Epimers vs. inverse epimers: the C-1 configuration in alnumycin A1†
Petri
Tähtinen
*a,
Terhi
Oja
b,
Nadine
Dreiack
ab,
Pekka
Mäntsälä
b,
Jarmo
Niemi
b,
Mikko
Metsä-Ketelä
b and
Karel D.
Klika
*a
aDepartment of Chemistry, University of Turku, Vatselankatu 2, FIN-20014, Turku, Finland. E-mail: klikakd@yahoo.co.uk; petri.tahtinen@utu.fi; Fax: +358 (0)2 333 6700; Tel: +358 (0)2 333 6804
bDepartment of Biochemistry and Food Chemistry, University of Turku, Vatselankatu 2, FIN-20014, Turku, Finland
First published on 26th March 2012
Abstract
The determination of whether two stereoisomers constitute an epimeric pair or if they differ in their configuration at all stereogenic centers bar one can be deceptive, and even more problematic than just absolute configuration determination per se. The latter stereochemical relationship is hereby defined as a pair of inverse epimers and is exemplified by alnumycin A1 to introduce the concept.
Bacterial aromatic polyketides exhibit high bioactivities and have a number of established medicinal applications,1 including use as antibiotics. Typically, a polyaromatic aglycone chromophore is substituted with various sugars, often a necessity for exhibiting biological activity. Alnumycin2 (1, Fig. 1) is an interesting case as it possesses a highly unusual 4′-hydroxy-5′-hydroxymethyl-2′,7′-dioxane moiety which is attached atypically via a C–C bond to the isochromanequinone aglycone (viz. prealnumycin, 2).
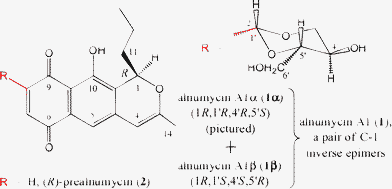 | ||
| Fig. 1 The structures of the inverse epimeric pair (2)-(1R,1′RS,4′RS,5′SR)-alnumycin A1 (1α and 1β) and prealnumycin (2). The recently described4 notation for indicating relative stereochemistry as an extension to the standard Natta projection system5 is used and where the ‘2’ near to the C-1′ atom and preceding the name indicates that the number of stereoisomers present in the sample or under consideration is two. | ||
The gross structures of the stereoisomers denoted herein as alnumycin A1 (1), isolated from a culture broth of Streptomyces sp. CM020,3 have been reported previously under various monikers, viz. BE-41956A,2a alnumycin,2b and K1115 B1.2c However, only one stereoisomer was indicated in the latter two reports and absolute configurations were not determined. Indeed, only the relative configuration of the dioxane ring was indicated by Bieber et al.2b (nominally 1′RS,4′RS,5′SR, thus equating to 1α or 1β) and though no stereochemistry was indicated by Naruse et al.,2c their compound clearly possessed the same relative dioxane ring configuration. Recently, Tatsuta et al.2d identified two stereoisomers, both with the same aforementioned2b relative dioxane ring configuration, from S. griseorubiginosus (Mer-K115) and determined their absolute configurations. Their compounds, denoted as K1115 B1α and K1115 B1β with absolute configurations of 1R,1′R,4′R,5′S and 1R,1′S,4′S,5′R, respectively, were assigned by stereospecific synthesis of the two compounds and their enantiomers followed by comparison of NMR spectra and optical rotations (ORs) to those of the natural products.‡ Herein, K1115 B1α and K1115 B1β are denoted as alnumycin A1α (1α) and alnumycin A1β (1β), respectively. Thus, intriguingly, 1α and 1β are not epimers of one another as one might surely have initially suspected, and their special relationship – in that they differ from each other in their configurations at all stereogenic centers bar one – has realized the genesis of the concept of inverse epimers as indicated in Fig. 1 and for which a definition is: Inverse epimers are stereoisomers containing more than two stereogenic centers that differ in their configuration at all stereogenic centers except one. In other words, the inverse of the epimeric case.
