Silk doped with a bio-modified dye as a viable platform for eco-friendly luminescent solar concentrators
Manuela
Melucci
*ab,
Margherita
Durso
a,
Laura
Favaretto
a,
Massimo L.
Capobianco
a,
Valentina
Benfenati
*ac,
Anna
Sagnella
a,
Giampiero
Ruani
c,
Michele
Muccini
c,
Roberto
Zamboni
a,
Valeria
Fattori
a and
Nadia
Camaioni
*a
aIstituto per la Sintesi Organica e la Fotoreattività (ISOF), Consiglio Nazionale Ricerche, via P. Gobetti 101, Bologna, 40129, Italy. E-mail: mmelucci@isof.cnr.it, camaioni@isof.cnr.it
bIstituto di Chimica dei composti Organometallici (ICCOM), Consiglio Nazionale delle Ricerche, via Madonna del Piano 10, Sesti Fiornetino, (FI) 50019, Italy
cIstituto per lo studio dei Materiali Nanostrutturati, (ISMN), Consiglio Nazionale Ricerche, via P. Gobetti 101, Bologna, 40129, Italy. E-mail: v.benfenati@ismn.bo.cnr.it
First published on 26th July 2012
Abstract
The potential of fluorescent silk fibroin (SF) as a fully water-processable platform for an application in luminescent solar concentrators (LSCs) is shown. SF preserves its mechanical properties when doped with a bio-modified dye and the dye shows enhanced fluorescence when embedded in silk. These features, combined with high optical transparency and high refractive index, make SF a viable eco-friendly matrix for LSCs.
Natural polymers are investigated in biotechnology because of their unique properties, such as biological compatibility and nontoxicity.1 In addition, such biopolymers are biodegradable, so do not contribute to the accumulation of chemical waste. Silk fibroin (SF) is a biopolymer,2 that can be obtained as pure regenerated solution from the silk fibers of Bombyx mori silkworm, by a water-based process. SF has being used for centuries for biomedical applications, but it is receiving increasing attention as technological material, integrated as a structural component of various devices, spanning from optical devices3 to field effect transistors,4 with promising features for implantable devices intended for neuroregenerative medicine, or as a food sensor as an environmental pathogen detector for food quality control. From a silk fibroin regenerated solution, mechanically robust films of fibroin can be easily prepared by drop casting, spin coating, or layer by layer deposition,5 avoiding the use of solvents hazardous for human health and environment. The mechanical properties of silk films strongly depend on the amount of highly crystalline β-sheets, acting as reinforcing fillers and physical cross-links6 and strategies for controlling the β-sheets vs. alpha domains have been developed, including a green post-processing method via water annealing.7 Concerning the optical properties, SF films show high optical transparency in the visible range and a high refractive index (n = 1.54 at 633 nm),8 so it can be used to make optical waveguides.9 Recently, directional and wavelength- specific fluorescent enhancement by means of nanoimprinting has been demonstrated for dye-doped silk thin films.10 Basing on this unique combination of mechanical and optical properties, we investigate the potential of SF as a transparent matrix for the development of fully water-processable and eco-friendly Luminescent Solar Concentrators (LSCs). LSCs are optical systems consisting of a flat plate made of a highly transparent matrix, doped with very small amounts of luminescent dyes.11 The embedded dyes absorb part of the incoming sunlight and isotropically re-emit it with their characteristic spectrum. Depending on the refractive index of the matrix, most of the emitted light is trapped in the plate and guided toward its side-edges by total internal reflection, thus realizing the concentration of solar light. A synthetic polymer, poly(methyl methacrylate), is commonly used as transparent matrix for LSCs. Following a green approach, we have recently reported on the effectiveness of poly-L-lactic acid (PLA), a carbohydrate derived from starch, as a renewable and eco-sustainable matrix for luminescent solar concentrators.12 SF as a transparent matrix for LSCs offers the following advantages compared to PLA: i) a higher refractive index (1.54 vs. 1.45); and ii) water processability for a very low impact on environment (PLA is processed from chloroform).
