open during adsorption of molecules. This high degree of elasticity has achieved adsorption values as high as 230 vol.% of gas molecules.15 An example of a breathing type material is the MIL-53 series (MIL stands for Materials from Institut Lavoisier), with changes of 100% in the unit cell volume upon adsorption/desorption. MIL-53 is built from M(OH)2 octahedra (where M can be Fe3+, Cr3+, Al3+, Ga3+, In 3+ or Sc3+) and 1,4-benzene-dicarboxylate (terephthalate) linkers.14 During adsorption of guest molecules, e.g. CO2 or H2O, the MIL-53 framework pore dimensions reversibly change. For the Cr or Al containing forms of MIL-53, MIL-53(Cr) or MIL-53(Al), the structure in which the pores are in the “open” form (long pore form, lp) is the most stable after thermal activation. Serre and co-workers induced the change lp→np on the MIL-53(Cr) framework using external pressure (mercury at 1 MPa).16 In spite of the proof of concept that flexible MOFs can be squeezed using external pressure, to the best of our knowledge, to date no more literature exists on the elastic properties of flexible MOFs.
During the last few years we have extensively investigated the adsorption and chemical properties of the amine functionalized version of MIL-53 (hereafter NH2-MIL-53).17–21 The NH2-MIL-53 framework is synthesized using amino-terephthalic acid as a linker instead of the unfunctionalized terephthalic linker. The inclusion of the amine functionality has very interesting consequences for the adsorptive performance of the NH2-MIL-53. Although we initially assumed the NH2-MIL-53 would behave similarly to MIL-53(Al or Cr), i.e. starting in the open pore configuration after thermal activation, followed by a transition to the narrow pore form after adsorption of CO2 molecules at relatively low pressures, a more detailed combined spectroscopic (XRD, IR) and theoretical (DFT) study indicated that NH2-MIL-53 behaves more like MIL-53(Fe), where a very narrow pore (vnp) form prevails after thermal activation. In the absence of guest molecules, the dehydrated form of amino-MIL-53 is in a vnp form because of the efficient hydrogen bonding between –NH2 groups and the framework hydroxyls when the framework is contracted.20,21 More recently, we discovered that the effect of amines is similar when the metal nodes in the NH2-MIL-53(X) structure are varied (X= Al, Fe, Ga, In, Sc).22
Encouraged by the outstanding CO2 separation performance of the functionalized NH2-MIL-53 series, and with an eye on possible large scale application either in the shape of pellets or as mixed matrix membranes,23 we investigated the mechanical properties of the newly synthesized NH2-MIL-53(In). The pressurization study was carried out using synchrotron-based powder X-ray diffraction in a diamond anvil cell (DAC) pressure apparatus at beamline X17C at the National Synchrotron Light Source (NSLS), Brookhaven National Laboratory. The DAC consists of two opposing diamonds with the sample compressed between the culets. In order to avoid axial tensions, a 0.25 mm pre-indented steel gasket is placed between the culets and filled with a fluid to apply a hydrostatic pressure. The pressure is generated when the DAC, filled with the sample and hydrostatic fluid, is sealed and squeezed.24 The pressure inside the chamber is monitored by observation of the fluorescence signal of a small ruby chip placed inside the DAC. The position of the fluorescence maximum of this material has a linear dependence up to 25 GPa.25 Prior to the insertion into the gasket hole, the sample was compacted; after that the chamber was loaded with a pressurization fluid and the pressure was increased in steps of 1 GPa. The pressure was measured before and after exposing the cell to the synchrotron radiation but no differences were found.
In the first series of experiments, we followed the evolution of the XRD pattern of the NH2-MIL-53(In) framework using a MeOH : EtOH = 1:1 mixture as pressurization fluid (Fig. 1). In the presence of a penetrating fluid, the original vnp structure expands to the NH2-MIL-53(In)lp (sp.gr. Imma). As the pressure in the DAC increased, a slight change in the XRD patterns of the solid could be observed that we attribute to a partial deformation closure of the structure (peak at 2θ = 2.2°, corresponding to the [2 0 0] plane of the vnp configuration, see below). It seems that in order to better withstand the external pressure, the structure collapses slightly. Apart from this change, already observed at relatively low pressures (1 GPa), no further changes were observed, demonstrating a high stability of the framework and evidencing that external pressure cannot be used to squeeze the material (at least when the adsorbed fluid and that used for pressurization are the same).