Alnumycins A1α (1α) and A1β (1β), isolated as part of our work elucidating their biogenesis,6 would otherwise constitute a pair of enantiomers if it were not for the chiral atom C-1. Since C-1 is quite distant from the other stereogenic centers, all located in the dioxane ring, there is only a minimal interaction between C-1 and the remaining stereogenic centers, resulting in exceedingly similar NMR spectra (see ESI†) for 1α and 1β and the compounds are almost enantiomers. For 1α and 1β, since they differ at all stereogenic centers bar C-1, they are C-1 inverse epimers. The intriguing question is, upon encountering such compounds, do the two stereoisomers constitute a pair of epimers or a pair of inverse epimers? Analytical challenges arise because the properties of a compound A relative to its epimer E under achiral conditions are exactly the same as those between A and its inverse epimer IE. This is because E and IE are enantiomers. This concept is depicted in Fig. 2 using the configurations for the pair of stereoisomers reported by Tatsuta.2d
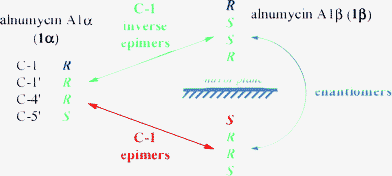 | ||
| Fig. 2 Schematic indicating the stereochemical relationships between alnumycin A1α (1α), its C-1 epimer, and its C-1 inverse epimer 1β. | ||
Whether a compound and its epimer/inverse epimer exhibit large discernible spectral or physicochemical property differences is dependent on the disposition of the pertinent chiral center (the chiral center that changes in the case of epimers or is invariant in the case of inverse epimers) and its relation to the other stereogenic centers in the molecule. But regardless of whether property differences are large or not, the premise of a sizable challenge in distinguishing between a case of epimers or inverse epimers is maintained; to effect distinction, by either chirooptical methods {e.g. OR, electronic circular dichroism (ECD), or vibrational circular dichroism (VCD)}, X-ray diffraction, chiral-sensitive methods {e.g. NMR using either chiral derivatizing agents (CDAs) or chiral solvating agents (CSAs)}, or asymmetric synthesis, is requisite. Indeed, such an analysis can transcend the determination of the absolute configuration of enantiomers given that determination of one enantiomer yields the other by default whereas for this case, both compounds may be required to have their absolute configurations independently determined, with the presence of enantiomers only compounding matters.§
To determine the C-1 configuration in prealnumycin (2) and alnumycin A1 (1), experimental ECD spectra were compared to theoretical spectra7 (Fig. 3) calculated for structures with a 1R configuration (see ESI† for modeling details and results: for 1, three conformers were located; for 2, only 1 low-energy conformer was found). Overall, the match8 between the predicted and observed spectra for 2 was high, thus yielding the C-1 configuration as R. Similarly, the match between the predicted and observed spectra for 1 was also high and therefore it too has an R configuration for C-1. Since the observed spectra for 1 and 2 are essentially identical, it implies that the C-1′ and other stereogenic centers in 1 perturb the ECD spectra only minimally and hence the spectra are dependent only on the C-1 configuration. This is further substantiated by the predicted spectra for the inverse epimers 1α and 1β (Fig. 3) which are extremely similar, and likewise as well for the individual conformers of 1α and 1β (data not shown).
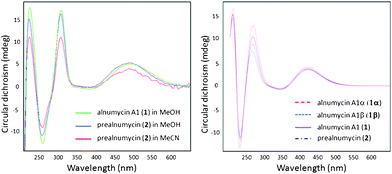 | ||
Fig. 3 Observed (left) and calculated (right) ECD spectra of alnumycin A1 (1) and (R)-prealnumycin (2). The calculated spectra of 1α and 1β are Boltzmann-calculated population-weighted averages9 of two conformers whilst 1 is an average of 1β and 1α based on a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively, obtained from 1H NMR. This figure, enlarged, is also shown in the ESI†. 2, respectively, obtained from 1H NMR. This figure, enlarged, is also shown in the ESI†. | ||
Intriguingly, not only is the 1R configuration proven for alnumycin A1 (1) and prealnumycin (2), but since in 1 the ratio of 1β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1α was 3
1α was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively, this then proves them to be C-1 inverse epimers as a consequence of the independence of the ECD spectra to configurations other than C-1. This is because a near equal amount of C-1 epimers would not yield comparably intense ECD (i.e., cf.2) as one stereoisomer would be replaced by its enantiomer (e.g.1β, Fig. 2), thus cancelling to a degree the ECD response of the remaining stereoisomer. Thus, one would not even have to utilize calculations if this were known a priori.
