Copper-catalysed addition of α-alkyl azaarenes to ethyl glyoxylate via direct C(sp3)–H activation†
Jia-Jia
Jin
a,
Hong-Ying
Niu
ab,
Gui-Rong
Qu
a,
Hai-Ming
Guo
*a and
John S.
Fossey
*ac
aCollege of Chemistry and Environmental Science, Key Laboratory of Green Chemical Media and Reactions of Ministry of Education, Henan Normal University, Xinxiang, 453007, Henan, China. E-mail: guohm518@hotmail.com; Fax: 86 373 3329276; Tel: 86 373 3329255
bSchool of Chemistry and Chemical Engineering, Henan Institute of Science and Technology, Xinxiang 453003, China
cSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, West Midlands B15 2TT, UK. E-mail: j.s.fossey@bham.ac.uk
First published on 14th May 2012
Abstract
A novel protocol for the copper-catalysed direct C(sp3)–H bond functionalisation of 2-alkyl azaarenes to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds has been developed, which expands the scope of C(sp3)–H bond activation reactions and provides new access to medicinally important azaarene derivatives.
O double bonds has been developed, which expands the scope of C(sp3)–H bond activation reactions and provides new access to medicinally important azaarene derivatives.
The functionalisation of pyridines and quinolines is a valuable chemical transformation in organic synthesis since derivatives of these aromatic heterocycles can display extremely potent biological, chemical and pharmaceutical properties.1 The C–H bond functionalisation of pyridines and quinolines2 is an expedient and atom economical synthetic strategy to access substituted azaarene derivatives. Among them, the direct C(sp3)–H bond functionalisation of 2-alkyl azaarenes is a challenging synthetic process due to the lower activity of alkyl groups.
Fagnou et al. and Charette et al. have reported the palladium catalysed C(sp3)–H activation of 2-alkyl pyridine N-oxides3 and N-iminopyridinium ylides4 through the introduction of an activating group onto the pyridine core to enhance the acidity of the α-H of 2-alkyl pyridine. However, this synthetic strategy requires the modification of substrates and multistep synthetic sequences. Recently, a cluster of reports have appeared on the direct C(sp3)–H bond functionalisation of 2-alkyl-substituted azaarenes catalysed by palladium and Lewis acid without an activating group.5–9 For example, Rueping et al. and Huang and co-workers reported the addition of α-alkyl azaarenes to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond of N-sulfonyl aldimines5a–b,7a(eq 1, Scheme 1). Matsunaga and Kanai and co-workers reported the direct addition of alkyl-substituted azaarenes to the C
N double bond of N-sulfonyl aldimines5a–b,7a(eq 1, Scheme 1). Matsunaga and Kanai and co-workers reported the direct addition of alkyl-substituted azaarenes to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds of enones promoted by Lewis acids7b (eq 2, Scheme 1). To the best of our knowledge, the addition of 2-alkyl azaarenes to C
C double bonds of enones promoted by Lewis acids7b (eq 2, Scheme 1). To the best of our knowledge, the addition of 2-alkyl azaarenes to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds catalysed by Lewis acids has not been reported. To address this apparent gap in synthetic capability it was reasoned that the addition of α-alkyl azaarenes to C
O double bonds catalysed by Lewis acids has not been reported. To address this apparent gap in synthetic capability it was reasoned that the addition of α-alkyl azaarenes to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds could be achieved when an appropriate Lewis acid10 was used as a catalyst. After the first draft of this manuscript was submitted, a paper by Li and co-workers appeared online describing the synthesis of azaarene-substituted 3-hydroxy-2-oxindoles catalysed by a Brønsted acid.11 Our continued interest in C–H bond activation12 led us to the present report where the copper-catalysed addition of α-alkyl azaarenes to the aldehyde group of ethyl glyoxylate via direct C(sp3)–H activation is detailed.
