Synthesis of novel magnetic silica supported hybrid ionic liquid combining TEMPO and polyoxometalate and its application for selective oxidation of alcohols†
Jie
Zhu
,
Peng-cheng
Wang
and
Ming
Lu
*
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094, China. E-mail: luming302@126.com; Fax: (+086)-025-84315514; Tel: (+086)-025-84315030
First published on 16th July 2012
Abstract
A novel magnetic silica supported bifunctional hybrid material (IL/SMNP) combining TEMPO based ionic liquid and polyoxometalate moieties was developed and used in the selective aerobic oxidation of various alcohols. A pseudo-homogeneous and environmental benign process was achieved with excellent activity and easy recovery of the catalyst.
The discovery of new environmentally friendly methods for selective catalytic oxidation of alcohol substrates to aldehydes and ketones is an important goal in the development of modern methods for chemical synthesis.1–3 Recently, utilization of the stable nitroxyl radical 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or its derivatives with a variety of terminal oxidants for oxidation of alcohols has attracted great attention.4,5 Especially common is the application of a co-oxidant such as NaOCl,6–9 NaOCl/NaClO2,10–13 trichloroisocyanuric acid,14,15m-chloroperbenzoic acid,16,17 oxone18,19 or hypervalent iodine compounds.20–22 Usually, even if TEMPO or its derivatives can be recycled, the co-oxidant is lost and must be re-added, which makes the process troublesome, tedious and less environmentally benign.
To obviate the disadvantages, we attempt to develop efficient hybrid catalysts with both TEMPO and co-oxidant moieties. In the last decade, ionic liquids (ILs) have attracted considerable attention as an alternative reaction medium, which show interesting properties such as high thermal stability, high loading capacity and easy recyclability.23–27 The characteristics of the ionic liquids can be tuned readily by modifying the structure of the cation or the anion, so that they can be adapted to special applications. On this basis, ILs are a good choice to investigate hybrid TEMPO and co-oxidant moieties. Also, the recovery of catalyst is of great importance. In recent years the use of magnetic nanoparticles has promised easy recycling of the catalyst by simply applying an external magnetic field28–31 and thus is also introduced into this study.
Herein, a task-specific IL was designed and synthesized combining a TEMPO moiety and a polyoxometalate (POM), H5PV2Mo10O40, as co oxidant for oxidation of various alcohols into their corresponding carbonyl compounds. Furthermore, silica coated magnetic nanoparticles (SMNP) were used as a support for the IL (IL/SMNP) to achieve a pseudo-homogeneous process and make the recovery and reuse of catalyst achievable and efficient. With the magnetic hybrid catalyst, both facilitated recovery of IL and excellent efficiency for selective oxidation of alcohols were achieved. The synthesis of catalyst is shown in Scheme 1.
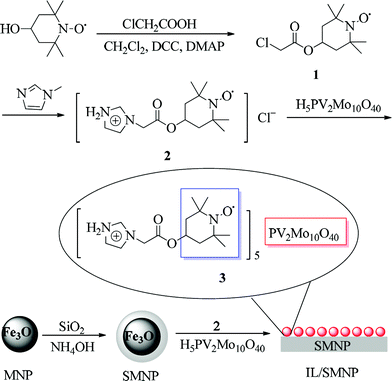 | ||
| Scheme 1 Synthesis of IL/SMNP | ||
Hybridization of the TEMPO moiety and co-oxidant was achieved to afford a bifunctionalized ionic liquid, with TEMPO being introduced into the imidazolium cation and [PV2Mo10O40]5− served as the anion (Scheme 1). The specific polyoxometalate (POM) H5PV2Mo10O40 has been shown to have catalytic activity in a myriad of applications,32,33 with, however, only limited reports for alcohol oxidation.34,35 Ben-Daniel36 described aerobic oxidation of alcohols in the combined presence of TEMPO and H5PV2Mo10O40, which inspired us to choose H5PV2Mo10O40 as the co-oxidant for TEMPO. To prepare the catalyst, H5PV2Mo10O40 was allowed to react with TEMPO based ionic liquid 2 and the POM anion [PV2Mo10O40]5− can be easily introduced into the ionic liquid through ion exchange process.