2, respectively, this then proves them to be C-1 inverse epimers as a consequence of the independence of the ECD spectra to configurations other than C-1. This is because a near equal amount of C-1 epimers would not yield comparably intense ECD (i.e., cf.2) as one stereoisomer would be replaced by its enantiomer (e.g.1β, Fig. 2), thus cancelling to a degree the ECD response of the remaining stereoisomer. Thus, one would not even have to utilize calculations if this were known a priori.
Furthermore, a strong dependency on the C-1 configuration only also holds for alnumycin A1 (1) with respect to OR,‡ and taken together with the ECD spectra being independent of configurations other than C-1 (an explanation for the dependency of the optical properties of 1 on C-1, and not C-1′, etc. is postulated further on), the following postulate can be formulated: If there are chirooptical or other changes in properties dominated by or localized to particular portions containing the stereogenic centers which are subject to the local chirality, then these can provide an indication of whether there is present a case of epimers or one of inverse epimers. One caveat to this tenet would be that if non-structurally altering methods, e.g. chirooptical methods, cannot be utilized, then singular changes are preferred, i.e. single derivatization rather than multiple derivatization, and that only singular effects are enacted, e.g. derivatization that does not cause concomitant alterations such as conformational changes. This aspect is currently under examination.
Of note, Bieber2b reported an OR for their alnumycin A1 (1) of only +170°, thus implicating a mixture of stereoisomers with variability at C-1 based on the large ORs reported.‡ The specific rotation (+1000°) reported by Tatsuta2d for their natural product, consisting of 1R,1′R,4′R,5′S and 1R,1′S,4′S,5′R stereoisomers 1α and 1β, was in concert with our measurement¶ of +855° and the determination of a pure 1R configuration. Whilst meaningful calculated ORs can be hard to obtain, in this instance, due to the sizeable magnitude of the ORs, they have provided unequivocal evidence (see ESI†) of the 1R configuration for our natural 1.
Regarding these large [α] values and their dependency, the primary reason for their size and why the C-1 configuration has a wholesale determinant effect on the sign of the rotation whilst the C-1′ configuration is ineffective because of the distortion to the conjugated system caused by the non-planarity of the pyran ring resulting in a curving of part of the system (primarily the C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C4 double bond but the axial electron lone pair of O-2 may be involved too) in one direction or the other. In the most stable conformation of prealnumycin (2) with an axially oriented n-propyl group, the dihedral angle, φ, of the C3
C4 double bond but the axial electron lone pair of O-2 may be involved too) in one direction or the other. In the most stable conformation of prealnumycin (2) with an axially oriented n-propyl group, the dihedral angle, φ, of the C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C4–C4a
C4–C4a![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C5 segment is 164°. For the conformer with an equatorially oriented n-propyl group, the skew is directed in the opposite sense {φ(C3
C5 segment is 164°. For the conformer with an equatorially oriented n-propyl group, the skew is directed in the opposite sense {φ(C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C4–C4a
C4–C4a![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C5), −158°} and calculation of its OR provided a near equal value relative to the more stable conformer, but tellingly of opposite sign. An analogous result was obtained for the calculated ECD spectra, with spectra for the two conformers almost mirror images of one another (see ESI†). However, 2 is in an anancomeric state favoring the axially oriented n-propyl group (ΔG, 4.40 kcal mol–1) and thus the skew is persistent and orientates only in the one fixed direction due to the axial conformer being stable and inflexible. Hence, the C-1 configuration determines the direction of the curvature, i.e. the sense of chirality of the chromophore is controlled by the absolute configuration at C-1 and thus is the sign determining factor for the ECD bands as well as the OR,10whilst it is the fixed curvature of the conjugated system that realizes the large magnitude of [α].||
