A polymeric membrane permeabilizer displaying densely packed arrays of crown ether lateral substituents†
Ming
Liu
a,
Anna
Bertova
b,
Nicolas
Illy
a,
Blandine
Brissault
a,
Jacques
Penelle
a,
Karol
Ondrias
b and
Valessa
Barbier
*a
aInstitut de Chimie et des Matériaux Paris-Est (ICMPE), CNRS and Université Paris-Est, 2-8 rue Henri Dunant, 94320 Thiais, France. E-mail: barbier@icmpe.cnrs.fr; Fax: +33 (0)1 49 78 12 08; Tel: +33 (0)1 49 78 11 94
bInstitute of Molecular Physiology and Genetics, Slovak Academy of Sciences, Vlarska 5, 833 34 Bratislava, Slovak Republic. E-mail: karol.ondrias@savba.sk; Fax: ++421 2 54773666; Tel: ++421 2 54774102
First published on 20th August 2012
Abstract
We report the design, synthesis and evaluation of a novel macromolecular membrane permeabilizer displaying geminally substituted crown-ethers (18-crown-6) on every third carbon alongside the backbone. The polymer has a rather high affinity with potassium as well as permeabilization properties towards K+, Na+ and Ca2+, including single-channel behavior.
Among the many molecular properties controlled by proteins in Nature, ion transport across biological membrane, as regulated by ion pumps, has always exerted a particular fascination. As a result, many efforts have been devoted to designing synthetic mimics whose efficiency in transporting inorganic ions such as Na+ or K+, two key players in biological processes, approaches the effectiveness of their natural counterparts.
The co-facial organization of crown-ethers on a specifically designed template is a common structural motif in artificial ion channels, and some key examples based on this design have been recently reported.1,2 Successful approaches have been developed. The first example makes use of oligo(p-phenylene) with eight phenylene units and pendant crown-ethers as ion transporting “relays”. These oligomers act as ionophores via depolarization experiments on polarized bilayer membranes in small unilamellar vesicles.3 In contrast to the octyphenyl rigid rods used in the above approach, Voyer's group has demonstrated the efficiency of semi-rigid α-helical peptidic scaffolds. The peptide includes side crown-ethers whose relative positions on the α-helix platform is controlled so as to generate a tubular stack parallel to the helix axis. It was recently suggested, based on preliminary in vitro studies, that these ion-channel mimics could be used as chemotherapeutic agents for cancer treatment.4 Finally, ion transport has also been evidenced for a more flexible system made of three aza-crown moieties linked together by alkyl spacers and dubbed “hydraphiles” by Gokel et al. This ionophore was also able to mediate cell death in vivo.5 More recently, novel hydraphiles made of four crowns, two of them linked by a ferrocene unit providing a more rigid structure, have been reported.6
Although an assessment of the relative efficiencies of the above structures cannot be made easily, as the ion transport measurements used in these studies are based on different techniques whose results cannot be directly compared, all the experimental data obtained thus far suggest that optimal distances among the crown-ether relays and a compromise between rigidity and flexibility play a key factor in the efficiency of the obtained ionophores.7
As a result, we report here that 18-crown-6 disubstituted poly(trimethylene-1,1-dicarboxylate), poly(1), yields a particularly efficient synthetic ion channel (Scheme 1). Poly(cyclopropane-1,1-dicarboxylates) have their lateral substituents located on every third carbon alongside the polymer backbone, which should generate intersubstituent distances of 0.75 nm for a backbone in a fully extended conformation. However, a structural study of poly(diethyl trimethylene-1,1-dicarboxylate) crystals revealed the existence of an equally stable semi-rigid 2-fold helix conformation that places the side substituents 0.585 nm apart.8 On the basis of these findings, one should expect an intersubstituent distance in between these two extreme values, i.e. in an optimal range for ion hopping as based on previous studies.