The main scope of the present work is to demonstrate the possibility of generating a novel class of fully water-processable, bio-derived, eco-friendly Luminescent Solar Concentrators. In this view, we sought to dope the silk matrix with a tailored newly developed oligothiophene dye, end-functionalized with a biocomponent as the amino-acid L-lysine (T4Lys, Scheme 1). Thiophene-based oligomers are a class of materials largely used for optical applications.13 Their chemical versatility allows for the fine tuning of the optical properties by proper molecular substitution.14 T4Lys was prepared using quaterthiophene bearing succinimidyl activated acid as symmetric ends (intermediate 3, Scheme 1) as a precursor. Succinimidyl-ended oligothiophenes have emerged as a valuable class of fluorescent probes for imaging and sensing purposes.15 Insertion of an amino acid moiety would be beneficial to impart water-solubility, a chance of further optical tuning by changing the charge of the zwitterionic component16 and eventually bio-recognition capability,17 thus increasing the range of applications of these materials. Of the amino acids, we chose L-lysine because its ε-amino group can spontaneously react with succinimidyl activated oligothiophene dyes. In addition, poly-L-lysine is a widely exploited substrate for cell adhesion and proliferation. Recently, the in vivo uptake of dyes into domesticated silkworms, has been demonstrated,18 leading to the direct production of intrinsically colored silk by the silkworms. Therefore, L-lysine substituted dyes could potentially improve the direct production of dye-doped silk by feeding silkworms a lysine-enriched diet.
![Synthetic route to l-lysine ended quaterthiophene T4Lys. i) Stille coupling, in situ Pd[AsPh3]4, refluxing toluene, 6 h. ii) DMF (NaOH aq), pH 9–10, room temperature, 48 h.](/image/article/2012/RA/c2ra21568c/c2ra21568c-s1.gif) | ||
| Scheme 1 Synthetic route to L-lysine ended quaterthiophene T4Lys. i) Stille coupling, in situ Pd[AsPh3]4, refluxing toluene, 6 h. ii) DMF (NaOH aq), pH 9–10, room temperature, 48 h. | ||
Scheme 1 shows the synthetic pathway employed for T4Lys preparation. Stille coupling between bistannyl bithiophene 1 and the brominated succinimidyl ended thiophene 2 led to the T4 derivative 3 that was then coupled with Lysine 4 in dimethylformamide (DMF)/water/NaOH solution at pH 9–10, affording the target T4Lys (HCl)2 in 30% yield.
At the above pH, the coupling between the activated acid and the ε NH2 of lysine is faster than the hydrolization of the succynymidyl group.15b T4Lys (HCl)2 dissolved in a H2O/Triethylamine (TEA) solution was then added to a SF solution obtained by Bombyx mori silkworm cocoons upon degumming and extraction procedures already described.19,1b The silk films were prepared by a drop-casting and slow-dying procedure according to the method reported in Ref. 19. The entire process is depicted in the sketch of Fig. 1, showing the silkworm and its cocoon (Fig. 1a), the SF upon the extraction and washing procedure (Fig. 1b) and the dying process (Fig. 1c, d). The SF solution doped with T4Lys (50 μg ml−1) was cast onto a glass substrate, to generate films with a thickness of around 20 μm (Fig. 1e). The as-cast silk films were desiccated for 12 h in a chemical hood. Fig. 1f shows a typical silk film under UV light irradiation (λ = 364 nm).