O double bonds could be achieved when an appropriate Lewis acid10 was used as a catalyst. After the first draft of this manuscript was submitted, a paper by Li and co-workers appeared online describing the synthesis of azaarene-substituted 3-hydroxy-2-oxindoles catalysed by a Brønsted acid.11 Our continued interest in C–H bond activation12 led us to the present report where the copper-catalysed addition of α-alkyl azaarenes to the aldehyde group of ethyl glyoxylate via direct C(sp3)–H activation is detailed.
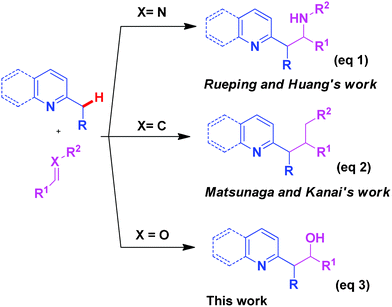 | ||
| Scheme 1 C(sp3)–H bond functionalisation with different double bond containing electrophiles. | ||
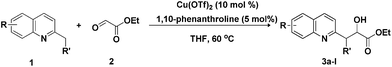
Initially, 2-methyl quinoline 1a and ethyl glyoxylate 2 were chosen as model substrates in a reaction designed to deliver product 3a, optimisation studies are summarised in Table 1. The reactions conducted at 120 °C in line with conditions reported for related reactions proceeded only in very low yields.5–9 The yield of the product 3a was satisfactory when the reaction was performed at 60 °C for 24 h. A screening of potential catalysts indicated that copper salts could permit the reaction to proceed smoothly with 10 mol% of Cu(OTf)2 to give product 3a in higher yields than any of the other copper salts13 tested (entries 1–5). Reducing the amount of Cu(OTf)2 resulted in diminished yields under otherwise unaltered conditions (entry 6). A survey of solvents revealed tetrahydrofuran (THF) to be an optimal selection (entries 7–11). Although the reaction gave a good yield (78%) in the absence of a ligand (entry 12), product 3a was obtained in a higher yield (94% after purification) in the presence of 5 mol% of 1,10-phenanthroline (entry 5). The use of 1,3-bis(diphenylphosphino)propane (dppp) as the ligand gave a lower yield than that with 1,10-phenanthroline (entry 13).
|
|
||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | Ligand | Yield/%b |
| a Unless otherwise stated, all reactions were carried out with 1a (0.6 mmol), 2 (0.2 mmol), ligand (5 mol%), solvent (0.8 mL) in a Schlenk tube at 60 °C for 24 h. b Isolated yield. c 5 mol% Cu(OTf)2 was used. | ||||
| 1 | CuI | THF | 1,10-phenanthroline | 68 |
| 2 | CuBr | THF | 1,10-phenanthroline | 65 |
| 3 | CuCl | THF | 1,10-phenanthroline | 60 |
| 4 | CuCN | THF | 1,10-phenanthroline | 72 |
| 5 | Cu(OTf)2 | THF | 1,10-phenanthroline | 94 |
| 6c | Cu(OTf)2 | THF | 1,10-phenanthroline | 75 |
| 7 | Cu(OTf)2 | DMF | 1,10-phenanthroline | 50 |
| 8 | Cu(OTf)2 | dioxane | 1,10-phenanthroline | 35 |
| 9 | Cu(OTf)2 | toluene | 1,10-phenanthroline | 43 |
| 10 | Cu(OTf)2 | i-PrOH | 1,10-phenanthroline | 55 |
| 11 | Cu(OTf)2 | CH2ClCH2Cl | 1,10-phenanthroline | 28 |
| 12 | Cu(OTf)2 | THF | none | 78 |
| 13 | Cu(OTf)2 | THF | dppp | 72 |
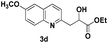
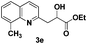
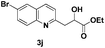
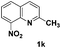
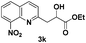
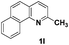
The tolerance of this reaction to various 2-alkyl quinolines with electron-neutral, electron-donating or electron-withdrawing groups attached was next examined under the optimised conditions (Table 2). The reaction of ethyl glyoxylate 2 and 2-alkyl quinolines 1a–1j proceeded smoothly and provided the corresponding products 3a–3j with yields of 68–94% (entries 1–10). In contrast, the C6-substituted 2-alkyl quinolines gave higher yields than the C8-substituted 2-alkyl quinolines, which might be as a result of steric factors at the C8 position of quinolines. 2-Methyl-8-nitroquinoline 1k gave a relatively low yield, which might again be due to steric effects compounded by deleterious electronic effects (entry 11). It was remarkable then that halide substituents were tolerated in the quinoline ring (entries 7, 8, and 10). When 2-methyl-7,8-benzoquinoline was used as the substrate, the yield of the corresponding adduct was only 54% (entry 12). Under the described conditions no main ring, C(sp2)–H, functionalisation was observed in the above reactions.