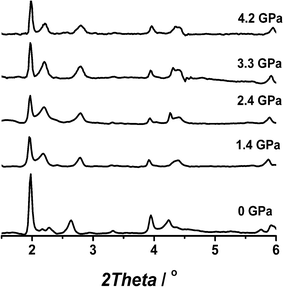 | ||
Fig. 1 Powder XRD patterns (λ = 0.37677 Å) of NH2-MIL-53(In) under different hydrostatic pressures using a MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH = 1 EtOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol. mixture as pressurization fluid. 1 vol. mixture as pressurization fluid. | ||
It has been demonstrated that the use of penetrating fluids enhances the stability of the framework under study.26 In the case of frameworks where several phases might exist, it therefore becomes crucial to compare results obtained using both penetrating and non-penetrating fluids. In a second series of experiments, mineral oil was used instead of the alcohol mixture. Fig. 2 shows the obtained XRD patterns under different hydrostatic pressures. The mineral oil does not enter the pores of the NH2-MIL-53(In), as evidenced by the initial vnp configuration. The obtained XRD pattern corresponds to the vnp configuration (sp.gr. Cc) of the NH2-MIL-53 series.21 The main reflections represent the [2 0 0] and [−1 1 1] planes (see Fig. 2, top inserts). Upon increasing the hydrostatic pressure inside the cell, a slight decrease in the reflection intensity along with a broadening and a shift to higher degrees (changes in d spacing higher than 0.1 Å) can be observed. The crystallinity of the structure, however, is remarkably maintained up to pressures as high as 23 GPa, much higher than any other MOF previously reported in the literature under similar experimental conditions.27 Experiments performed at decreasing pressure demonstrate that the compression of the framework is fully reversible up to pressures of 15 GPa. This is a clear advantage for the prospective use of similar materials, since external pressures of several GPa are commonplace in industrial applications (i.e. during shaping or formulation).
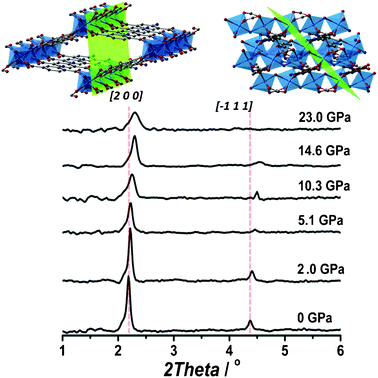 | ||
| Fig. 2 Powder XRD patterns (λ = 0.37677 Å) of NH2-MIL-53(In) under different hydrostatic pressures using a non-penetrating fluid (mineral oil). The planes corresponding to the main reflections are depicted in green. | ||
A third-order Birch–Murnaghan equation of state (see eqn (S1), ESI†) describing the correlation between unit cell volume and solid energy, was fitted to the hydrostatic data, yielding a bulk modulus, K0, for NH2-MIL-53(In) of 10.9 GPa (Fig. 3), with a K0′ value of 126. The low value obtained for the bulk modulus demonstrates that the NH2-MIL-53(In) framework is highly compressible, even when starting from a very narrow pore form. The high compressibility together with the high mechanical stability infers an outstanding structural robustness. When comparing the obtained bulk modulus versus the physical density of the material (obtained by He pycnometry) with several major classes of materials (Fig. 3), it can be observed that the NH2-MIL-53(In) framework falls at the interface between metals and polymers and surprisingly much closer to the behavior of zeolites than to that of other MOFs.
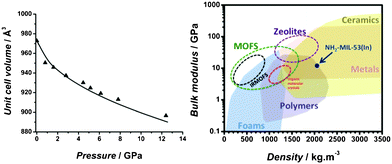 | ||
| Fig. 3 Evolution of the NH2-MIL-53(In) cell volume under different hydrostatic pressures using a non-penetrating fluid (mineral oil) and fitting to a third-order Birch–Murnaghan equation of state (left). Bulk modulus versus physical density map for NH2-MIL-53(In), plotted alongside major classes of materials (adapted from ref. 27). | ||
Summarizing, the NH2-MIL-53(In) framework presents a much higher resistance to amorphization than any previously reported MOF. Experiments performed in the presence of penetrating and non-penetrating fluids demonstrated that both vnp and lp forms of the material are highly robust. When starting from the vnp form and in the absence of adsorbates in the pores, the NH2-MIL-53(In) displays a remarkable compressibility. All together, these results demonstrate that framework flexibility is not a priori a limitation for MOF processing. In addition, matching mechanical properties of MOFs with other materials for the processing of composites (i.e. together with polymers for applications as membranes or in optoelectronics) should become easier if flexible frameworks are used.