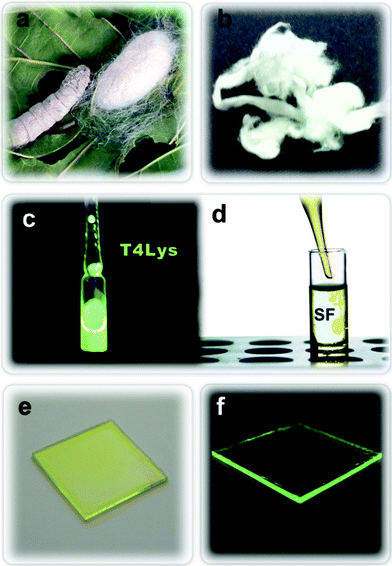 | ||
| Fig. 1 Sketch of the processing of T4Lys doped silk films: a) silkworm and its cocoon; b) SF fibers; c) solution of T4Lys in water (3 mg ml−1) under illumination at λmax = 364 nm; d) doping SF water solution with T4Lys; e) cast film of doped silk on glass and f) the same film under UV light (λ = 364 nm). | ||
It is known that SF molecular conformation affects the mechanical properties of silk films.20 Thus, the structure of un-doped and T4Lys-doped silk films were determined by Fourier transform infrared spectroscopy (FT-IR) (Fig. 2a, in which the spectra of L-lysine and L-lysine doped silk films are also reported as references). The infrared spectral region within 1200–1700 cm−1 had been previously assigned to the absorption of the peptide backbones of amide I (1600–1700 cm−1), amide II (1500–1600 cm−1) and amide III (1200–1350 cm−1), and used for the analysis of different secondary structures of silk fibroin. The amide I band appeared as a strong peak at 1660 cm−1, corresponding to the silk I structure. In the amide II region, peaks were seen at 1531 cm−1 (silk I) and at 1515 cm−1 (silk II). In the amide III region, a peak of 1235 cm−1, generally assigned to random coil-structures, is observed in both plain and T4Lys-doped silk films. No variation of the typical silk fingerprints was revealed upon doping (also after three months from the preparation), as the spectra resembled those previously reported.20 This result indicates that T4Lys-doping does not alter the conformational structure of the protein in SF films. This is a great advantage as it enables the processing of doped silk through a wide variety of already described methods, aimed at tightly controlling its mechanical properties and at preventing its dissolution in water or saline solutions. It should be pointed out that these kind of processes, such as water annealing inducing conformational transition of SF from silk I to silk II with consequent water insolubility, are necessary for outdoor applications such as LSCs.
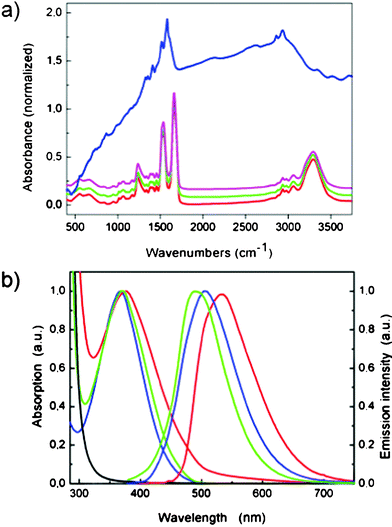 | ||
| Fig. 2 a) FT-IR spectra of films of silk (red); T4Lys-doped silk (green); lysine-doped silk (magenta); lysine (blue). b) Absorption (left) and emission (right) spectra of: T4Lys-doped silk film (green); a pure T4Lys film (red); and T4Lys in water solution (blue). The absorption of an un-doped 20 μm silk film (black) is also shown. | ||
The photophysical behaviour of silk films doped with T4Lys was inspected by measuring the absorption and photoluminescence spectra as well as the luminescence quantum yield (Φ and compared with those obtained for T4Lys, both in water solution and in pure film, in order to assess how the light emission properties of the dye (in particular, emission wavelength range and Φ) were affected by embedding in the silk matrix (Fig. 2b). The absorption features of T4Lys in the silk matrix are similar to the solution ones (peaks at 369 and 368 nm respectively), while the pure film presents a slight red shift of the absorption peak (377 nm). Concerning the emission behaviour, both solution (peak at 506 nm) and pure film (peak at 534 nm) show red-shifted bands with respect to doped silk (peak at 491 nm).