C5), −158°} and calculation of its OR provided a near equal value relative to the more stable conformer, but tellingly of opposite sign. An analogous result was obtained for the calculated ECD spectra, with spectra for the two conformers almost mirror images of one another (see ESI†). However, 2 is in an anancomeric state favoring the axially oriented n-propyl group (ΔG, 4.40 kcal mol–1) and thus the skew is persistent and orientates only in the one fixed direction due to the axial conformer being stable and inflexible. Hence, the C-1 configuration determines the direction of the curvature, i.e. the sense of chirality of the chromophore is controlled by the absolute configuration at C-1 and thus is the sign determining factor for the ECD bands as well as the OR,10whilst it is the fixed curvature of the conjugated system that realizes the large magnitude of [α].||
As a final consideration, it is worth contemplating why earlier accounts2b,c reported only the presence of a single stereoisomer of alnumycin A1 (1). This may have been due to the different Streptomyces strains utilized or the differing growing conditions yielding greater stereospecificity. The presence of a pair of stereoisomers for 1 may have also quite easily eluded2b,c detection since the degree of signal disparity varies greatly with the prevailing sample conditions under which they are examined** and in this particular examination the spectra for 1α and 1β were exceedingly similar (see Table S1, ESI† and accompanying notes). Incidentally, the diastereomeric predominance, 1α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1β, observed here was reversed in comparison to Tatsuta,2d though variation in the 1α
1β, observed here was reversed in comparison to Tatsuta,2d though variation in the 1α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1β ratio was evident from sample to sample. Slight perturbation due to chromatography was in fact evident to account for this, in addition of course to any variation arising from bacterial strain production.
1β ratio was evident from sample to sample. Slight perturbation due to chromatography was in fact evident to account for this, in addition of course to any variation arising from bacterial strain production.
In conclusion, it has been realized that it is beneficial to define a new stereochemical relationship, viz. that of inverse epimers. A conundrum arises in distinguishing between a case of a pair of epimers or one of inverse epimers because the epimer and the inverse epimer of a compound are enantiomeric, thus indistinguishable outside of a chiral environment and bearing the same relationship to the compound in question. In this work, distinction between the two cases was ascertained in the case of alnumycin A1 (1) by evaluation of experimental and predicted ECD spectra and the conundrum was resolved with an unequivocal result demonstrating that with considered application of an appropriate technique pertinent to the structure at hand, and bearing in mind the aforementioned caveat, the problem of distinguishing between a case of an epimeric pair or an inverse-epimeric pair of stereoisomers can be expeditiously surmounted.
Pertinently, the problem of epimers vs. inverse epimers is not limited to this special case however, and is conceivably wide ranging as doubtless there are likely to be numerous analogous cases of inverse epimeric pairs hidden in the vast arsenal of natural products. For example, due to the preponderance of certain moieties, e.g.D-sugars,†† workers are apt to merely assume such and perhaps not even consider the availability of alternatives. Thus it is perhaps usual that epimers are simply assumed and the notion of another stereoisomer, viz. the inverse epimer, is not even entertained let alone having the case proven one way to the other. Thus, the conundrum could easily be the cause of erroneous conclusions by chemists since they may just assume the simplest case (Ockham's razor§), or be overly reliant on predisposed presumptions (e.g.D- vs.L-sugars), or be even unaware that there could be a problem as the scenario is non-intuitive (i.e. assume that proving a case of epimers is all that is required without even the need for consideration of inverse epimers). The problem is not only of significant concern from a structural perspective as there are biochemical implications as well if the chirality deviates unknowingly from the expected.
Acknowledgements
Academy of Finland (grant no. 121688, 127844, and 136060), National Graduate School in Informational and Structural Biology, and Turku University Foundation are all thanked for financial support, and the CSC–IT Center for Science Ltd. is thanked for providing computational resources.References
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2007, 70, 461 CrossRef CAS
.
