A facile room-temperature route to flower-like CuO microspheres with greatly enhanced lithium storage capability†
Zhengqiu
Yuan
*,
Yan
Wang
and
Yitai
Qian
Department of Chemistry, University of Science and Technology of China, Hefei, 230026, P.R. China. E-mail: yuanzhengqiu@gmail.com; Fax: +86-551-3607402; Tel: +86-551-3601589
First published on 31st July 2012
Abstract
Pure-phase flower-like CuO porous microspheres with a sheet-like subunit-assembled micro/nano structure are prepared by a facile “alkali-oxidizing solution etching assisted crystallization based on microscale Cu particles” route, which manifest greatly enhanced Li storage properties: the initial discharge capacity of ∼1220 mAh g−1 and reversible capacity of ∼800 mAh g−1 at a current density of 50 mA g−1. Even cycled at a current density of 400 mA g−1, they still deliver stable capacity with a comparable value of 666 mAh g−1 after 200 cycles.
Lithium-ion batteries, a fast-developing technology in electric energy storage, are the dominant power source for a wide range of portable electronic devices.1–3 Compared to the relatively low Li storage capability (370 mAh g−1) of graphite used in commercial lithium-ion batteries, transition-metal oxides have been attracted intensive interest because they exhibited higher capacities and better rate capabilities in the past decade.4–6 However, they often exhibit poor kinetics and severe capacity fading upon cycling, mainly because of the low conductivity and the severe pulverization during cycling. Therefore, many efforts have been taken to overcome these problems, such as preparing composites with carbon,7–11 forming metal-oxide-graphene hybrid nanostructures,12–14 introducing one-dimensional nanostructures or nanocomposites15–21 and so on. Especially, the electrodes with micro/nano structures have attracted growing research interests because they have exhibited enhanced lithium storage capacities.22–24 There are two superiorities of the electrodes with micro/nano structures: (1) their large surface area endows them with high capacity and a favorable response to high-rate cycling due to the dramatic increment of reaction sites and the interface between the active materials and the electrolyte, as the nanoscale ingredient makes lithium ion diffusion much easier by shorting the diffusion length effectively to grain size; (2) the interior space among the microscale structures allows the volume variation upon insertion/extraction of lithium ions to be better accommodated.
Various synthetic methods can be taken into consideration to prepare micro/nano structured metal oxides, such as the hydrothermal method (e.g., CuO hollow micro/nano structure25), soft templates method (e.g., α-Fe2O3 hollow spheres26), hard templates method (e.g., SnO2 nanoboxes22) and hierarchical self-assembly on a metal thin-film (e.g., cog-like CuO on copper film24), etc. Can we prepare the micro/nano structures from the microscale units, like the artist carving the sculpture using wood as raw materials? We want to control the oxidizing reaction and the hierarchical self-assembly of the products on the surfaces of the microscale metal particles to prepare micro/nano structured metal oxides. Here, we choose CuO to demonstrate the concept in view of its much higher theoretical capacity of about 670 mAh g−1, improved safety compared to graphite, low cost, nontoxicity, and its expanding technological applications in various fields. As an example, we show that the prepared flower-like CuO exhibits improved electrochemical properties when evaluated as an anode material in lithium-ion batteries.
Although the CuO also suffers very rapid capacity decay caused by huge and uneven volume variations during the lithium uptake/release process (only 300 mAh g−1 retained after 20 cycles),25 most encouragingly, a few recent reports on tailored nanostructures have documented good cell stability (i.e., high capacities without a discernible decline for many cycles). These nanostructures generally are grouped into two classes: carbon composites27 and thin films24,28–30. For example, a hierarchical superstructure of microscale cog-like CuO assembled on copper film attained an initial discharge capacity of 1052 mAh g−1 and a reversible capacity of 810 mAh g−1.24 However, both classes have several disadvantages. In carbon composites, the following drawbacks were evident: (1) the presence of low-capacity carbon suppresses the overall energy density; (2) the synthesis usually involves multiple complicated steps and a high temperature process; and (3) the intact surface coating decreases the electrode's kinetics. On the other hand, thin films are suitable only for microbatteries.