A red shift of emission upon aggregation has been reported for oligothiophenes21 and the red shifted emission in pure T4Lys film must be assigned to stacking of the quaterthiophene moieties, leading to aggregates which have a lower energy and quenched emission compared to the isolated molecules. These aggregates are largely present in the pure film of T4Lys, as the terminal lysine groups are not effective in hindering molecular stacking, and are also formed in water solution where the hydrophobic thiophene chains are not effectively dissolved in the water medium.16 On the contrary, silk seems to be an effective dispersing medium for the T4Lys dye, keeping the quaterthiophene moieties well separated even at this relatively high doping level.22 The low luminescence quantum yields of the T4Lys dye in water solution (Φ = 0.06) and in pure film (Φ = 0.04) support the assumption of aggregation and, most importantly, the higher quantum yield obtained for the doped silk film (Φ = 0.14) demonstrates prevention of aggregation by silk embedding. Clearly, the silk matrix is a good dispersing medium for the T4Lys molecules, it effectively prevents luminescence quenching induced by aggregation and encourages the extension of the proposed approach to other Lys-substituted dyes with high luminescence quantum yield.
In summary, maintaining its molecular conformation upon doping, SF acts as an excellent biocompatible dispersing medium for T4Lys. The typical optical features of the dye in solution are preserved in the silk matrix, which effectively prevents dye aggregation with subsequent quenching of the luminescence quantum yield. These features, combined with its high optical transparency and refractive index, make silk a novel candidate as a transparent matrix for fully water-processable and eco-friendly luminescent solar concentrators, properly treated to avoid dissolution under outdoor conditions.
In addition, the L-Lysine approach can be extended to other non-toxic dyes with very high emission quantum yields required for efficient solar flux concentration, and paves the way for the direct production by silkworms of dye-doped silk. Collectively, our results highlight how the combination of SF with a bio-derived luminescent dye represents a viable platform toward a biocompatible and fully-green approach for a renewable energy technology.
Experimental section
General
(3,3′-Dimethyl-[2,2′-bithiophene]-5,5′-diyl)bis(tributylstannane), 1, and L-lysine 4 were purchased by Aldrich. 2,5-dioxopyrrolidin-1-yl 5-bromothiophene-2-carboxylate, 2 was prepared according to already described procedures.23ESI-MS was performed on a Bruker 3000+ spectrometer. FT-IR absorption measurements were performed using a Bruker IFS 113v in vacuum at 2 cm−1 resolution, averaging over 256 spectra; to avoid saturation of the IR spectra in transmission due to the large absorption of amides peaks, FS thin films were deposited on KBr substrates.
Emission quantum yields in solution were determined by comparing corrected emission spectra with quinine sulfate in air-equilibrated 1N H2SO4 (Φ = 0.55) as the reference, while the measurements of absolute quantum yield for the films were done using a SPEX Fluorolog II spectrofluorimeter equipped with a custom integrating sphere accessory.
Synthesis
Bis(2,5-dioxopyrrolidin-1-yl) 3′′,4′-dimethyl-[2,2′:5′,2′′:5′′,2′′′-quaterthiophene]-5,5′′′-dicarboxylate, 3: To a refluxing toluene solution (16.6 ml) of compound 2 (0.5 g, 1.64 mmol) and in situ-prepared Pd(AsPh3)4 (8 mol%, 31 mg of Pd2(dba)3 and 73 mg of AsPh3) under N2 atmosphere, compound 1 (575 mg, 0.745 mmol) in toluene (14.5 ml), was added dropwise. The solution was refluxed for 6h, then the precipitate formed was separated from the solution and washed with pentane and purified by flash chromatography on silica gel using a solution of DCM/AcOEt = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 as eluent. Crystallization from toluene afforded compound 3 as an orange powder (331 mg, yield 69%). M.p. = 268 °C, EIMS m/z: 640 (M+), 526 (M+- C4H4O3N), UV-Vis (CH2Cl2) λmax = 384 nm, PL (CH2Cl2, λexc = 380 nm), λmax = 517 nm. 1H NMR (400 MHz, CDCl3, TMS/ppm) δ 7.92 (d, J = 4.0 Hz, 2H), 7.21 (d, J = 4.0 Hz, 2H), 7.20 (s, 2H), 2.91 (br. s, 8H), 2.25 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 169.1, 157.1, 147.2, 138.4, 137.6, 135.1, 130.4, 129.2, 124.4, 124.3, 25.6, 15.1. Anal. calcd for C28H20N2O8S4: C, 52.49; H, 3.15; found: C, 52.65; H, 3.26.