A magnetic support was applied to achieve easy recovery of catalyst and a pseudo-homogeneous procedure. Fe3O4 magnetic nanoparticles (MNPs) were chosen as the core magnetic support because of their simple synthesis, low cost, and relatively large magnetic susceptibility. The IR spectrum (Fig. 1) of SMNP supported IL 3 (IL/SMNP) shows signals that are characteristic of TEMPO and [PV2Mo10O40]5− groups, which clearly differs from that of the bare magnetic nanoparticles (bare MNPs) and unfunctionalized silica-coated nanomagnets. XRD analysis was also applied to the prepared bare MNP, SMNP and IL/SMNP. The XRD patterns in Fig. 2 confirm that the coating process did not induce any phase change of Fe3O4. IR analysis thus indicated the successful loading of the IL on the surface of magnetic nanobeads and XRD analysis suggested phase retention of Fe3O4. To further confirm the composite nature of the catalyst, Raman spectra were also recorded and shown in ESI†.
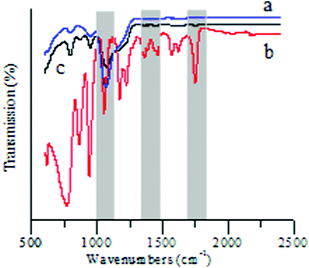 | ||
| Fig. 1 FTIR spectra of (a) bare MNP, (b) SMNP and (c) IL/SMNP. | ||
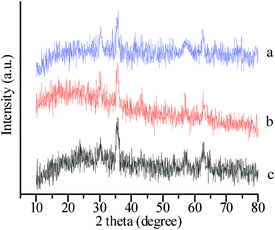 | ||
| Fig. 2 XRD patterns of (a) bare MNP, (b) SMNP and (c) IL/SMNP. | ||
The catalytic activity of SMNP supported difunctionalized IL was first tested in the oxidation of benzyl alcohol with toluene as solvent under 2 atm O2 in the absence of any other additives. To screen the reaction conditions, exploratory experiments were conducted and the results are summarized in Table 1. Preliminary results indicate that high temperature is favorable for the reaction and excellent catalytic performance of the catalyst were achieved at 80 °C (entries 5–8). The reaction efficiency increased directly with the amount of IL/SMNP and was almost independent of SMNP. A catalytic amount of IL/SMNP (<5 mol% of substrate) was sufficient to obtain good yields (entries 2–4). Comparing the bulk and supported IL on Al2O3 and SiO2, it appears that the enhancement in catalytic performance is due to the increased surface area (entries 9–11). SMNP supported IL, with an increased surface area, showed much higher oxidation activity than that of unsupported IL. IL/SMNP also exhibited better performance than Al2O3 supported IL, since SiO2 has a larger surface area than that of Al2O3.37 Hybridization of TEMPO and H5PV2Mo10O40 renders the oxidation step of the TEMPO moiety with H5PV2Mo10O40 more efficient probably because the reaction would change to an intramolecular process (entry 12).
| Entry | Catalyst (10−2 mmol) | T/°C | Conv. (%) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 2 mmol benzyl alcohol, 15 mL toluene, 2 atm O2, 5 h; loading of IL = 20 wt% in entries 2–8, 10 and 11. b 1 × 10−2 mmol TEMPO and H5PV2Mo10O40. | ||||
| 1 | — | 80 | Trace | Trace |
| 2 | IL/SMNP (0.2) | 80 | 78 | 76 |
| 3 | IL/SMNP (1) | 80 | 98 | 97 |
| 4 | IL/SMNP (2) | 80 | 99 | 97 |
| 5 | IL/SMNP (1) | 50 | 31 | 31 |
| 6 | IL/SMNP (1) | 60 | 63 | 92 |
| 7 | IL/SMNP (1) | 70 | 90 | 88 |
| 8 | IL/SMNP (1) | 90 | 98 | 98 |
| 9 | IL (1) | 80 | 76 | 76 |
| 10 | IL/SiO2 (1) | 80 | 99 | 99 |
| 11 | IL/Al2O3 (1) | 80 | 85 | 84 |
| 12 | TEMPO/H5PV2Mo10O40 (1b) | 80 | 72 | 72 |
The catalytic performance of IL/SMNP at different loadings was also studied. As illustrated in Fig. 3, the support SMNP alone hardly showed any activity in the oxidation of benzyl alcohol, and a maximum activity was observed at a loading of 20 wt% IL. At lower loading, despite the high dispersion of catalyst, the surface of SMNP was not fully covered. On the other hand, at higher loading, the aggregation of IL lowered the dispersion, leading to reduced surface area. Therefore, 20 wt% IL/SMNP was used in the following investigations.