|
|
|||
|---|---|---|---|
| Entry | Substrate | Product | Yield/%b |
| a Unless otherwise stated, all reactions were carried out with 1a (0.6 mmol), 2 (0.2 mmol), catalyst (10 mol%), ligand (5 mol%), THF (0.8 mL) in a Schlenk tube at 60 °C for 24 h. b Isolated yield. c The diastereomeric ratio in parentheses as determined by HPLC. | |||
| 1 |
|
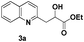
|
94 |
| 2 |
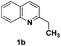
|

|
88(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)c 8)c |
| 3 |
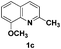
|
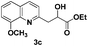
|
78 |
| 4 |
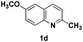
|
|
84 |
| 5 |
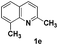
|
|
68 |
| 6 |
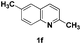
|
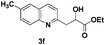
|
73 |
| 7 |
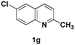
|
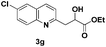
|
79 |
| 8 |
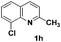
|
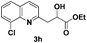
|
68 |
| 9 |
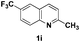
|
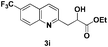
|
80 |
| 10 |

|
|
84 |
| 11 |
|
|
36 |
| 12 |
|
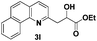
|
54 |
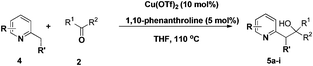
The use of 2-alkyl pyridines as substrates under the earlier optimised conditions for quinolines is summarised in Table 1. With regards to the different properties of quinoline and pyridine, the reaction temperature for the 2-alkyl pyridines was surveyed and 110 °C emerged as the best. Next, the scope of the 2-alkyl pyridines was also examined. The reaction of ethyl glyoxylate 2 and the 2-alkyl pyridines 4a–4i, bearing either electron-neutral or electron-withdrawing groups, provided the corresponding products 5a–5i in moderate to good yields (entries 1–9, Table 3). The addition of 2,6-lutidine 4b to ethyl glyoxylate 2 gave a high yield of the mono functionalised product 5b (entry 2). However, when ethyl glyoxylate 2 was used in excess the double C(sp3)–H functionalised product increased. Chloride and CF3 substituents were also tolerated on the ring (entries 7, 8 and 9, Table 2). Unfortunately, the observed ratio was often close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in reactions with the potential to form mixtures of diastereoisomers (entries 3–5, Table 3). The addition of 2-alkyl azaarenes to ethyl pyruvate, aliphatic aldehydes or aromatic aldehydes failed to deliver the desired addition products under the same reaction conditions (entries 10, 11 and 12, Table 3).
1 in reactions with the potential to form mixtures of diastereoisomers (entries 3–5, Table 3). The addition of 2-alkyl azaarenes to ethyl pyruvate, aliphatic aldehydes or aromatic aldehydes failed to deliver the desired addition products under the same reaction conditions (entries 10, 11 and 12, Table 3).