15 as eluent. Crystallization from toluene afforded compound 3 as an orange powder (331 mg, yield 69%). M.p. = 268 °C, EIMS m/z: 640 (M+), 526 (M+- C4H4O3N), UV-Vis (CH2Cl2) λmax = 384 nm, PL (CH2Cl2, λexc = 380 nm), λmax = 517 nm. 1H NMR (400 MHz, CDCl3, TMS/ppm) δ 7.92 (d, J = 4.0 Hz, 2H), 7.21 (d, J = 4.0 Hz, 2H), 7.20 (s, 2H), 2.91 (br. s, 8H), 2.25 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 169.1, 157.1, 147.2, 138.4, 137.6, 135.1, 130.4, 129.2, 124.4, 124.3, 25.6, 15.1. Anal. calcd for C28H20N2O8S4: C, 52.49; H, 3.15; found: C, 52.65; H, 3.26.
(2R,2′R)-6,6′-((3′′,4′-dimethyl-[2,2′:5′,2′′:5′′,2′′′-quaterthiophene]-5,5′′′- dicarbonyl)bis(azanediyl))bis(2-aminohexanoic acid), T4Lys: L-lysine 4 (35 mg, 0.24 mmol) is added to a DMF solution (3 ml) of compound 3 (70 mg, 0.11 mmol), then conc. NaOH aqueous solution was added until pH 9–10 was reached. The yellow solution so obtained was stirred at room temperature for 48 h. After this time, the crude mixture was washed with CH2Cl2 (× 3) and to the collected aqueous phases HCl aq. was added until chloridrate precipitation. The orange precipitate was further washed twice i) with an aqueous solution of HCl, ii) acetone and the phases separated by centrifugation. The solid phase was dried under vacuum, affording T4Lys(HCl)2 as an orange powder (22 mg, 30% yield). M.p. 280 °C (decomposes after this value), IR (Vaseline) ν (cm−1) 3325 (NH st), 2920 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C Thiophene bd), 1617 (amidic CO st), 1485 (NH3+ st), 1534 (NH3+ st); UV-Vis (H2O) λmax = 270 nm (lysine), 364 nm (T4), ESI-MS: calculated for [M+H]+: 703.18, found : 703.3, calculated for [M − H]−: 701.16, found: 701.2), 1H NMR (400 MHz, D2O+NaOH/ppm) δ 7.17 (br s, 2H), 6.47 (br s, 4H), 3.03 (br s, 4H + 2H in α to N), 1.8–1.2 (m, 12 H of Lys and 6H of CH3 on thiophenes). Elementary analysis calculated for T4Lys (HCl)2 [C32H40Cl2N4O6S4] C, 49.54; H, 5.20; N, 7.22; S, 16.53, found: C, 48.42; H, 4.68; N, 6.15; S, 17.04.
C Thiophene bd), 1617 (amidic CO st), 1485 (NH3+ st), 1534 (NH3+ st); UV-Vis (H2O) λmax = 270 nm (lysine), 364 nm (T4), ESI-MS: calculated for [M+H]+: 703.18, found : 703.3, calculated for [M − H]−: 701.16, found: 701.2), 1H NMR (400 MHz, D2O+NaOH/ppm) δ 7.17 (br s, 2H), 6.47 (br s, 4H), 3.03 (br s, 4H + 2H in α to N), 1.8–1.2 (m, 12 H of Lys and 6H of CH3 on thiophenes). Elementary analysis calculated for T4Lys (HCl)2 [C32H40Cl2N4O6S4] C, 49.54; H, 5.20; N, 7.22; S, 16.53, found: C, 48.42; H, 4.68; N, 6.15; S, 17.04.
Preparation of silk solution and film
Silk solutions were prepared from Bombyx mori silkworm cocoons (supplied by Tajima Shoji, Co., Yokohama, Japan) according to the procedures described in previous studies.8aThe silk film was prepared according to the drop-casting and slow-dying procedure we described previously.1 An aliquot of 1 ml of 8% of dye doped (50 μg ml−1) silk solution was cast on a 2 × 2 cm2 glass substrate, to generate films with a thickness of around 20 μm. The as-cast films were desiccated for 12 h in a chemical hood.