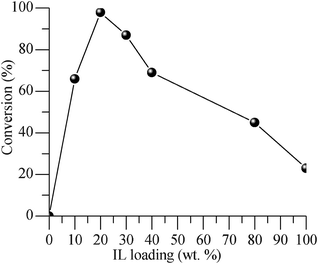 | ||
| Fig. 3 Performance of bulk and SMNP supported IL at different loadings in the aerobic oxidation of benzyl alcohol at 80 °C, 5 h. | ||
To examine the utility and generality of this methodology for the oxidation of alcohols, we applied the present catalyst system to a variety of alcohols as shown in Table 2. Substrate scope was extended to benzylic, allylic, heterocyclic, alicyclic and aliphatic alcohols. In all cases, ketones or aldehydes were the only detected reaction products. All the primary benzylic alcohols tested were converted into their corresponding aldehydes in high yields and no over-oxidation to acids was observed (entries 1–7). Further, it was found that the oxidative efficiency was not affected by the presence of a double bond (entries 11 and 12) or substituent group on the benzene ring (entries 2–7). It is noteworthy that a variety of heterocyclic alcohols, which are often less active in many reported systems, worked well in this system (entries 8–10). In addition, the activities exhibited by secondary alcohols were also good in the IL/SMNP catalyzed system (entries 16 and 17). Together with the good results with benzylic and heteroaryl alcohols, the yields obtained from the reaction of aliphatic alcohols under the same conditions were also satisfactory upon prolonging the reaction time (entries 14 and 15).
| Entry | Alcohol | Product | t/h | Conv. (%) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 2 mmol alcohol, 1 mmol IL/SMNP (20 wt% loading IL), toluene (15 mL), 2 atm O2, 80 °C. | |||||
| 1 |
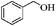
|
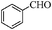
|
5 | 98 | 97 |
| 2 |
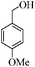
|
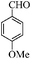
|
5 | 97 | 95 |
| 3 |
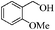
|
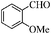
|
6 | 96 | 93 |
| 4 |
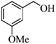
|
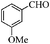
|
6 | 93 | 92 |
| 5 |
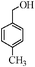
|
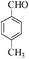
|
5 | 99 | 98 |
| 6 |
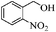
|
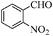
|
6 | 91 | 90 |
| 7 |
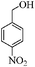
|
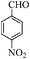
|
4 | 93 | 87 |
| 8 |
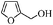
|
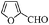
|
5 | 91 | 88 |
| 9 |
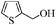
|
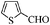
|
6 | 87 | 84 |
| 10 |
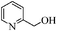
|
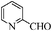
|
6 | 82 | 79 |
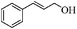
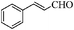
| 11 |
|
|
8 | 77 | 77 |
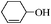
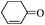
| 12 |
|
|
6 | 94 | 92 |
| 13 |
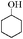
|
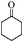
|
12 | 80 | 73 |
| 14 | 1-C8H17OH | 1-C7H13CHO | 16 | 78 | 77 |
| 15 |
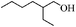
|
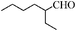
|
16 | 76 | 72 |
| 16 |
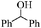
|
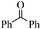
|
18 | 66 | 65 |
| 17 |
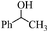
|
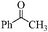
|
18 | 70 | 68 |
The advantage of the magnetic hybrid catalyst lies in not only the high catalytic activity and exclusion of any additives during oxidation process, attributed to the combination of TEMPO and H5PV2Mo10O40 moieties, but also in the ease of separation and recyclability provided by the SMNP support. Simply by applying an external magnet to the reaction vessel a separation of the catalyst is achieved within 5 s and the resulting clear supernatant can be decanted, which minimizes the loss of catalyst during separation. The recovered IL/SMNP can be reused for at least 10 times without significant loss of activity. The conversion of substrate decreased gradually with the recycling process going on and finally dropped to about 80% in the 10th run. The cycle times can be seen in Figure S8 from supporting information.† The SiO2 coating ensured the stability of the Fe3O4 core against oxidation in the reaction, so as to obtain a high recyclability. Beyond 10 times of reuse, the SiO2 coating starts to break up and the Fe3O4, losing the protection of SiO2 is more readily oxidized. This would lessen the magnetism and so the catalyst would be lost in solution and the recovery efficiency decreases gradually.
All synthesized particles have small coercivities, which indicate that they are superparamagnetic in nature. As shown in Fig. 4, saturation magnetizations for MNP, SMNP and IL/SMNP are 62.56, 37.98 and 31.42 emu g−1, respectively, with the decreasing values reflecting the increasing amount of nonmagnetic material on the particle surface. For SMNP the nonmagnetic fraction, as reflected by the silica![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4 ratio (wt%) is 47.54
Fe3O4 ratio (wt%) is 47.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.46 as obtained from XRF analysis (ESI†). A direct result of this effect is that it takes longer to separate SMNP and IL/SMNP than bare MNP from solution.
