Novel solvent-free synthesis and modification of polyaspartic acid hydrogel
Yuming
Wang
,
Mingyuan
Xue
,
Jun
Wei
,
Chunling
Li
,
Rui
Zhang
,
Hui
Cao
,
Jing
Yang
and
Tianwei
Tan
*
Beijing Key Lab of Bioprocess, Beijing University of Chemical Technology, Beijing0, 100029, People's Republic of China. E-mail: twtan@mail.buct.edu.cn
First published on 17th October 2012
Abstract
Polyaspartic acid (PASP) hydrogel, a hydrolysis derivative of polysuccinimide (PSI), has been previously synthesized by using organic solvents such as dimethyl formamide (DMF) or dimethyl sulfoxide (DMSO), making the process environmentally and economically unattractive. The present paper presents a “green” synthesis, without using organic solvents. It demonstrates that hydrolysis and modification can be combined with in situ cross-linking. Introducing an aqueous soluble cross-linker, experimental results would broaden the application area of PASP at significantly reduced preparation cost, whilst moreover improving the required properties. The PASP hydrogel obtained by this method has a 600 g g−1 water-swelling ratio against 420 g g−1 as obtained by the conventional method using DMF. To reduce the ionic strength sensitivity of the hydrogel, sulfo-groups from, e.g. taurine, can be grafted onto the main chain of PASP and produce a modified PASP hydrogel with a 300 g g−1 water-swelling ratio in a 0.9% sodium chloride solution, against only 80 g g−1 for the non-grafted PASP hydrogel.
1. Introduction
1.1 PASP hydrogel: uses, traditional solvent-based synthesis and novel “green” production
Polyaspartic acid (PASP) is a hydrophilic biodegradable polymer, containing a large amount of carboxyl side chains. PASP is classified into linear and cross-linked structures.1–3 Commercial non-cross-linked PASP has a low molecular weight and is mainly used as crop growing accelerant, whereas the cross-linked PASP and its derivates are applied as superabsorbent resin, separation medium or drug delivery vehicle. Although the study of linear PASP started in the 1950s by the Donlar Company, the commercial application of cross-linked PASP hydrogel is only rarely reported upon in detail.Conventional synthesis1 uses excessive4–10 amounts of organic solvents, such as dimethyl sulfoxide (DMSO) or dimethyl formamide (DMF)11,12 with associated problems of waste solvent production and required recycling and of toxicity and cost. These problems have limited the applications of cross-linked PASP hydrogel. Cross-linked PASP was conventionally prepared from polysuccinimide (PSI) by two subsequent steps: PSI was firstly dissolved and cross-linked in DMF or DMSO; the cross-linked PSI was thereafter hydrolyzed to obtain cross-linked PASP. Polysuccinimide (PSI) is virtually insoluble in water or weak alkali until heterogeneous hydrolysis occurs.13 This synthesis uses 20 to 40 mL of organic solvent to crosslink 1 g PSI.14
As a hydrolysis product of non-porous PSI particles, PASP is readily soluble. The traditional organic solvents used in the cross-linking step are hence the critical obstacle in the widespread production and marketing due to their toxicity and recycling cost. By replacing the organic solvent and performing the cross-linking step in an aqueous solution according to the traditional 2-step process, only the surface of the PSI particle will be cross-linked, while the inner core will not have the chance to be crosslinked.
The present study uses a novel method of synthesizing PASP without organic solvents, as shown in Fig. 1(b), with R = CxHy–NH2 (0 ≤ x ≤ 6) being simultaneously and co-currently hydrolyzed and cross-linked. The traditional DMF process is also illustrated for comparison in Fig. 1(a).
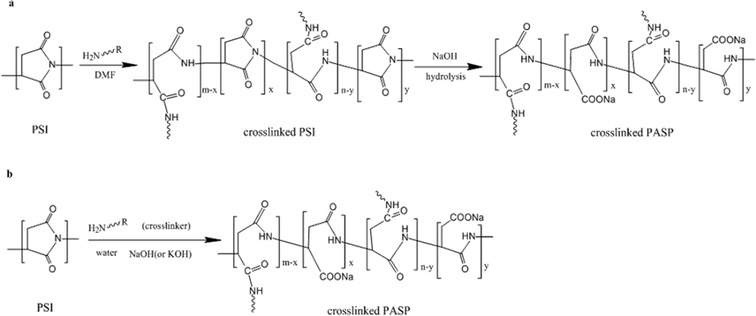 | ||
| Fig. 1 (a) Procedure of synthesizing crosslinked PASP in organic solvent; (b) procedure of synthesizing crosslinked PASP in water. | ||
While PSI is hydrolyzed and built into the main chain of PASP in an alkaline environment, the amine function of the cross-linker attacks the five-membered ring of PSI, opens the ring and connects chains of PASP into a network. The single and combined hydrolysis and cross-linking reactions produce a hydrophilic ring-opening product, swellable in aqueous solution. The unreacted PSI particle is then exposed to the cross-linker and base, thus maintaining a more complete participation in the reaction.