This work was partially supported by the Consorzio MIST-ER through project FESR-tecnopolo AMBIMAT. MM thanks Dr A. Tolomelli, Dr P. Galletti and Dr P. Dambruoso for the helpful discussion.
References
- (a) V. Benfenati, S. Toffanin, R. Capelli, L. M. Camassa, S. Ferroni, D. L. Kaplan, F. G. Omenetto, M. Muccini and R. Zamboni, Biomaterials, 2010, 31, 7883 CrossRef CAS; (b) V. Benfenati, K. Stahl, C. Gomis-Perez, S. Toffanin, A. Sagnella, R. Torp, D. L. Kaplan, G. Ruani, F. G. Omenetto, R. Zamboni and M. Muccini, Adv. Funct. Mater., 2012, 22, 1871 CrossRef CAS; (c) G. H. Altman, F. Diaz, C. Jakuba, T. Calabro, R. L. Horan, J. Chen, H. Lu, J. Richmond and D. L. Kaplan, Biomaterials, 2003, 24, 401 CrossRef CAS.
- D. N. Rockwood, R. C. Preda, T. Yücel, X. Wang, M. L. Lovett and D. L. Kaplan, Nat. Protoc., 2011, 6, 1612 CrossRef CAS.
- (a) D. H. Kim, Y. S. Kim, J. Amsden, B. Panilaitis, D. L. Kaplan and F. G. Omenetto, Appl. Phys. Lett., 2009, 95, 133701 CrossRef; (b) D. H. Kim, J. Viventi, J. J. Amsden, J. Xiao, L. Vigeland, Y. S. Kim, J. A. Blanco, B. Panilaitis, E. S. Frechette, D. Contreras, D. L. Kaplan, F. G. Omenetto, Y. Huang, K. C. Hwang, M. R. Zakin, B. Litt and J. A. Rogers, Nat. Mater., 2010, 9, 511 CrossRef CAS; (c) H. Tao, M. A. Brenckle, M. Yang, J. Zhang, M. Liu, S. M. Siebert, R. D. Averitt, M. S. Mannoor, M. C. McAlpine, J. A. Rogers, D. L. Kaplan and F. G. Omenetto, Adv. Mater., 2012, 24, 1067 CrossRef CAS.
- R. Capelli, J. J. Amsden, G. Generali, S. Toffanin, V. Benfenati, M. Muccini, D. L. Kaplan, F. G. Omenetto and R. Zamboni, Org. Electron., 2011, 12, 1146 CrossRef CAS.
- C. Jiang, X. Wang, R. Gunawidjaja, Y. Lin, M. K. Gupta, D. L. Kaplan, R. R. Naik and V. V. Tsukruk, Adv. Funct. Mater., 2007, 17, 2229 CrossRef CAS.
- K. A. Karvea, E. S. Gil, S. P. McCarthyd and D. L. Kaplan, J. Membr. Sci., 2011, 383, 44 CrossRef.
- J. Nam and Y. H. Park, J. Appl. Polym. Sci., 2001, 81, 3008 CrossRef CAS.
- (a) B. D. Lawrence, F. Omenetto, K. Chui and D. L. Kaplan, J. Mater. Sci., 2008, 43, 6967 CrossRef CAS; (b) H. Perry, L. D. Negro, A. Gopinat, D. L. Kaplan and F. G. Omenetto, Adv. Mater., 2008, 20, 3070 CrossRef CAS.
- S. T. Parker, P. Domachuk, J. Amsden, J. Bressner, J. A. Lewis, D. L. Kaplan and F. G. Omenetto, Adv. Mater., 2009, 21, 2411 CrossRef CAS.