In this Communication, we report a room-temperature and novel process, “alkali-oxidizing solution etching assisted crystallization”, to synthesize porous flower-like CuO. The synthesis scheme of the flower-like CuO is shown in Fig. 1a, which is confirmed by the extra experiments below. In a typical experiment, 0.128 g copper powder (∼3250 mesh) was added into the mixed solution consisting of 10 ml deionized water and 6 ml 13–14 M NaOH (aq.) in a 50 ml beaker, then the mixture was stirred. Vigorous stirring was maintained throughout the entire process. After one hour, 5 ml 2 M (NH4)2S2O8 (aq.) was added into the above mixture, drop by drop. The products were prepared after 20 h of stirring at room temperature. After the reaction was finished, the resulting black solid products were centrifuged, washed with distilled water and ethanol to remove the ions possibly remaining in the final products, then dried at 60 °C under vacuum for 6 h. The obtained samples were characterized by X-ray powder diffraction (XRD) and scanning electron microscopy (SEM).
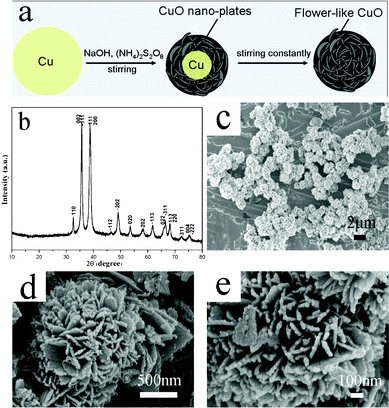 | ||
| Fig. 1 (a) Schematic illustration of the formation of flower-like CuO microspheres. (b) XRD pattern and (c, d, e) SEM images of the flower-like CuO synthesized at room temperature from microscale copper particles. | ||
The crystallographic structure and phase purity of the flower-like products were examined by X-ray powder diffraction (XRD), with the result shown in Fig. 1b. All the identified diffraction peaks can be unambiguously assigned to the phase-pure monoclinic CuO (tenorite, Joint Committee on Powder Diffraction Standards (JCPDS) card No. 41-0254, space group: C2/c, a = 4.6850 Å, b = 3.4230 Å, c = 5.1320 Å, β = 99.520°). No signals from possible impurities such as Cu, Cu(OH)2, or Cu2O were detected.
The size and morphology of the products were examined by scanning electron microscopy (SEM). Fig. 1c shows that most of the CuO sample consists of monodispersed spherical particles with flower-like texture. The diameter of the microspheres is 1–2 μm (Fig. 1d). It can be clearly seen that these flower-like microspheres are composed of many nanoplate petals with an average thickness of about 25–35 nm (Fig. 1e); these nanoplates interweave together forming an open porous structure and are expected to facilitate electrolyte penetration into the electrode particles, thus providing more interface area between the electrode materials and the electrolyte. The BET surface area of the flower-like CuO is 7.8 m2 g−1 and the average crystalline size is about 18 nm.
We have also carried out experiments under different reaction conditions. For a shorter reaction time of 8 h, the monodispersed flower-like particles with the size of about 1–2 μm are generated, the outside parts of which are composed of many thin nanoplates and the insides are microscale copper particles; the SEM images are shown in Fig. S1b and c†. The results can also be certified by XRD. From the X-ray spectrogram of the obtained products, not only the diffraction peaks of CuO can be detected, but also the signals from copper co-exist in the XRD spectrogram, which is shown in Fig. S1a†. At the same time, the diffraction peaks of Cu are much sharper than CuO, which shows that the size of the copper particle in the interior of the flower-like particles is much larger than CuO. This experiment shows that the stirring time of the reaction is essential to prepare pure flower-like CuO and confirms the synthesis scheme above. At the same time, we have carried out an interesting experiment. Keeping the given reaction conditions for preparing pure flower-like CuO unchanged, the reaction system is placed in an ultrasonic environment. The obtained products characterized by XRD are pure CuO, and the morphology looks like destroyed flowers with the petals everywhere, shown in Fig. S2†. Although the products are generated in ultrasonic conditions, SEM images show that there are still many particles composed of nanoplates. The experiment shows that the tendency to form flower-like products is very intense. On the other hand, the fact reveals that the structure of the flower-like CuO is relatively stable, which may be favorable for improving the cycling stability.