1.2 Further modification of PASP hydrogel
As an ionic type hydrogel, PASP hydrogel gets an outstretched network structure when it touches water because of hydrophilic group dissociation. The increase of ionic strength inside the network will result in an osmotic pressure between the network inside and the solution outside, which makes the water molecule filter into it. However, the ions in the solution will restrain the dissociation so that they decrease the osmotic pressure,15 which will obviously decrease the swelling ratio of the hydrogel. Since there is rarely pure water in nature, the ionic strength sensitivity is an important consideration for hydrogels.In order to improve the swelling ratio of PASP hydrogel in a saline solution, a diversification of the hydrophilic bases is an effective method.3,16 With more kinds of hydrophilic bases, the hydrogel will also get a higher swelling ratio in deionized water. The present study adds taurine into the solution where the hydrolysis and cross-linking are processed. The amine function of taurine will attack the five-membered ring of PSI during cross-linking and will graft taurine onto the main chain of PASP, creating a derivative with a lateral sulfo group, as shown in Fig. 1 for the case of –NH. This derivative is represented as R = CxHy–SO3H (0 ≤ x ≤ 6). The new derivative is supposed to have a higher swelling ratio in both deionized water and ionic solution.
1.3 Objectives of the research
The research will prove that the “green” synthesis of PASP cross-linked hydrogel is not only feasible, but also provides a hydrogel with better characteristics than the product manufactured by using the solvent-based synthesis. The taurine grafting moreover further improves the properties.2. Experimental
2.1 Materials
Polysuccinimide (PSI) was prepared in our laboratory. In a previous study, the authors discussed the effect of different PSI molecular weights on the properties of PASP.2,6,17 For the present study, we selected a specific PSI with molecular weight, Mw, of 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da and with Mw/Mn = 2.31, with Mw and Mn defined as weight-average molecular weight and number-average molecular weight, respectively. 1,6-hexanediamine, hydrazine hydrate, NaOH and taurine were all of analytical grade and purchased from Beijing Chemical Reagent Company (China). They were used as supplied.
000 Da and with Mw/Mn = 2.31, with Mw and Mn defined as weight-average molecular weight and number-average molecular weight, respectively. 1,6-hexanediamine, hydrazine hydrate, NaOH and taurine were all of analytical grade and purchased from Beijing Chemical Reagent Company (China). They were used as supplied.
2.2 Preparation of cross-linked PASP in organic solvent
To better illustrate the novelty of the solvent-free synthesis, the present section summarizes the solvent-based production process, as illustrated in Fig. 1(a). The cross-linked PASP hydrogel was produced by dispersing 1.0 g of PSI in 28 mL DMF and 8 mL de-ionized water with magnetic stirring in a 200 mL beaker. Then, 10 mL 1,6-hexamethylene diamine solution (0.50 mol L−1) and 10.0 mL NaOH solution (1 mol L−1) were added into the beaker. The mixture containing PSI, 1,6-hexamethylene diamine, NaOH, DMF and de-ionized water was stirred for 48 h. The cross-linking and hydrolysis were carried out at 25.0 °C. Subsequently, 100 mL ethanol was added, and the precipitate (crosslinked PASP hydrogel) was purified by washing with ethanol or water for 3 cycles to remove the unreacted chemicals. The hydrogel was finally freeze-dried.2.3 Novel “green” preparation of cross-linked PASP in an aqueous solution
The cross-linked PASP hydrogel was synthesized by the steps of Fig. 1(b). PSI was milled to 100 μm particles. The particles of PSI were dispersed in the aqueous solutions of dissolved cross-linker and base. When the temperature of the 48 h stirred slurry reached the selected value between 0 and 60 °C (selected in view of studying the effect of temperature on properties), the slurry became transparent and viscous. To remove the unreacted chemicals, the hydrogel in solution was washed with ethanol and swelling was allowed to proceed for 3 washing cycles until the pH remained constant. The freeze-dried product was used in further measurements. For practical use, however, ethanol can be replaced by water since unreacted crosslinker and base are aqueous soluble while hydrogel is insoluble and will only swell when water is added.2.4 Modification of cross-linked PASP in an aqueous solution with taurine
The process of modification is identical to section 2.3 above, except for the addition of a certain amount of taurine into the solution at the same time, which is also a kind of “green” chemical. Since taurine is connected to the five-membered ring of PSI as the cross-linking agent, the amount of hydrazine will be reduced proportionally.2.5 Determination of the hydrogel properties
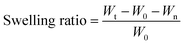 | (1) |
where Wt is the weight of the tea bag including swollen hydrogel, W0 is the weight of the dry sample and Wn is the weight of the wet nylon net.