- (a) Y. Tsuboi, H. Adachi, K. Yamada, H. Miysaka and A. Itaya, Jpn. J. Appl. Phys., 2002, 41, 4772 CrossRef CAS; (b) K. Agarwal, D. A. Hoagland and R. J. Farris, J. Appl. Polym. Sci., 1997, 63, 401 CrossRef.
- W. G. J. H. M. van Sark, K. W. J. Barnham, L. H. Slooff, A. J. Chatten, A. Büchtemann, A. Meyer, S. J. McCormack, R. Koole, D. J. Farrell, R. Bose, E. E. Bende, A. R. Burgers, T. Budel, J. Quilitz, M. Kennedy, T. Meyer, C. De Mello Donegá, A. Meijerink and D. Vanmaekelbergh, Opt. Express, 2008, 16, 21773 CrossRef CAS.
- V. Fattori, M. Melucci, L. Ferrante, M. Zambianchi, I. Manet, W. Oberhauser, G. Giambastiani, M. Frediani, G. Giachi and N. Camaioni, Energy Environ. Sci., 2011, 4, 2849 CAS.
- (a) Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics, I. F. PerepichkaD. F. Perepichka, Ed. Wiley 2009. Search PubMed; (b) A. Mishra, C.-Q. Ma and P. Bauerle, Chem. Rev., 2009, 109, 1141 CrossRef CAS; (c) G. Barbarella, M. Melucci and G. Sotgiu, Adv. Mater., 2005, 17, 1581 CrossRef CAS.
- M. Melucci, M. Zambianchi, L. Favaretto, V. Palermo, E. Treossi, M. Montalti, S. Bonacchi and M. Cavallini, Chem. Commun., 2011, 47, 1689 RSC.
- (a) G. Sotgiu, M. Galeotti, C. Samori', A. Bongini and A. Mazzanti, Chem.–Eur. J., 2011, 17, 7947 CrossRef CAS; (b) M. Zambianchi, F. D. Maria, A. Cazzato, G. Gigli, M. Piacenza, F. D. Sala and G. Barbarella, J. Am. Chem. Soc., 2009, 131, 10892 CrossRef CAS; (c) M. Zambianchi, A. Barbieri, A. Ventola, L. Favaretto, C. Bettini, M. Galeotti and G. Barbarella, Bioconjugate Chem., 2007, 18, 1004 CrossRef CAS.
- K. P. R. Nilsson, M. R. Andersson and O. Inganas, J. Phys.: Condens. Matter, 2002, 14, 10011 CrossRef CAS.
- A. Åslund, A. Herland, P. Hammarström, K. P. R. Nilsson, B.-H. Jonsson, O. Inganäs and P. Konradsson, Bioconjugate Chem., 2007, 18, 1860 CrossRef.
- N. C. Tansil, L. D. Koh and M. -Y. Han, Adv. Mater., 2011, 24, 1388 CrossRef.
- V. Benfenati, S. Toffanin, R. Capelli, L. M. Camassa, S. Ferroni, D. L. Kaplan, F. G. Omenetto, M. Muccini and R. Zamboni, Biomaterials, 2010, 31, 7883 CrossRef CAS.
- Q. Lu, B. Zhang, M. Li, B. Zuo, D. L. Kaplan, Y. Huang and H. Zhu, Biomacromolecules, 2011, 12, 1080 CAS.
- (a) J. Cornil, D. Beljonne, J.-P. Calbert and J.-L. Brødas, Adv. Mater., 2001, 13, 1053 CrossRef CAS; (b) M. Melucci, M. Zambianchi, A. Zanelli, N. Camaioni, M. Gazzano, A. Bongini and G. Barbarella, ChemPhysChem, 2007, 8, 2621 CrossRef CAS.
- M. Berggren, P. Bergman, J. Fagerstrom, O. Inganas, M. Andersson, H. Weman, M. Granstrom, S. Stafstrom, O. Wennerstrom and T. Hjertberg, Chem. Phys. Lett., 1999, 304, 84 CrossRef CAS.
- A. Cazzato, M. L. Capobianco, M. Zambianchi, L. Favaretto, C. Bettini and G. Barbarella, Bioconjugate Chem., 2007, 18, 318 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