Although the flower-like structure is not a novel morphology for CuO, the growth process of our flower-like CuO is from outside to inside and different from the previous reported flower-like CuO which adopts the opposite process. The growth process of outside to inside is in accord with the electrolyte permeating into electrode material from outside into the inside. The pores in the flower-like CuO formed in etching process from outside to inside provide unblocked paths for electrolyte permeating into the interior of the electrode.
We next study the electrochemical properties of the material. Fig. S3† shows the representative cyclic voltammograms (CVs) of the sample between 5 mV and 3 V at a scan rate of 0.5 mV s−1. There are four peaks at 1.6, 1.05, 0.8 and 0.5 V during the first reduction scan, corresponding to the formation of a Cu2+1−xCu1+xO1−x/2 (0 ≤ x ≤ 0.4) solid solution; the conversion from CuO to Cu2O, the decomposition of Cu2O into Cu and Li2O, and the formation and decomposition of a polymeric gel-like film on the surface of the electrodes, respectively; there are two oxidation peaks at about 2.5 and 2.7 V appearing in the first oxidation scan, which are related to the oxidation process 2Cu + Li2O → Cu2O + 2Li and the oxidation of Cu2O to CuO, respectively.31–33 Apparently, the peak intensity drops significantly in the second cycle, indicating the occurrence of some irreversible processes in the electrode material in the first cycle. In addition, it can be seen that in the third scan, the peak intensity is nearly unchanged and the reduction peaks are located at 2.3, 1.2 and 0.75 V, indicating that the electrode reactions become more reversible.
The galvanostatic discharge-charge curves of the pure-phase flower-like CuO electrode, measured between 0.005 V and 3 V at a current density of 50 mA g−1, are shown in Fig. 2a. This reaction provides the dominant contribution to the Li storage capability of the material, giving rise to a high first-cycle discharge capacity of about 1220 mAh g−1 (see Fig. 2a). From the second cycle onward, the flower-like CuO spheres exhibit excellent cyclic capacity retention, with a stable capacity of about 800 mAh g−1 (see Fig. 2b). The irreversible capacity in the first cycle may be mainly ascribed to diverse irreversible processes such as interfacial lithium storage, inevitable formation of a solid electrolyte interface (SEI layer) and organic conductive polymer, as well as the electrolyte decomposition, which are common for most anode materials.4,33,34 These values are considerably higher than the initial discharge capacity (970 mAh g−1) and reversible capacity (560 mAh g−1), reported for negative electrodes consisting of a surface network of CuO nanofibres,28 and are larger than the theoretical capacity of CuO. The extra capacity can probably be attributed to the long slope below 0.75 V which represents reversible formation and decomposition of a polymeric gel-like film on the internal surface of flower-like microspheres.33 In the charging process, Fig. 2a shows a capacitive behavior before the plateau, which might correspond to the Li interfacial storage. At a current density of 200 mA g−1, they still deliver stable capacity with a comparable value of 674 mAh g−1 after 100 cycles, shown in Fig. 2c. Even cycled at a current density of 400 mA g−1, the final discharge capacity is 666 mAh g−1 after 200 cycles, see Fig. 2d. As a comparison, the commercial CuO powders show a much lower first-cycle discharge capacity (940 mAh g−1), and the capacity drops quickly to 360 mAh g−1 after 10 cycles at the current density of 200 mA g−1, which is shown in Fig. S4†.
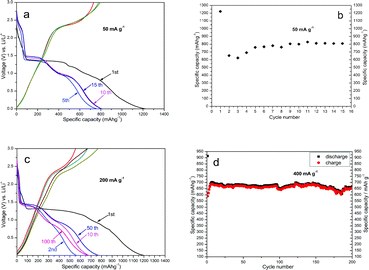 | ||
| Fig. 2 Electrochemical measurements of the sample. (a, c) Voltage profiles for the first galvanostatic discharge curve and charge-discharge curves. (b, d) Cycling performance of the flower-like CuO microspheres. The galvanostatic tests were respectively performed at current rates of 50 mA g−1 (a, b), 200 mA g−1 (c) and 400 mA g−1 (d), between 0.005 V and 3 V. | ||
The rate capability of flower-like CuO microspheres is presented in Fig. 3. The cell was cycled between 0.005 and 3 V at various current densities from 50 to 800 mA g−1. Even at a current density of 800 mA g−1, the flower-like CuO still retained a discharge capacity of 626 mAh g−1, which was considerably higher than determined for electrodes made from CuO powers and films. At last, when the current density returns back to 200 mA g−1, the final discharge and charge capacities of our CuO are 675 and 670 mAh g−1, respectively, which are similar to the theoretical capacity of CuO. The SEM image of our flower-like CuO after cycling test at various rates is shown in Fig. S5†, which basically retains the flower-like morphology. We could probably attribute this superior performance of our flower-like porous spheres to the thin nanoplates providing a fast and efficient transport of Li ions, and the porous interior allowing the material to effectively buffer the stress induced during the charge-discharge process.