3. Results and discussion
3.1 Scheme for the ring-opening of PSI
Mosig13 proposed a hydrolysis model of PSI. This is a fair model as a guide for plant process studies and to develop reactor control schemes in producing the linear PASP. For cross-linked PASP hydrogel, the model is invalid since cross-linked PASP was conventionally obtained from cross-linked PSI, synthesized in DMF or DMSO. Both the base and cross-linkers used in the present study were water soluble and they react with PSI by the scheme shown in Fig. 2. We therefore propose a novel scheme for the ring-opening of PSI as an alternative to the Mosig model.13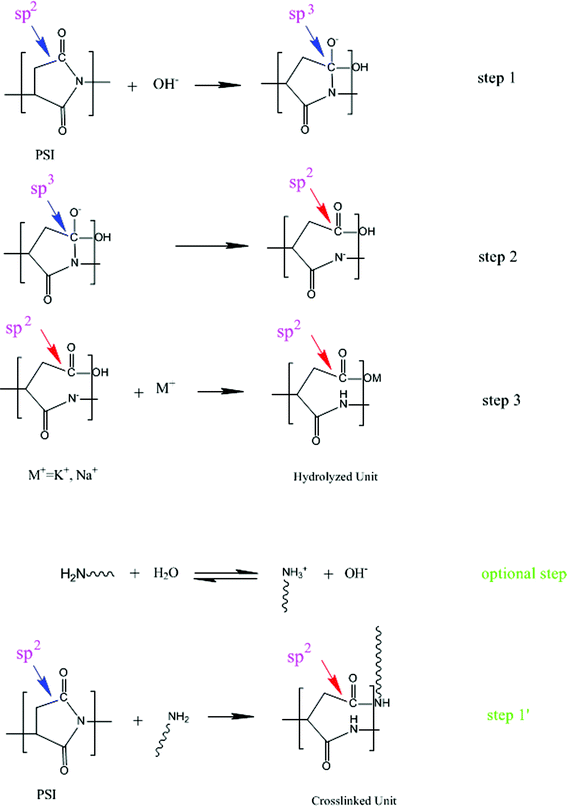 | ||
| Fig. 2 Hydrolyzing and crosslinking scheme. | ||
PSI is virtually insoluble in water or in a weak base until ring-opening occurs, which reacts in the heterogeneous environment. The PSI particles are non-porous, which is dissolved in DMF, not in water. In DMF, PSI particles are all dispersed then hydrolyzed and crosslinked, while in water, the exposed parts of PSI particles are hydrolyzed and crosslinked first, and then the ring-opening product, cross-linked PASP, swells in water and disperses throughout the whole reacting system. Then the inner parts are exposed and the ring-opening process continues. Moreover, during the ring-opening procedure, although the base and cross-linkers attack the PSI ring directly from the beginning, they have to permeate the cross-linked PASP membrane to form an interface solution in order to attack the PSI ring after the cross-linked PASP membrane has been formed.18–20
3.2 Effect of the method of the solution
The SEM results of Fig. 3 illustrate that PASP are formed into network structures both in DMF and in water, which are basically the same. For further applications, the PASP produced in water can be used for protein delivery after using ultrafiltration as purification agent21,22 or as swelling resin in sand fixing23,24 due to its hydrophilic, nontoxic and biodegradable character.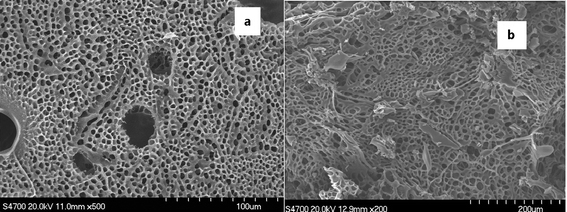 | ||
| Fig. 3 SEM images of crosslinked PASP (a) in DMF by 1,6-hexanediamine and (b) in aqueous solvent by hydrazine hydrate. | ||
The conventional method to synthesize cross-linked PASP from PSI involved two steps. PSI was cross-linked in DMF solution with 1,6-hexanediamine. PASP was thereafter prepared by hydrolysis of cross-linked PSI in base solution. Fig. 4 illustrates 1H NMR spectra of PSI (Fig. 4-a) and PASP (Fig. 4-b) prepared in water and cross-linked by hydrazine.25–27 As the ring of PSI has been attacked by base and crosslinker, the 5.2 ppm and 2.5–3.2 ppm proton shift numbers will decrease under the effect of the crosslinking structure. In the 1H NMR spectrum of PASP, the typical proton chemical shifts at 4.4–4.6 ppm and 2.4–2.8 ppm are indicated, although the 3.0–2.6 ppm shift has decreased since the cross-linker is free of –NH–NH– groups.