48.46 as obtained from XRF analysis (ESI†). A direct result of this effect is that it takes longer to separate SMNP and IL/SMNP than bare MNP from solution.
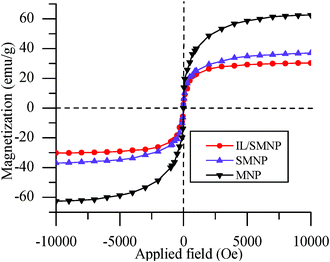 | ||
| Fig. 4 Magnetization curves for MNP, SMNP and IL/SMNP. | ||
The EPR spectra were recorded with the same mass amount of ILs 2 and 3 for convenience, but the data was converted for comparison in the following explanation. As shown in Fig. 5, the spectra revealed an unexpectedly lower signal for the nitroxyl radical of IL 3 compared with that of TEMPO based IL 2. This indicates that in 3 the POM is partially reduced and TEMPO is partially oxidized. The yellowish green color of IL/SMNP also confirms the redox reaction. The structure of IL/SMNP is thus not as simple as that shown in Scheme 1. We surmise that on average one out of five nitroxyl radicals is oxidized by the POM into to a nitrosonium cation, which is the active alcohol oxidizing species which is responsible for converting the alcohol into the corresponding carbonyl compound. If so, the molal ratio of nitroxyl radical in IL 2 to IL 3 would be 2.43 which matches with the determined value of 2.51. While, in contrast to V(V), V(IV) is EPR active, because the associated signal is of much lower intensity compared with that of nitroxyl radical, the typical spectrum for reduced H5PV2Mo10O40 is hard to identify when both species are present. The V(IV) signal only can be seen using EPR software after magnifying the signal value. Together with previous studies,36,38 a possible mechanism for the aerobic oxidation of alcohols in the system is proposed as shown in Scheme 2. The catalytic cycle was completed when oxidized form of POM is regenerated by molecular oxygen.
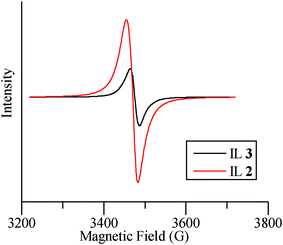 | ||
| Fig. 5 ESR spectra for ILs 2 and 3. | ||
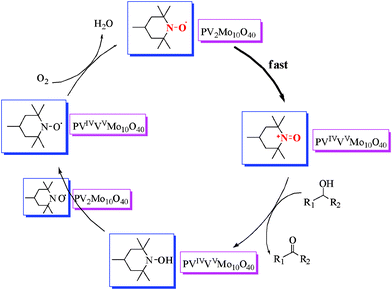 | ||
| Scheme 2 Proposed mechanism of the oxidation. | ||
Conclusions
In conclusion, we have designed and synthesized a magnetic silica supported bifunctional hybrid-type ionic liquid catalyst (IL/SMNP) bearing two catalyst sites, TEMPO and polyoxometalate moieties. This was proved to be efficient for selective oxidation of a wide set of aliphatic, allylic, heterocyclic and benzylic alcohols with good to excellent yields. In particular, attributed to the magnetic Fe3O4/SiO2 system, it was a pseudo-homogeneous process and after reaction, IL/SMNP could be easily recovered with the help of an external magnet to be reused.References
- R. A. Sheldon, I. W. C. E. Arends and A. Dijksman, Catal. Today, 2000, 57, 157–166 CrossRef CAS.
- S. Caron, R. W. Dugger, S. G. Ruggeri, J. A. Ragan and D. H. Brown Ripin, Chem. Rev., 2006, 106, 2943–2989 CrossRef CAS.
- R. W. Dugger, J. A. Ragan and D. H. Brown Ripin, Org. Process Res. Dev., 2005, 9, 253–258 CrossRef CAS.
- L. Tebben and A. Studer, Angew. Chem., Int. Ed., 2011, 50, 5034–5068 CrossRef CAS.
- R. Ciriminna and M. Pagliaro, Org. Process Res. Dev., 2010, 14, 245–251 CrossRef CAS.
- P. L. Anelli, C. Biffi, F. Montanari and S. Quici, J. Org. Chem., 1987, 52, 2559–2562 CrossRef.