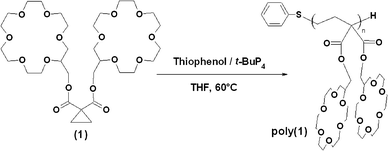 | ||
| Scheme 1 Monomer and polymer synthesis. | ||
Monomer (1) was prepared according to a two-step synthesis (Scheme S1†). First, a hydroxylmethyl-substituted crown-ether was coupled with malonyl dichloride. Then, the cyclopropane moiety was introduced via the condensation of the formed malonate with 1,2-dibromoethane. (1) was characterized by 1H NMR, 13C NMR, IR spectroscopy and elemental analysis (Fig. S1, S2, Table S1†). Poly(1) was prepared according to a recent metal-free (living) polymerization methodology.9 A phosphazenium thiophenolate, which had been generated in situ by mixing the t-BuP4 phosphazene base with thiophenol, initiates the polymerization (Scheme 1). The polymerization conversion Cv was determined by removing an aliquot from the polymerization reactor, quenching the carbanion with hydrochloric acid, and monitoring the disappearance of the monomer protons at 1.41 ppm (cyclopropyl ring) and the appearance of the –CH2CH2–C protons of the polymer backbone at 1.77 ppm. The theoretical molecular weight Mn,th was calculated from the [1]0/[PhSH]0 ratio (i.e., assuming quantitative initiation) taking into account the conversion. All synthesized polymers were purified by dialysis and fully characterized by 1H, 13C NMR (Fig. S3†) and IR spectroscopy. Molecular weights were obtained by SEC (THF + 0.1% tetrabutylammonium bromide). Mn values were also determined by end-group analysis (1H NMR in d6-acetone) using protons characteristic of the phenylthio initiator residue (at 7.15 and 7.29 ppm) and the CH2 protons located on the α-position of the ester function in each repeat unit (4.06–4.18 ppm). Two batches were synthesized. The results are reported in Table 1.
| Run | Time/h | Cv (%) | M n,th× 103 | M n,exp × 103 | M w/Mn | |
|---|---|---|---|---|---|---|
| SEC | 1H NMR | |||||
| 1 | 24 | 98 | 10.0 | 3.6 | 7.9 | 1.39 |
| 2 | 28 | 80 | 8.2 | 2.3 | 10.9 | 1.10 |
In all cases, spectroscopic evidences were fully compatible with the structure expected for poly(1). The 1H NMR spectrum shows rather broad peaks compatible with an oligomeric structure and a high steric hindrance resulting from the large substituents (Fig. 1). Experimental absolute Mn values, as determined by 1H NMR, are in good agreement with theoretical ones, i.e. assuming livingness for the polymerization and quantitative initiation. SEC analysis were performed in the presence of tetra(n-butyl)ammonium ions to decrease the adsorption of the macromolecules on the column. Nevertheless, relative Mn values determined by SEC (using polystyrene standards) were underestimated, as expected from this densely substituted system. The first synthesized batch showed a quantitative conversion and a polydispersity index Mw/Mn of 1.39. This second value is rather high for a controlled living polymerization, and is probably due to a transfer reaction involving an impurity. For the second run a narrow molecular weight distribution was observed (1.10), as expected for a living polymerization.
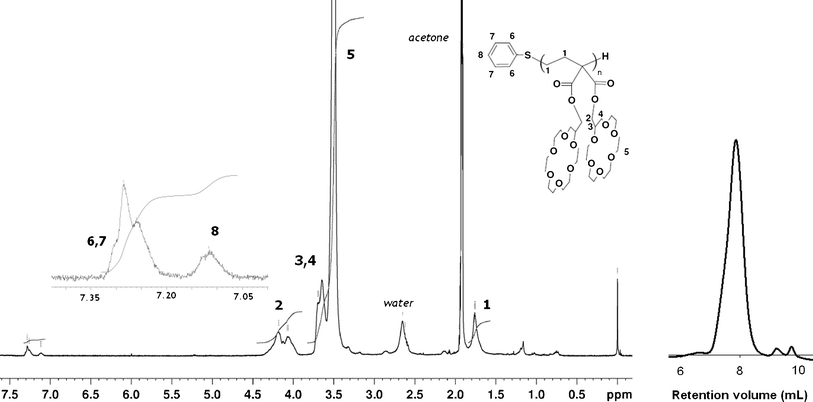 | ||
| Fig. 1 Characterization of poly(1) (Table 1, run 2). (left) 1H NMR spectrum of poly(1) in d6-acetone; inset: thiophenoxyl aromatic signals. (right) Size exclusion chromatogram of poly(1) (in THF + 0.1% tetrabutylammonium bromide). | ||
Picrate extraction tests, in particular saturation experiments,10 were performed to evaluate the binding efficiencies of both (1) and poly(1) to alkali ions. The method used is based on the extraction of metal picrates initially dissolved in water by a CH2Cl2 solution containing the crown ether molecule. The metal ions considered in the present study were lithium, sodium and potassium. The extent of binding was determined indirectly by measuring the amount of UV-active picrate counterion in the dichloromethane solution.