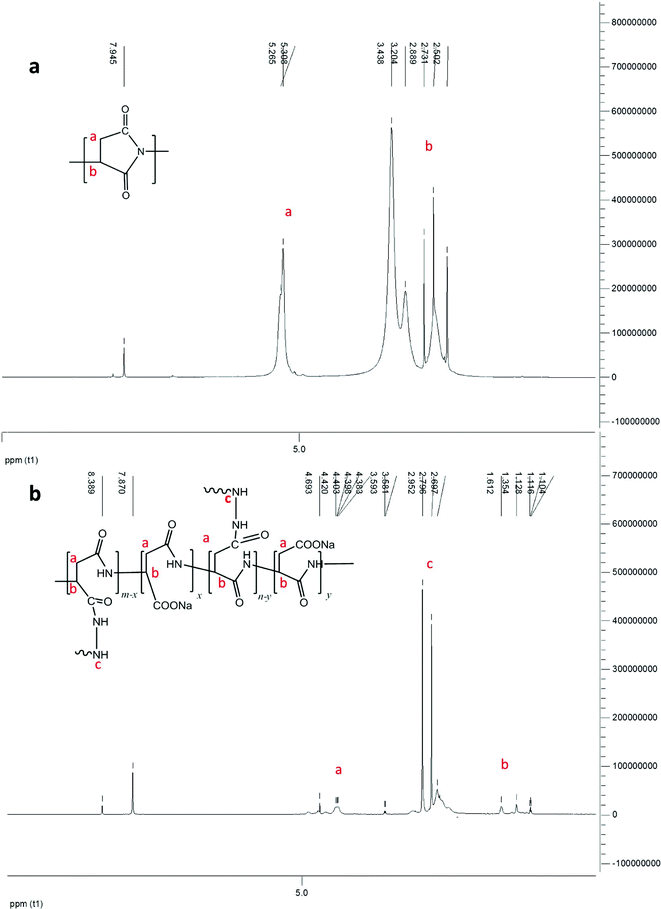 | ||
| Fig. 4 1H NMR spectrum of (a) PSI and (b) PASP, cross-linked by hydrazine in water. | ||
3.3 Effect of cross-linkers
As Fig. 4 revealed, the five membered ring was firstly activated by base to a sp2 structure during hydrolysis of PSI. The imide bond cleavage then generates α or β carboxyl groups and the N atom become part of the amine structure. The effect of the amount of crosslinkers is clearly shown in the DSC results of Fig. 5.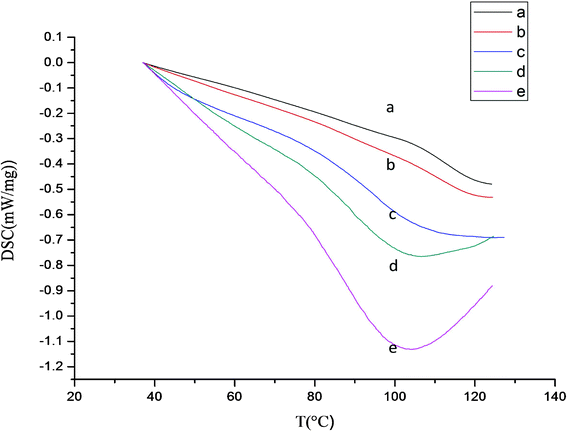 | ||
| Fig. 5 DSC result of different PASP hydrogel crosslinked by hydrazine at pH = 12.0 and 20 °C with molar ratio of crosslinker to PSI of (a) 10%, (b) 20%, (c) 30%, (d) 40% and (e) 50%. | ||
According to PSI hydrolysis characteristics, the Lewis base cross-linking agent (such as double amine crosslinkers hydrazine or 1,6-hexanediamine) would lead to a similar result. The difference is that the cross-linkers do not provide OH− to attack the PSI but attack the PSI directly themselves because of their nucleophilic character. Another difference between the hydrolysis and cross-linking reaction is the ionization of the cross-linkers. Compared with the limited ionization of the cross-linkers in DMF, the cross-linkers in water tend to ionize OH− and require more energy13 to attack the PSI ring as the optional step shown in Fig. 2. Therefore, the presence of the base suppressed the ionization of the cross-linker and helped the crosslinking reaction.