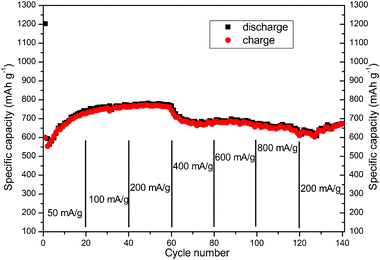 | ||
| Fig. 3 Rate performance of pure flower-like CuO. Specific capacity as a function of cycle number and discharging (black square symbols) or charging (red round symbols) at various rates. The rate was respectively 50, 100, 200, 400, 600, 800 and 200 mA g−1 in a voltage range of 0.005–3 V. | ||
In summary, we have developed a simple, novel and room-temperature process “alkali-oxidizing solution etching assisted crystallization based on microscale metal particles” for preparing porous CuO microspheres with well-defined flower-like morphologies on a large scale. This flower-like morphology with thin nanoplates provides interconnected open pores that allow electrolyte penetration, reducing the diffusion path of the lithium ions; the porous and relatively stable microscale structure can allow volume variation upon insertion/extraction of lithium ions. To the best of our knowledge, the capacity and cycle stability of our pure flower-like CuO spheres without carbon coating, heat treatment or supporting on copper substrate in lithium-ion batteries testing are excellent. This novel method can also be extended to prepare other micro/nano structured transition-metal oxides (e.g., ZnO) for use in energy storage and conversion, as well as catalysis.
Acknowledgements
This work is financially supported by the National Nature Science Fund of China (No. 91022038) and the 973 Project of China (No. 2011CB935901).References
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS.
- J. S. Chen, L. A. Archer and X. W. Lou, J. Mater. Chem., 2011, 21, 9912–9924 RSC.
- J. Maier, Nat. Mater., 2005, 4, 805–815 CrossRef CAS.
- Y. M. Kang, K. T. Kim, K. Y. Lee, S. J. Lee, J. Y. Jung and J. Y. Lee, J. Electrochem. Soc., 2003, 150, A1538–1543 CrossRef CAS.
- R. Dedryvere, S. Laruelle, S. Grugeon, P. Poizot, D. Gonbeau and J. M. Tarascon, Chem. Mater., 2004, 16, 1056–1061 CrossRef CAS.
- P. Lian, X. Zhu, H. Xiang, Z. Li, W. Yang and H. Wang, Electrochim. Acta, 2009, 55, 834–839 Search PubMed.
- Y. J. Mai, X. L.Wang, J. Y. Xiang, Y. Q. Qiao, D. Zhang, C. D. Gu and J. P. Tu, Electrochim. Acta, 2011, 56, 2306–2311 CrossRef CAS.
- J. Y. Xiang, J. P. Tu, L. Zhang, Y. Zhou, X. L. Wang and S. J. Shi, J. Power Sources, 2010, 195, 313–319 CrossRef CAS.
- W. J. Yu, P. X. Hou, L. L. Zhang, F. Li, C. Liu and H. M. Cheng, Chem. Commun., 2010, 46, 8576–8578 RSC.
- B. Wang, X. L. Wu, C. Y. Shu, Y. G. Guo and C. R. Wang, J. Mater. Chem., 2010, 20, 10661–10664 RSC.
- Y. Y. Liang, Y. G. Li, H. L. Wang, J. G. Zhou, J. Wang, T. Regier and H. J. Dai, Nat. Mater., 2011, 10, 780–786 CrossRef CAS.
- H. L. Wang, L. F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, Y. Y. Liang, Y. Cui and H. J. Dai, J. Am. Chem. Soc., 2010, 132, 13978–13980 CrossRef CAS.