The results are graphically depicted in Fig. 2, where the amount of complexed cations per the available crown units is plotted vs. the amount of cations in the initial aqueous solution per crown-ether units. For all cations investigated, i.e. Li+, Na+ and K+, poly(1) is less efficient in extracting the metallic cations compared with the corresponding monomer (1) (Fig. 2). Charges introduced on the oligomer by ion binding increases the Coulombic repulsion exerted on the metallic cations and makes further binding more and more difficult. Interactions with (1) and poly (1) decrease in the order K+ > Na+ > Li+. This trend could be expected on the basis of the well-known preference displayed by 18-crown-6 crown-ethers for potassium cations.11 In contrast, sodium and lithium cations are smaller than the cavity size and thus less effectively bound to the crown-ether. For monomer (1), the complexed K+ to crown ratio approaches a plateau value of 0.5–0.6, suggesting the formation of a relatively stable 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sandwich-type complex involving two crown ethers for a single potassium cation, with a possible coexistence of both this complex and a 1
1 sandwich-type complex involving two crown ethers for a single potassium cation, with a possible coexistence of both this complex and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex at higher initial concentrations in potassium ion.
1 complex at higher initial concentrations in potassium ion.
![Binding of Na+ to (1) (○) and poly(1) (●) and K+ to (1) (□) and poly(1) (■) as a function of picrate concentration; [crown units] = 7 × 10−5 M (data not shown for Li+).](/image/article/2012/RA/c2ra20548c/c2ra20548c-f2.gif) | ||
| Fig. 2 Binding of Na+ to (1) (○) and poly(1) (●) and K+ to (1) (□) and poly(1) (■) as a function of picrate concentration; [crown units] = 7 × 10−5 M (data not shown for Li+). | ||
The permeabilization and single channel properties induced by poly(1) were investigated on a bilayer lipid membrane using one of the two synthesized samples (Table 1, run 1). All experimental details can be found as supporting information.† The experiment consists in recording the current flow through the membrane in response to an applied voltage.12 The introduction of poly(1) (1–1000 fmol L−1) in one of the compartments, named cis and trans, in the set-up (inset, Fig. 3A) decreased the BLM capacity and increased its stability, indicating that the polymer indeed interacted with the membrane, but modification of the membrane permeability could rarely be observed under these conditions. However, when higher voltages were applied (±100–200 mV) or when the BLM was broken (or rinsed with ethanol) and formed again, BLM overall ion selective current and/or single channels could be observed, corresponding to 80% of all recorded events. Among them, 30% of the observations were correlated to single-channel activities (Fig. S4–S8†). The overall or the single channel behavior sustained for more than 10 min and in some cases for more than 40 min (∼15%). An example of Na+ single-channel current observed after incorporation of poly(1) within a BLM is shown in Fig. 3B. The Na+ conductance showed variations over a 1–3 pA range (Fig. S4–S6;† ∼70% of the single channel records) what may indicate that there are several events relating to “open”, “closed”, or “partially open” channel states.