As the crosslinker amount increases, Fig. 4 illustrates that the glass transition temperatures, Tg, decrease from 113.0 to 86.9 °C at greater crosslinker amounts, i.e. by increasing the amount of crosslinker in the network. This trend can be ascribed to the presence of crosslinker bridges which reduce the interactions between free sites of the neighboring polymeric chains and cause a decrease of the glass transition temperature of the polymer.
The effect of the crosslinker type and amount is also clearly determined from measurements of the swelling ratio. When hydrazine is used as PASP crosslinker, a higher swelling ratio is obtained because 1,6-hexanediamine has hydrophobic methylene groups while hydrazine does not. The crosslinking density in the polymer network is related to the amount of crosslinkers: Torma28 illustrates that the network of PASP determines the swelling ratio. Fig. 6 illustrates that the water swelling ratio is proportional to the crosslinker amount: when the crosslinking amount is relatively low, the cross-linked PASP has a relatively low swelling ratio because the network is not completed. As the crosslinking amount increases, the PASP hydrogel absorbs more water due to the formation of the hydrophilic network. However, when PASP is overly cross-linked, a lower swelling ratio will be obtained due to the confinement of the PASP network.
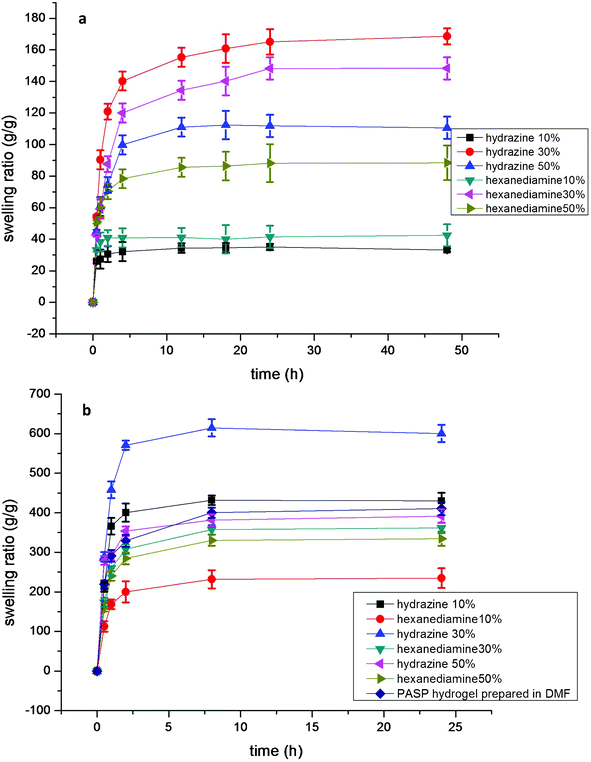 | ||
| Fig. 6 (a) Swelling ratio of PASP obtained at 0.0 °C, pH = 9.0, (b) Swelling ratio of PASP obtained at 0.0 °C, pH = 12.0, including the PASP prepared in DMF. Percentages are the molar ratio of crosslinker to PSI. | ||
3.4 Effect of pH
As Fig. 6 demonstrates, when the pH is relative low, the amount of base reactant in the reaction system is insufficient to limit the ionization, though the PSI ring can be opened. When the pH increases, the ionization of cross-linkers can be restrained and the hydrogel structure will be formed more completely, and the maximum swelling ratio will increase from 170 g g−1 to 600 g g−1 at pH = 12.0. However, when the pH reaches 14.0, the swelling ratio cannot be measured because the OH− groups have a significantly higher opportunity to attack the PSI rings and this procedure is more likely than the direct hydrolysis of PSI.29–32 According to the swelling ratio results of Fig. 6, the PASP network is more complete at pH = 12. Fig. 7 shows the FTIR spectrum of the hydrogel synthesized by hydrazine hydrate at different molar ratios. These experiments were carried out at the same pH. As the PSI five-member rings begin to break, the absorption peak at ∼1664 cm−1 from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in the carboxamide functional group and the peak at 1550 cm−1 from the N–C bond in the carboxamide functional group become more and more intense because of the formation of cross-linking points. Meanwhile, the peak at 1790 cm−1 of the imide disappears during the ring-opening procedure, which indicates that in the environment of pH = 12, PSI can be totally converted.