- D. H. Wang, Daiwon Choi, J. Li, Z. G. Yang, Zimin Nie, R. Kou, D. H. Hu, C. M. Wang, L. V. Saraf, J. G. Zhang, Ilhan A. Aksay and J. Liu, ACS Nano, 2009, 3, 907–914 CrossRef CAS.
- Z. Ying, Q. Wan, H. Cao, Z. T. Song and S. L. Feng, Appl. Phys. Lett., 2005, 87, 113108 CrossRef.
- H. X. Zhang, C. Feng, Y. C. Zhai, K. L. Jiang, Q. Q. Li and S. S. Fan, Adv. Mater., 2009, 21, 2299–2304 CrossRef CAS.
- S. M. Paek, E. J. Yoo and I. Honma, Nano Lett., 2009, 9, 72–75 CrossRef CAS.
- D. H. Wang, R. Kou, D. Choi, Z. G. Yang, Z. Nie, J. Li, L. V. Saraf, D. H. Hu, J. G. Zhang, G. L. Graff, J. Liu, M. A. Pope and A. Aksay, ACS Nano, 2010, 4, 1587–1595 CrossRef CAS.
- A. M. Chockla, J. T. Harris, V. A. Akhavan, T. D. Bogart, V. C. Holmberg, Chet Steinhagen, C. B. Mullins, K. J. Stevenson and B. A. Korgel, J. Am. Chem. Soc., 2011, 133, 20914–20921 CrossRef CAS.
- M. M. Rahman, J. Z. Wang, X. L. Deng, Y. Li and H. K. Liu, Electrochim. Acta, 2009, 55, 504–510 CrossRef CAS.
- J. J. Zhu, Z. H. Lu, S. T. Aruna, D. Aurbach and A. Gedanken, Chem. Mater., 2000, 12, 2557–2566 CrossRef CAS.
- Z. Y. Wang, D. Y. Luan, Freddy Yin Chiang Boey and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 4738–4741 CrossRef CAS.
- C. W. Sun, Shreyas Rajasekhara, J. B. Goodenough and F. Zhou, J. Am. Chem. Soc., 2011, 133, 2132–2135 CrossRef CAS.
- W. X. Zhang, M. Li, Q. Wang, G. D. Chen, M. Kong, Z. H. Yang and S. Mann, Adv. Funct. Mater., 2011, 21, 3516–3523 CrossRef CAS.
- S. Y. Gao, S. X. Yang, J. Shu, S. X. Zhang, Z. D. Li and K. J. Jiang, J. Phys. Chem. C, 2008, 112, 19324–19328 CAS.
- B. Wang, J. S. Chen, H. B. Wu, Z. Y. Wang and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 17146–17148 CrossRef CAS.
- X. H. Huang, C. B. Wang, S. Y. Zhang and F. Zhou, Electrochim. Acta, 2011, 56, 6752–6756 CrossRef CAS.
- H. B. Wang, Q. M. Pan, J. W. Zhao, G. P. Yin and P. J. Zuo, J. Power Sources, 2007, 167, 206–211 CrossRef CAS.
- Z. Y. Wang, F. B. Su, S. Madhavi and X. W. Lou, Nanoscale, 2011, 3, 1618–1623 RSC.
- Q. M. Pan, H. Z. Jin, H. B. Wang and G. P. Yin, Electrochim. Acta, 2007, 53, 951–956 CrossRef CAS.
- S. Grugeon, S. Laruelle, R. Herrera-Urbina, L. Dupont, P. Poizot and J. M. Tarascon, J. Electrochem. Soc., 2001, 148, A285–292 CrossRef CAS.
- A. Débart, L. Dupont, P. Poizot, J. B. Leriche and J. M. Tarascon, J. Electrochem. Soc., 2001, 148, A1266–1274 CrossRef.
- S. Laruelle, S. Grugeon, P. Poizot, M. Dollé, L. Dupont and J. M. Tarascon, J. Electrochem. Soc., 2002, 149, A627–634 CrossRef CAS.
- X. W. Lou, C. M. Li and L. A. Archer, Adv. Mater., 2009, 21, 2536–2539 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: XRD, SEM, electrode preparation and testing. See DOI: 10.1039/c2ra21267f/ |
| This journal is © The Royal Society of Chemistry 2012 |