Acknowledgements
J.G. gratefully acknowledges the Dutch National Science Foundation (NWO-CW VENI) for financial support. We are grateful to Zhiqiang Chen for his help with XRD experiments. This research was partially supported by COMPRES, the Consortium for Materials Properties Research in Earth Sciences under NSF Cooperative Agreement EAR 10-43050. Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886.References
- T. J. Barton, L. M. Bull, W. G. Klemperer, D. A. Loy, B. McEnaney, M. Misono, P. A. Monson, G. Pez, G. W. Scherer, J. C. Vartuli and O. M. Yaghi, Chem. Mater., 1999, 11, 2633–2656 CrossRef CAS.
- Z. Q. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC.
- A. Corma, H. Garcia and F. X. L. Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Ferey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS.
- K. W. Chapman, G. J. Halder and P. J. Chupas, J. Am. Chem. Soc., 2008, 130, 10524–10526 CrossRef CAS.
- E. C. Spencer, R. J. Angel, N. L. Ross, B. E. Hanson and J. A. K. Howard, J. Am. Chem. Soc., 2009, 131, 4022–4026 CrossRef CAS.
- S. A. Moggach, T. D. Bennett and A. K. Cheetham, Angew. Chem., Int. Ed., 2009, 48, 7087–7089 CrossRef CAS.
- K. W. Chapman, G. J. Halder and P. J. Chupas, J. Am. Chem. Soc., 2009, 131, 17546–17547 CrossRef CAS.
- Y. H. Hu and L. Zhang, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 174103 CrossRef.
- F. Birch, J. Geophys. Res., 1952, 57, 227–286 CrossRef CAS.
- C. Serre, S. Bourrelly, A. Vimont, N. Ramsahye, G. Maurin, P. Llewellyn, M. Daturi, Y. Filinchuk, O. Leynaud, P. Barnes and G. Férey, Adv. Mater., 2007, 19, 2246–2251 CrossRef CAS.
- C. Serre, F. Millange, C. Thouvenot, M. Nogues, G. Marsolier, D. Louer and G. Ferey, J. Am. Chem. Soc., 2002, 124, 13519–13526 CrossRef CAS.
- S. Surble, C. Serre, C. Mellot-Draznieks, F. Millange and G. Ferey, Chem. Commun., 2006, 284–286 RSC.
- I. Beurroies, M. Boulhout, P. L. Llewellyn, B. Kuchta, G. Férey, C. Serre and R. Denoyel, Angew. Chem., Int. Ed., 2010, 49, 7526–7529 CrossRef CAS.
- S. Couck, J. F. M. Denayer, G. V. Baron, T. Remy, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2009, 131, 6326–6327 CrossRef CAS.
- S. Couck, T. Remy, G. V. Baron, J. Gascon, F. Kapteijn and J. F. M. Denayer, Phys. Chem. Chem. Phys., 2010, 12, 9413–9418 RSC.
- A. Boutin, S. Couck, F.-X. Coudert, P. Serra-Crespo, J. Gascon, F. Kapteijn, A. H. Fuchs and J. F. M. Denayer, Microporous Mesoporous Mater., 2011, 140, 108–113 CrossRef CAS.
- E. Stavitski, E. A. Pidko, S. Couck, T. Remy, E. J. M. Hensen, B. M. Weckhuysen, J. Denayer, J. Gascon and F. Kapteijn, Langmuir, 2011, 27, 3970–3976 CrossRef CAS.
- S. Couck, E. Gobechiya, C. E. A. Kirschhock, P. Serra-Crespo, J. Juan-Alcañiz, A. Martinez-Joaristi, E. Stavitski, J. Gascon, F. Kapteijn, G. V. Baron and J. Denayer, ChemSusChem, 2012, 5, 740–750 CrossRef CAS.
- Unpublished results.
- B. Zornoza, A. Martinez-Joaristi, P. Serra-Crespo, C. Tellez, J. Coronas, J. Gascon and F. Kapteijn, Chem. Commun., 2011, 47, 9522–9524 RSC.
- A. Katrusiak, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 135–148 CrossRef.
- R. A. Forman, G. J. Piermarini, J. Dean Barnett and S. Block, Science, 1972, 176, 284–285 CAS.
- A. J. Graham, D. R. Allan, A. Muszkiewicz, C. A. Morrison and S. A. Moggach, Angew. Chem., Int. Ed., 2011, 50, 11138–11141 CrossRef CAS.
- J. C. Tan and A. K. Cheetham, Chem. Soc. Rev., 2011, 40 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details and additional results. See DOI: 10.1039/c2ra20528a/ |
| This journal is © The Royal Society of Chemistry 2012 |