O stretching in the carboxamide functional group and the peak at 1550 cm−1 from the N–C bond in the carboxamide functional group become more and more intense because of the formation of cross-linking points. Meanwhile, the peak at 1790 cm−1 of the imide disappears during the ring-opening procedure, which indicates that in the environment of pH = 12, PSI can be totally converted.
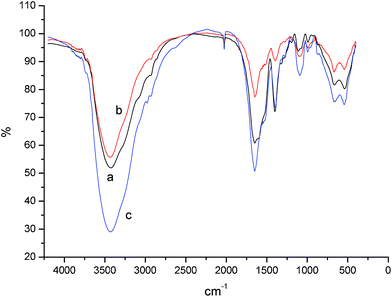 | ||
| Fig. 7 FTIR spectrum of PASP crosslinked by hydrazine hydrate in water: (a) hydrazine hydrate at 10% molar ratio of PSI, pH = 12.0, (b) hydrazine hydrate at 30% molar ratio of PSI, pH = 12.0, (c) hydrazine hydrate at 50% molar ratio of PSI, pH = 12.0. | ||
The cross-linked PASP has been washed several times with water and ethanol alternately. The cross-linkers with a single end group are not physically removable. The absorption peak at ∼1514 cm−1 confirms the –NH2 formation in the PASP hydrogel, which is the end group of the cross-linkers. This absorption peak increases as the cross-linking density increases. When the cross-linkers are abundant, i.e. 50% of the PSI ring unit quantitatively, the “half-crosslinking” reaction tends to occur because the cross-linkers themselves have to compete with each other to bind with the main chain of PSI.28–33
3.5 Effect of temperature
The reactions were also conducted by controlling the PSI ring-opening temperature at 0, 20, 40 and 60 °C. The swelling ratio results are illustrated in Fig. 8. When crosslinker and pH stay the same, the swelling ratio decreases significantly as the temperature increases and this regression is the result of both backbone hydrolysis, which occurs at the more extreme temperature, and cross-linkers not having enough opportunity to attack the PSI ring. For the commercial use of PASP hydrogel, it is suggested to apply milder, relatively low temperature to protect product quality.34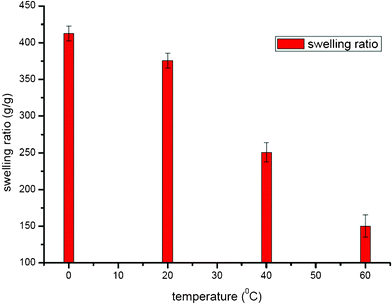 | ||
| Fig. 8 Swelling ratio of PASP obtained at different temperatures. For pH = 12.0, crosslinked by hydrazine hydrate (20% molar ratio of PSI). | ||
3.6 Effect of the grafted taurine-sulfo group on the swelling properties in a saline solution
As Fig. 9 reveals, the swelling ratio of taurine-modified PASP hydrogel in deionized water and a solution of 0.9 wt% NaCl both increase with an increasing taurine concentration, expressed as mol% of PSI. However, a maximum is achieved at 8 mol%, with all other conditions of crosslinker, pH and temperature remaining the same. The maximum swelling ratio obtained is 900 g g−1 in deionized water and 300 g g−1 in saline solution compared with 500 g g−1 in deionized water and only 80 g g−1 in saline solution for the non-grafted PASP hydrogel. This result indicates that taurine grafted into PASP hydrogel will improve the swelling ratio of the hydrogel when its content is moderate, since taurine has a sulfo-group which is strongly hydrophilic and enhances the hydrogel dissociation against the ions of the solution. However, when the content of taurine is high, the swelling ratio will decrease because taurine has the same reaction position as the cross-linking agent: too much taurine will block the cross-linking agent from opening the ring of PSI, which will limit the forming of cross-linked structures of PASP hydrogel.35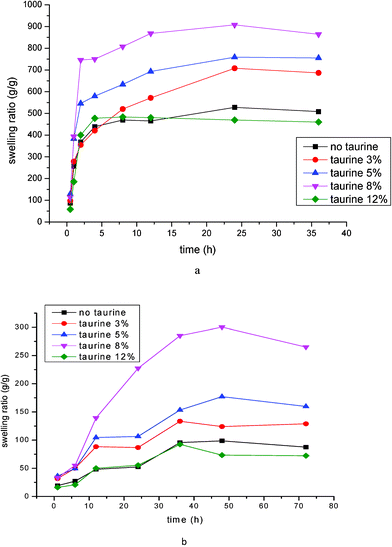 | ||
| Fig. 9 Swelling ratio of PASP grafted with different amounts of taurine: (a) in deionized water; (b) in a solution of 0.9 Wt% NaCl. | ||
According to the FTIR results of Fig. 10, the peak at 1050 cm−1 from the –SO3Na in sample b which was grafted with taurine does not show in sample a that was synthesized in the aqueous solution but without modification. The other peaks are almost identical. This result indicates that taurine has been grafted onto the chain of PASP without breaking the structure of PASP.