-
(a) M. Tsukamoto, M. Hirayama, S. Nakajima, T. Okabe, K. Ojiri and H. Suda, Jpn. Kokai Tokkyo Koho, 1997 Search PubMed
, JP 09241257; (b) B. Bieber, J. Nüske, M. Ritzau and U. Gräfe, J. Antibiot., 1998, 51, 381 CrossRef CAS
; (c) N. Naruse, M. Goto, Y. Watanabe, T. Terasawa and K. Dobashi, J. Antibiot., 1998, 51, 545 CrossRef CAS
; (d) K. Tatsuta, S. Tokishita, T. Fukuda, T. Kano, T. Komiya and S. Hosokawa, Tetrahedron Lett., 2011, 52, 983 CrossRef CAS
.
- T. Oja, K. Palmu, H. Lehmussola, O. Leppäranta, K. Hännikäinen, J. Niemi, P. Mäntsälä and M. Metsä-Ketelä, Chem. Biol., 2008, 15, 1046 CrossRef CAS
.
- K. D. Klika, Int. J. Org. Chem., 2011, 1, 215 CrossRef CAS
.
- Giulio Natta (1903–1979).
- T. Oja, K. D. Klika, L. Appassamy, J. Sinkkonen, P. Mäntsälä, J. Niemi and M. Metsä-Ketelä, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 6024 CrossRef CAS
.
- Calculated using time-dependent DFT (TD-DFT) at the M06-2X/6-311G+(d)//M06-2X/6-31G(d) level of theory with 24 excited states.
- The experimental ECD were difficult to reproduce theoretically with respect to absolute wavelength, but were left unscaled nevertheless.
- P. Tähtinen, A. Bagno, K. D. Klika and K. Pihlaja, J. Am. Chem. Soc., 2003, 125, 4609 CrossRef
.
- An analogous deduction has similarly been made for β-lactams (see ref. 11).
- R. Łysek, K. Borsuk, M. Chmielewski, Z. Kałuża, Z. Urbańczyk-Lipkowska, A. Klimek and J. Frelek, J. Org. Chem., 2002, 67, 1472 CrossRef
.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Modeled structures of 1 and 2 including Cartesian coordinates, characterization data, and experimental methods. See DOI: 10.1039/c2ra20537h/ |
| ‡ Tatsuta2d did not separate the two isomers of the natural product but a valid deduction was facilitated by very large specific rotations which depend seemingly only on the C-1 configuration and are essentially invariant to the dioxane ring stereochemistry. For the two synthesized 1R stereoisomers 1α and 1β, specific rotations of +1100° and +1000° were obtained whilst −900° was found for both unnatural 1S stereoisomers. |
| § Ockham's razor intimates that a pair of C-1 epimers would prevail over inverse epimers since the latter requires configurational changes at a number of centers instead of just one. One can speculate on the number of times it may have simply been assumed that the former must be the case. |
| ¶ The specific rotation of +855° measured here for the natural product alnumycin A1 (1) (lit.2d +1000°) was measured at much lower concentrations than reported due to the availability of only 10 cm cells. Thus, the lower value here does not necessarily imply a scalemic mixture for C-1, but does at the very least insinuate that the C-1 configuration must be R dominated. The holemicity (enantiopurity) of C-1 in both 1 and prealnumycin (2) was proven by CDA analysis and thus the large positive rotation is well in accordance with the 1R configuration determined by Tatsuta.2d |
| || Even though the λ used for the OR measurement (589.3 nm) was not far from an absorption band, calculated OR values at λ well away from the absorption range were still very sizeable nonetheless (see ESI†). |
| ** In fact, the 1H NMR spectra of 1α and 1β were remarkable in that for most signals it was extremely difficult to even gauge the presence of a pair of stereoisomers despite the fine linewidth of some signal lines. The results were found to be very concentration, solvent, and temperature dependent. The peak “splittings” due to stereoisomers could also be easily mis-interpreted as long-range scalar couplings in some instances. Thus, recognition of the presence of stereoisomers is dependent on many factors including a high dependency on the acquisition and processing parameters (particularly so for 13C NMR spectra) as well as B0 homogeneity given the extreme similarity of the compounds' spectra. Attention is drawn to specific signals and particular technical aspects in the text following the table of NMR data compiled in the ESI†. |
| †† Of the vast array of sugars (glycosides) found in Nature, many are (derived from) D-sugars but they can also be (composed from) L-sugars. |
| This journal is © The Royal Society of Chemistry 2012 |