|
|
|||
|---|---|---|---|
| Entry | Substrate | Product | Yield/%b |
| a Unless otherwise stated, reactions were carried out with 1a (0.6 mmol), 2 (0.2 mmol), catalyst (10 mol%), ligand (5 mol%), THF (0.8 mL) in a Schlenk tube at 60 °C for 24 h. b Isolated yield. c 4 equiv. of 4a was used. d The diastereomeric ratio in parentheses as determined by HPLC. e N.R. is no reaction. | |||
| 1 |

|
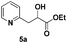
|
67c |
| 2 |
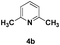
|
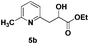
|
86 |
| 3 |
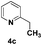
|

|
89(5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)d 6)d |
| 4 |
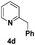
|

|
64(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)d 1)d |
| 5 |

|
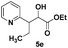
|
78(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)d 1)d |
| 6 |
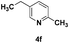
|
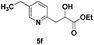
|
74 |
| 7 |
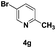
|
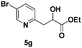
|
50 |
| 8 |
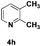
|
|
55 |
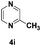
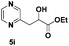
| 9 |
|
|
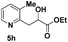
54 |
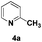
| 10 |
|
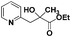
|
N.R.e |
| 11 |
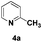
|
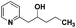
|
N.R.e |
| 12 |
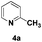
|
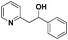
|
N.R.e |
In conclusion, we have developed an efficient copper-catalysed addition of 2-alkyl azaarenes to aldehyde esters through C(sp3)–H bond functionalisation. This example of the addition of 2-alkyl azaarenes to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond catalysed by a Lewis acid expands the scope of C(sp3)–H bond activation reactions. This protocol provides a simple and rapid approach to access a variety of azaarene derivatives, which are of great interest and importance in medicinal chemistry applications. Further studies to expand the scope of generating new C–C bonds via C–H bond activation strategies are ongoing.
O double bond catalysed by a Lewis acid expands the scope of C(sp3)–H bond activation reactions. This protocol provides a simple and rapid approach to access a variety of azaarene derivatives, which are of great interest and importance in medicinal chemistry applications. Further studies to expand the scope of generating new C–C bonds via C–H bond activation strategies are ongoing.
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (Grant Nos 21072047, and 21172059), the Program for New Century Excellent Talents from the University of Ministry of Education (No. NCET-09-0122), Excellent Youth Foundation of Henan Scientific Committee (No. 114100510012), the Program for Innovative Research Team from the University of Henan Province (2012IRTSTHN006), the Program for Changjiang Scholars and Innovative Research Team in the University (IRT1061), and the Excellent Youth Program from Henan Normal University. JSF thanks Henan Normal University for a visiting professorship and the University of Birmingham for support.References
- (a) P. N. W. Baxter, J. M. Lehn, J. Fischer and M. T. Youinou, Angew. Chem., Int. Ed. Engl., 1994, 33, 2284 CrossRef; (b) J. M. Lehn, Science, 2002, 295, 2400 CrossRef CAS; (c) D. Henry, Tetrahedron, 2004, 60, 6043 CrossRef; (d) J. P. Michael, Nat. Prod. Rep., 2005, 22, 627 RSC; (e) M. C. Bagley, C. Glover and E. A. Merritt, Synlett, 2007, 2459 CrossRef CAS.
- (a) F. Kakiuchi and N. Chatani, Adv. Synth. Catal., 2003, 345, 1077 CrossRef CAS; (b) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC; (c) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS; (d) X. Chen, K. M. Engle, D. H. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS; (e) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS; (f) C. L. Sun, B. J. Li and Z. J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS; (g) S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626 CrossRef CAS.
- (a) L. C. Campeau, D. J. Schipper and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 3266 CrossRef CAS; (b) D. J. Schipper, L. C. Campeau and K. Fagnou, Tetrahedron, 2009, 65, 3155 CrossRef CAS.
- J. J. Mousseau, A. Larivée and A. B. Charette, Org. Lett., 2008, 10, 1641 CrossRef CAS.
- (a) B. Qian, S. Guo, J. Shao, Q. Zhu, L. Yang, C. Xia and H. Huang, J. Am. Chem. Soc., 2010, 132, 3650 CrossRef CAS; (b) B. Qian, S. Guo, C. Xia and H. Huang, Adv. Synth. Catal., 2010, 352, 3195 CrossRef CAS; (c) B. Qian, P. Xie, Y. Xie and H. Huang, Org. Lett., 2011, 13, 2580 CrossRef CAS.