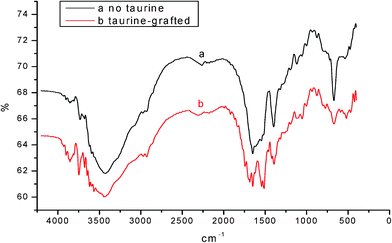 | ||
| Fig. 10 FTIR spectrum of modified PASP with the lateral sulfo-group crosslinked by hydrazine hydrate in water. | ||
4. Conclusions
The paper presents a novel solvent-free method of synthesizing PASP hydrogel, and its modification by taurine-grafting. Omitting solvents makes the production environmentally friendly, more economical, and with better properties in ionic solutions.The essence of cross-linking and hydrolysis is the ring-opening procedure and the transformation of the imide to amide bond. With the presence of aqueous soluble cross-linker and base, the five numbered ring of PSI will be activated and becomes unstable in the aqueous solution where no organic solvent was added. The pH, temperature and types of cross-linkers are critical factors for the swelling ability of PASP hydrogel, and operation at pH = 12 and moderate temperature is recommended: the crosslinking reaction is indeed exothermic and a lower temperature leads to a more completely reacted PSI albeit at a reduced reaction rate; a high temperature leads to a less complete reaction albeit at a higher reaction rate.
The cross-linking density of the PASP network is controlled by the amount of cross-linking agent: at weak cross-linking, the network is open and can entrap water molecules. When the added volume of cross-linking agent increases, the water absorption is initially increased until a compact network starts to limit the water absorption.
Taurine can be grafted onto PASP and the sulfo-group of taurine will be connected with the main chain of PASP to become a derivative, making the hydrogel more dissociative in ionic solution and improving the properties, although excessive amounts of taurine will restrict the crosslinking from happening, which will affect the formation of the hydrogel, since the reaction positions of grafting and crosslinking are the same. Taurine-grafted hydrogel has the highest swelling ratio in the solution of 0.9 wt% NaCl at a 8 mol% concentration of taurine, relative to PSI.
Acknowledgements
The authors express their thanks for the support from the Natural Science Foundation of China (50373003) (20876011) (21076017) (21106005), the National High Technology Research and Development of China (863 Program) (Grant No. 2009AA03Z232) and National Key Development Program for Basic Research (973 Program) (Grant No. 2009CB724703, 2011CB710800), Beijing Education Committee funding.References
- Y. Zhao, J. Kang and T. Tan, Polymer, 2006, 47, 7702–7710 CrossRef CAS.
- Y. Zhao, H. Su, L. Fang and T. Tan, Polymer, 2005, 46, 5368–5376 CrossRef CAS.
- M. J. Zohuriaan-Mehr, A. Pourjavadi, H. Salimi and M. Kurdtabar, Polym. Adv. Technol., 2009, 20, 655–671 CrossRef CAS.
- G. Pitarresi, F. S. Palumbo, G. Cavallaro, S. Faré and G. Giammona, J. Biomed. Mater. Res., Part A, 2008, 87A, 770–779 CrossRef CAS.
- S. Sun, H. Cao, H. Su and T. Tan, Polym. Bull., 2009, 62, 699–711 CrossRef CAS.
- H. Cao, X. Ma, S. Sun, H. Su and T. Tan, Polym. Bull., 2010, 64, 623–632 CrossRef CAS.