- X. E. Wu, L. Ma, M. X. Ding and L. X. Gao, Synlett, 2005, 4, 607–610 Search PubMed.
- A. Gheorghe, A. Matsuno and O. Reiser, Adv. Synth. Catal., 2006, 348, 1016–1020 CrossRef CAS.
- O. Holczknecht, M. Cavazzini, S. Quici, I. Shepperson and G. Pozzi, Adv. Synth. Catal., 2005, 347, 677–688 CrossRef CAS.
- M. Zhao, J. Li, E. Mano, Z. Song, D. M. Tschaen, E. J. J. Grabowski and P. J. Reider, J. Org. Chem., 1999, 64, 2564–2566 CrossRef CAS.
- M. M. Zhao, J. Li, E. Mano, Z. J. Song and D. M. Tschaen, Org. Synth., 2004, 81, 195–203 Search PubMed.
- A. Zanka, Chem. Pharm. Bull., 2003, 51, 888–889 CrossRef CAS.
- M. Shibuya, T. Sato, M. Tomizawa and Y. Iwabuchi, Chem. Commun., 2009, 1739–1741 RSC.
- L. De Luca, G. Giacomelli, S. Masala and A. Porcheddu, J. Org. Chem., 2003, 68, 4999–5001 CrossRef CAS.
- L. De Luca, G. Giacomelli and A. Porcheddu, Org. Lett., 2001, 3, 3041–3043 CrossRef CAS.
- J. A. Cella, J. A. Kelley and E. F. Kenehan, J. Org. Chem., 1975, 40, 1860–1862 CrossRef CAS.
- S. D. Rychovsky and R. Vaidyanathan, J. Org. Chem., 1999, 64, 310–312 CrossRef.
- C. Bolm, A. S. Magnus and J. P. Hildebrand, Org. Lett., 2000, 2, 1173–1175 CrossRef CAS.
- P. L. Bragd, A. C. Besemer and H. van Bekkum, Carbohydr. Polym., 2002, 49, 397–406 CrossRef CAS.
- T. Yakura and A. Ozono, Adv. Synth. Catal., 2011, 353, 855–859 CrossRef CAS.
- C. Zhu, Y. Wei and L. Ji, Synth. Commun., 2010, 40, 2057–2066 CrossRef CAS.
- X. Q. Li and C. Zhang, Synthesis, 2009, 1163–1169 CAS.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS.
- R. Sheldon, Chem. Commun., 2001, 2399–2407 RSC.
- J. S. Wilkes, Green Chem., 2002, 4, 73–80 RSC.
- W. Miao and T. H. Chan, Acc. Chem. Res., 2006, 39, 897–908 CrossRef CAS.
- K. Bica and P. Gaertner, Eur. J. Org. Chem., 2008, 3235–3250 CrossRef CAS.
- A. K. Tucker-Schwartz and R. L. Garrell, Chem.–Eur. J., 2010, 16, 12718–12726 CrossRef CAS.
- B. Karimi and E. Farhangi, Chem.–Eur. J., 2011, 17, 6056–6060 CrossRef CAS.
- C. X. Miao, J. Q. Wang, B. Yu, W. G. Cheng, J. A. Sun, S. Chanfreau, L. N. He and S. J. Zhang, Chem. Commun., 2011, 47, 2697–2699 RSC.
- M. Ma, Q. Zhang, D. Yin, J. Dou, H. Zhang and H. Xu, Catal. Commun., 2012, 17, 168–172 CrossRef CAS.
- H. Lu, J. Gao, Z. Jiang, Y. Yang, B. Song and C. Li, Chem. Commun., 2007, 150–152 Search PubMed.
- S. Zhao, X. Wang and M. Huo, Appl. Catal., B, 2010, 97, 127–134 CrossRef CAS.
- A. M. Khenkin, I. Vigdergauz and R. Neumann, Chem.–Eur. J., 2000, 6, 875–882 CrossRef.
- A. Bordoloi, S. Sahoo, F. Lefebvre and S. B. Halligudi, J. Catal., 2008, 259, 232–239 CrossRef CAS.
- R. Ben-Daniel, P. Alsters and R. Neumann, J. Org. Chem., 2001, 66, 8650–8653 CrossRef CAS.
- J. Zhang, A. Wang, X. Li and X. Ma, J. Catal., 2011, 279, 269–275 CrossRef CAS.
- A. M. Khenkin, A. Rosenberger and R. Neumann, J. Catal., 1999, 182, 82–91 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20588b |
| This journal is © The Royal Society of Chemistry 2012 |