- For the Pd-catalyzed C(sp3)–H bond functionalisation of pyridines, see: (a) D. Shabashov and O. Daugulis, Org. Lett., 2005, 7, 3657 CrossRef CAS; (b) T. Niwa, H. Yorimitsu and K. Oshima, Org. Lett., 2007, 9, 2373 CrossRef CAS; (c) P. M. Burton and J. A. Morris, Org. Lett., 2010, 12, 5359 CrossRef CAS; (d) H. F. Jiang, H. J. Chen, A. Wang and X. H. Liu, Chem. Commun., 2010, 46, 7259 RSC; (e) D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965 CrossRef CAS; (f) G. Y. Song, Y. Su, X. Gong, K. L. Han and X. W. Li, Org. Lett., 2011, 13, 1968 CrossRef CAS; (g) S. Duez, A. K. Steib, S. M. Manolikakes and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 7686 CrossRef CAS; (h) K. J. Stowers, K. C. Fortner and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 6541 CrossRef CAS.
- For Lewis acid catalysed reactions, see: (a) M. Rueping and N. Tolstoluzhsky, Org. Lett., 2011, 13, 1095 CrossRef CAS; (b) H. Komai, T. Yoshino, S. Matsunaga and M. Kanai, Org. Lett., 2011, 13, 1706 CrossRef CAS.
- (a) N. Chatani, T. Asaumi, S. Yorimitsu, T. Ikeda, F. Kakiuchi and S. Murai, J. Am. Chem. Soc., 2001, 123, 10935 CrossRef CAS; (b) S. Pan, K. Endo and T. Shibata, Org. Lett., 2011, 13, 4692 CrossRef CAS; (c) Y. Z. Yan, K. Xu, Y. Fang and Z. Y. Wang, J. Org. Chem., 2011, 76, 6849 CrossRef CAS; (d) H. Tsurugi, K. Yamamoto and K. Mashima, J. Am. Chem. Soc., 2011, 133, 732 CrossRef CAS.
- For recent reviews, see: O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902 RSC.
- (a) Y. Nakao, K. S. Kanyiva and T. Hiyama, J. Am. Chem. Soc., 2008, 130, 2448 CrossRef CAS; (b) M. Tobisu, I. Hyodo and N. Chatani, J. Am. Chem. Soc., 2009, 131, 12070 CrossRef CAS.
- R. Niu, J. Xiao, T. Liang and X. W. Li, Org. Lett., 2012, 14, 676 CrossRef CAS.
- (a) H. M. Guo, L. L. Jiang, H. Y. Niu, W. H. Rao, L. Liang, R. Z. Mao, D. Y. Li and G. R. Qu, Org. Lett., 2011, 13, 2008 CrossRef CAS; (b) H. M. Guo, W. H. Rao, H. Y. Niu, L. L. Jiang, G. Meng, J. J. Jin, X. N. Yang and G. R. Qu, Chem. Commun., 2011, 47, 5608 RSC; (c) H. M. Guo, W. H. Rao, H. Y. Niu, L. L. Jiang, L. Liang, Y. Zhang and G. R. Qu, RSC Adv., 2011, 1, 961 RSC.
- (a) P. Rémy, M. Langer and C. Bolm, Org. Lett., 2006, 8, 1209 CrossRef; (b) C. Girard, E. Onen, M. Aufort, S. Beauvière, E. Samson and J. Herscovici, Org. Lett., 2006, 8, 1689 CrossRef CAS; (c) I. Ban, T. Sudo, T. Taniguchi and K. Itami, Org. Lett., 2008, 10, 3607 CrossRef CAS; (d) Y. Li, Z. Yu and S. Wu, J. Org. Chem., 2008, 73, 5647 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, compound characterizations, and copies of the 1H NMR and 13C NMR spectra. See DOI: 10.1039/c2ra20627g/ |
| This journal is © The Royal Society of Chemistry 2012 |