- H. Cheng, Y.-Y. Li, X. Zeng, Y.-X. Sun, X.-Z. Zhang and R.-X. Zhuo, Biomaterials, 2009, 30, 1246–1253 CrossRef CAS.
- Y. S. Jeon, J. Lei and J.-H. Kim, J. Ind. Eng. Chem., 2008, 14, 726–731 CrossRef CAS.
- L. Dong, A. K. Agarwal, D. J. Beebe and H. Jiang, Nature, 2006, 442, 551–554 CrossRef CAS.
- H. Ajiro, Y. Takemoto, T.-a. Asoh and M. Akashi, Polymer, 2009, 50, 3503–3507 CrossRef CAS.
- T. Gyenes, V. Torma, B. Gyarmati and M. Zrínyi, Acta Biomater., 2008, 4, 733–744 CrossRef CAS.
- T. Gyenes, V. Torma and M. Zrínyi, Colloids Surf., A, 2008, 319, 154–158 CrossRef CAS.
- J. Mosig, C. H. Gooding and A. P. Wheeler, Ind. Eng. Chem. Res., 1997, 36, 2163–2170 CrossRef CAS.
- G. Pitarresi, G. Cavallaro, B. Carlisi, G. Giammona, D. Bulone and P. L. San Biagio, Macromol. Chem. Phys., 2000, 201, 2542–2549 CrossRef CAS.
- J. Yang, L. Fang and T. Tan, J. Appl. Polym. Sci., 2006, 102, 550–557 CrossRef CAS.
- L. Zhang, C. Liao, Z. Yin and Y. Huang, Appled Chemical Industry, 2009, 38, 282–285 CAS.
- R. Zhang, Z. Huang, M. Xue, J. Yang and T. Tan, Carbohydr. Polym., 2011, 85, 717–725 CrossRef CAS.
- S. Ladet, L. David and A. Domard, Nature, 2008, 452, 76–79 CrossRef CAS.
- A. Montembault, C. Viton and A. Domard, Biomaterials, 2005, 26, 933–943 CrossRef CAS.
- N. Boucard, C. Viton and A. Domard, Biomacromolecules, 2005, 6, 3227–3237 CrossRef CAS.
- T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993–2007 CrossRef CAS.
- S. Bontha, A. V. Kabanov and T. K. Bronich, J. Controlled Release, 2006, 114, 163–174 CrossRef CAS.
- J. Yang, H. Cao, F. Wang and T. Tan, Environ. Pollut., 2007, 150, 381–384 CrossRef CAS.
- J. Yang, F. Wang, L. Fang and T. Tan, Environ. Pollut., 2007, 149, 125–130 CrossRef CAS.
- M. Nakanishi and K. Kataoka, Macromol. Symp., 2009, 279, 14–20 CrossRef CAS.
- C. Dispenza, G. Tripodo, C. LoPresti, G. Spadaro and G. Giammona, React. Funct. Polym., 2009, 69, 565–575 CrossRef CAS.
- J. Elisseeff, K. Anseth, R. Langer and J. S. Hrkach, Macromolecules, 1997, 30, 2182–2184 CrossRef CAS.
- V. Torma, T. Gyenes, Z. Szakács and M. Zrínyi, Acta Biomater., 2010, 6, 1186–1990 CrossRef CAS.
- Y. Wang, Y. Wang, G. Wu, Y. Fan and J. Ma, Colloids Surf., B, 2009, 68, 13–19 CrossRef CAS.
- K. Y. Lee and S. H. Yuk, Prog. Polym. Sci., 2007, 32, 669–697 CrossRef CAS.
- C. J. F. Rijcken, O. Soga, W. E. Hennink and C. F. V. Nostrum, J. Controlled Release, 2007, 120, 131–148 CrossRef CAS.
- M. Malmsten, H. Bysell and P. Hansson, Curr. Opin. Colloid Interface Sci., 2010, 15, 435–444 CrossRef CAS.
- F. Sanda, K. Terada and T. Masuda, Macromolecules, 2005, 38, 8149–8154 CrossRef CAS.
- F. Castelli, O. La Camera, G. Pitarresi and G. Giammona, Int. J. Pharm., 1998, 174, 81–90 CrossRef CAS.
- J. Guo, J. Zhu, Y. Ti and L. Li, Journal of Tianjin University, 2006, 39, 1145–1150 CAS.
| This journal is © The Royal Society of Chemistry 2012 |