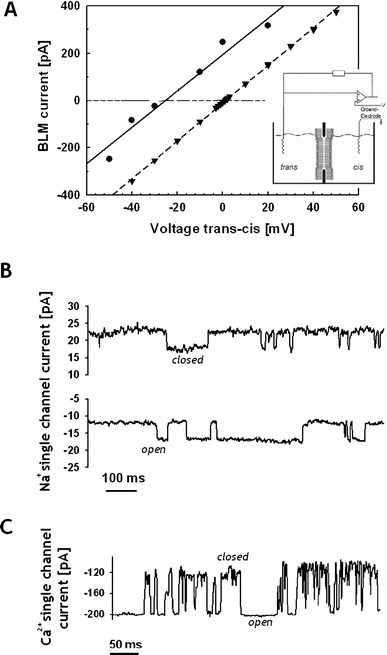 | ||
Fig. 3 Activity of poly(1) in BLM: (A) macroscopic IV profile; (●) 50/250 mmol L−1 NaCl cis/trans solutions, g = 7.7 nS, Erev = −27 mV, PNa+/PCl− = 6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ; (▼) 250/250 mmol L−1 KCl/NaCl cis/trans solutions, g = 8.0 nS, Erev = 1.5 mV, PNa+/PK+ ∼ 1. Inset: experimental set-up. (B) Na+ single channel current, 50/250 mmol L−1 NaCl cis/trans solutions; voltage = −10 mV (up) and − 40 mV (down). (C) Ca2+ single channel current, 250/250 mmol L−1 CaCl2/NaCl cis/trans solutions ; voltage = −100 mV. 1 ; (▼) 250/250 mmol L−1 KCl/NaCl cis/trans solutions, g = 8.0 nS, Erev = 1.5 mV, PNa+/PK+ ∼ 1. Inset: experimental set-up. (B) Na+ single channel current, 50/250 mmol L−1 NaCl cis/trans solutions; voltage = −10 mV (up) and − 40 mV (down). (C) Ca2+ single channel current, 250/250 mmol L−1 CaCl2/NaCl cis/trans solutions ; voltage = −100 mV. | ||
Similar experiments also identified short-lived Ca2+ single-channel currents (Fig. 3C). In the experiments with Ca2+ in cis and K+ in trans, Ca2+ channels were observed at 0 mV (Fig. S7†). At the applied voltages, when Ca2+ current flowed from cis to trans side, the single Ca2+ current fluctuation was more regular than the one carried by K+ ion from the trans to the cis side (Fig. S8†). This may indicate that Ca2+ partially stabilized a “channel like formation” of the poly(1) chains. An ohmic behavior was observed in the current–voltage relationship (IV profile) (Fig. 3A). When exposed to a salt gradient, poly(1) revealed a preference for cations over anions. A permeability ratio PNa+/PCl−, calculated from the reversal potential Erev13, ranged from 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 11.2
1 to 11.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereas the ratio PNa+/PK+ was close to unity (Fig. 3A). The equal permeability for K+ and Na+ suggests the ion transport does not occur through crown-ethers. Experiments gave rise to several values for the conductance that increased with the decrease in Erev (Fig. S9†). The conductance variation suggests some barrel pore formation. These results also clearly indicate that most (or a sufficiently substantial amount) of the ions passing though the pore interact with its inner surface. In order to interact with the pore surface the ion must lose part or all of its hydrated water shell. This indicates that the smallest pore diameter observed is in the range of the ion hydrated shells, or between the ion's hydrated and deshydrated shells, or in extreme cases at the range of ion's radius. Taking into account the radius of the hydrated and dehydrated ions (in nm: K+ 0.201 and 0.138; Na+ 0.178 and 0.102; Ca2+ 0.253 and 0.123; Cl− 0.195 and 0.181, respectively),14 we may estimate the smallest radius of the pore in the range of ∼0.3 ± 0.1 nm. Finally, the single channel current amplitude was not uniform for different experiments and varied with time. This sample heterogeneity is more frequently observed with supramolecular than with unimolecular ion channels and pores.12 Thus, a “channel structure unit” obtained from the aggregation of varying number of poly(1) chains is a reasonable hypothesis. Moreover, the closed state of the single channel was not at zero-current, and was often imposed by the BLM current, which did not display single-channel characteristics. The results suggest that at least two distinct structural polymer–lipid complexes are formed in the BLM, one resulting from the BLM overall current, with pores open most of the time, and the other one from single-channel current resulting from a gating of two conformational states in the polymer–lipid structure.
1, whereas the ratio PNa+/PK+ was close to unity (Fig. 3A). The equal permeability for K+ and Na+ suggests the ion transport does not occur through crown-ethers. Experiments gave rise to several values for the conductance that increased with the decrease in Erev (Fig. S9†). The conductance variation suggests some barrel pore formation. These results also clearly indicate that most (or a sufficiently substantial amount) of the ions passing though the pore interact with its inner surface. In order to interact with the pore surface the ion must lose part or all of its hydrated water shell. This indicates that the smallest pore diameter observed is in the range of the ion hydrated shells, or between the ion's hydrated and deshydrated shells, or in extreme cases at the range of ion's radius. Taking into account the radius of the hydrated and dehydrated ions (in nm: K+ 0.201 and 0.138; Na+ 0.178 and 0.102; Ca2+ 0.253 and 0.123; Cl− 0.195 and 0.181, respectively),14 we may estimate the smallest radius of the pore in the range of ∼0.3 ± 0.1 nm. Finally, the single channel current amplitude was not uniform for different experiments and varied with time. This sample heterogeneity is more frequently observed with supramolecular than with unimolecular ion channels and pores.12 Thus, a “channel structure unit” obtained from the aggregation of varying number of poly(1) chains is a reasonable hypothesis. Moreover, the closed state of the single channel was not at zero-current, and was often imposed by the BLM current, which did not display single-channel characteristics. The results suggest that at least two distinct structural polymer–lipid complexes are formed in the BLM, one resulting from the BLM overall current, with pores open most of the time, and the other one from single-channel current resulting from a gating of two conformational states in the polymer–lipid structure.
In conclusion, poly(crown-ether)s with a unique topology and a high density of 18-crown-6 substituents have been successfully synthesized by metal-free AROP of a cyclopropane-1,1-dicarboxylate. Monodisperse oligomers can be prepared with a good control over the molecular weight. As far as we know, this is the first time that a synthetic membrane permeabilizer is obtained by a controlled polymerization methodology. This novel oligo(crown-ether) appears to form aggregated ion-channels of various conductance. The formed pores are permeable to K+, Na+ and Ca2+ and selective for cations over anions. Current studies involving poly(crown-ether)s of different structures (i.e., modifying the polymer size and the crown-ether's number and size) will be presented in an upcoming full paper.
Acknowledgements
We thank the ANR (2011 JS08 007 01), the French Ministry of Foreign Affairs (PHC Stefanik 2012/n°26317ZA) and the Slovak Grant Agency APVV (SK-FR-0014-11) for financial support of this research. We thank Paris-Est University for a post-doctoral grant to M. Liu. We are also grateful to the French Ministry for Research for the PhD fellowship of N. Illy.References
- T. M. Fyles and J. K. W. Chui, Chem. Soc. Rev., 2012, 41, 148–175 RSC.
- S. Matile, A.V. Jentzsch, J. Montenegro and A. Fin, Chem. Soc. Rev., 2011, 40, 2453–2474 RSC.
- J.-Y. Winum and S. Matile, J. Am. Chem. Soc., 1999, 121, 7961–7962 CrossRef CAS.
- P-L. Boudreault, F. Otis and N. Voyer, Chem. Commun., 2008, 2061–2168 Search PubMed.
- B.A. Smith, M. M. Daschbach, S. T. Gammon, S. Xiao, S. E. Chapman, C. Hudson, M. Suckow, D. Piwnica-Worms, G. W. Gokel and W. M. Leevy, Chem. Commun., 2011, 47, 7977–7979 RSC.
- M. Tsikolia, A. C. Hall, C. Suarez, E. O. Nylander, S. M. Wardlaw, M. E. Gibson, K. L. Valentine, L. N. Onyewadume, D. A. Ahove, M. Woodbury, M. M. Mongare, C. D. Hall, Z. Wang, B. Draghici and A. R. Katritzky, Org. Biomol. Chem., 2009, 7, 3862–3870 CAS.
- F. Otis, C. Racine-Berthiaume and N. Voyer, J. Am. Chem. Soc., 2011, 133, 6481–6483 CrossRef CAS.
- P. Sikorski, E. D. T.Atkins, L. C. Kagumba and J. Penelle, Macromolecules, 2002, 35, 6975–6984 CrossRef CAS.
- N. Illy, S. Boileau, W. Buchmann, J. Penelle and V. Barbier, Macromolecules, 2010, 43(21), 8782–8789 CrossRef CAS.
- S. Kopolow, T. E. Hogen Esch and J. Smid, Macromolecules, 1973, 6, 133–142 CrossRef CAS.
- G. W. Gokel, W. M. Leevy and M. E. Weber, Chem. Rev., 2004, 104, 2723–2750 CrossRef CAS.
- S. Matile and N. Sakai, The characterization of synthetic ion channels and pores, in Analytical Methods in Supramolecular Chemistry, ed. C. Schalley, Wiley, Weiheim, 2007, pp. 391–418 Search PubMed.
- K. A. Hayman, T. D. Spurway and R. H. Ashley, J. Membr. Biol., 1993, 136, 181–190 CrossRef CAS.
- M. Y. Kiriukhin and K. D. Collins, Biophys. Chem., 2002, 99, 155–168 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details on monomer synthesis, polymerization, saturation experiments and BLM conductance measurements; full characterization of (1) and poly(1) (1H NMR, 13C NMR, elemental analysis). Further examples of BLM current and single channel measurements. See DOI: 10.1039/c2ra20548c |
| This journal is © The Royal Society of Chemistry 2012 |
