Coordination behaviour and biopotency of metal NN salen complexes†
Nadia E.A.
El-Gamel
*
Chemistry Department, Faculty of Science, Cairo University, 12613, Giza, Egypt. E-mail: nadinealy@hotmail.com; Fax: 0020235727556; Tel: 0020235676624
First published on 28th May 2012
Abstract
Coordination of Co(II) (1), Fe(III) (2) and UO2(IV) (3) with N,N′-bis(2-methoxybenzylidene)ethylenediamine Schiff base is structurally characterized using different spectroscopic and thermal studies. The reported compounds are examined as antimicrobial agents by screening their biological interactions; they showed enhanced antimicrobial activity compared with that of the free ligand. The cytotoxicity of the prepared compounds against L929 mouse fibroblasts and HEK 293 cells is determined by the MTT assay. The cytotoxic behavior is investigated for their possible use in tracking of cells and tissues. As observed in the in vitro assay, the complexes exhibited a low extent of cytotoxicity on the selected cells; they displayed remarkable potential viability compared with the same cells treated with the salen Schiff base. The cytotoxicity of the uranyl complex is lower than that of the other prepared complexes at a defined concentration, which indicates a synergistic effect upon coordination to the uranium ion. This study reveals that metal ions have quite an important role in the cytotoxicity. The results revealed that coordination may be considered as an interesting strategy to significantly reduce the cytotoxic dose, which may help to overcome the limitations of use of any metal and to understand their metabolism in living beings. In this work, the variation in chemical and coordination behaviour that has successfully enabled the integration of the target candidates into biological environments, which allows further in vivo developments in therapeutical and medical fields is discussed.
Introduction
With the continuous growth of industrial and biomedical applications around the world, Schiff bases have tremendous potential for a host of applications; their enormous effects on different models have raised serious possibilities recently for their use in technical, analytical and biological processes.1–6 For this reason many industries and manufacturers are now introducing Schiff base models in their mainstream products to exploit many capabilities emerging from their functionalities across a wide range of industrial and catalytic applications. Recently some of these issues have been addressed by employing Schiff bases in oxidation, asymmetric catalysis and epoxidation processes.7–9The interaction of metal ions with Schiff bases becomes a subject worthy of pursuit and has demonstrated great promise for their extensive applications in catalytic activity in chemical and enzymatic reactions;10–14 in addition, the use of the metal complexes as therapeutics has become one of the central issues over the last few years. This has given urgency to research to develop a variety of interesting compounds as drugs used in many fields, this is related to the presence of a variety of donor atoms in the system which can interact with metal ions in many different ways and generate novel compounds with a specific design; along these lines, the conjugation between metal ions and biologically active compounds can potentially enhance their biological and biomedical activities.15,16 The rapidly increasing functionality of biologically active complexes allows this class of compounds to be used more broadly in clinical and biomedical applications.
Schiff bases derived from salicylaldehyde and diamines are among the most relevant synthetic salen ligands with great potential applications in asymmetric catalysis. Unparalleled attention has been devoted to these materials due to their low cost, ease of fabrication and their stability. Bidentate salen ligands containing imine groups hold great promise as modulators in the construction of transition metal centers,17–20 therefore exploring the chemical and physical properties of salen metal complexes represents a significant scientific challenge worthy of pursuit.
Metal salen complexes are reported to be efficient homogeneous catalysts in asymmetric catalytic reactions including oxidations, epoxidation of olefins, epoxide ring opening, and Diels–Alder reactions;21–27 moreover, their framework has been specifically designed for applications as functional materials,28 therefore their potential catalytic activity has been reported and exploited in industrial fields. Herein, Co(II) and Fe(III) species have been chosen in this study due to their potential importance and useful applications in the biological field, whereas UO2(IV) ions were selected to compare between the obtained results in both cases and to evaluate the effect of different metal ions on biological processes and the biocomptability profile.
The present work reports the synthesis and characterization of N,N′-bis(2-methoxybenzylidene)ethylenediamine Schiff base (Fig. 1) and its metal complexes. The structural characterization of the complexes is discussed using different physico-chemical and spectroscopic tools. The antimicrobial activity of these complexes has been screened against two Gram-positive and two Gram-negative bacteria. Antifungal activity against two different fungi has been evaluated and compared with the reference salen Schiff base. In vitro cytotoxicity is examined using two cell lines, HEK 293 and L929 mouse fibroblasts. Herein in this study, the author has discussed and demonstrated the versatility of the compounds under study for further use in in vitro constructs that direct cell behaviour into therapeutic cell based complexes to stimulate biomedical applications in vivo, this perspective should be considered and drawn for further utilization in biomedical fields, which can be exploited in conjunction with the promising catalytic activity of the reported Schiff base and/or its complexes.
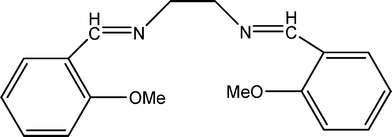 | ||
| Fig. 1 The structure of the salen Schiff base. | ||
Results and discussion
Structural analysis of the complexes
All the complexes are stable at room temperature and insoluble in water. The ratio of metal ions to salen Schiff base is found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Molar conductivity measurements in DMF were recorded as 13.40 and 13.15 Ω2 cm−1 mol−1 for (1) and (2) respectively, indicating the non-electrolytic nature of these compounds,29 while the uranyl complex is a 2
1. Molar conductivity measurements in DMF were recorded as 13.40 and 13.15 Ω2 cm−1 mol−1 for (1) and (2) respectively, indicating the non-electrolytic nature of these compounds,29 while the uranyl complex is a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolyte, with a conductivity of 160 Ω2 cm−1 mol−1 due to the presence of uncoordinated NO3− ions.29
1 electrolyte, with a conductivity of 160 Ω2 cm−1 mol−1 due to the presence of uncoordinated NO3− ions.29
In FT-IR spectra, the free Schiff base ligand showed a strong band at 1632 cm−1, which is characteristic of the azomethine (–HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) group.30 Coordination of the Schiff base to the metal through the nitrogen atom led to reduction of electron density in the azomethine link and lowered the νC
N) group.30 Coordination of the Schiff base to the metal through the nitrogen atom led to reduction of electron density in the azomethine link and lowered the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N absorption frequency. The band due to νC
N absorption frequency. The band due to νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N is shifted to lower frequencies and appears at 1600, 1598 and 1603 cm−1 for (1), (2) and (3), respectively, indicating coordination of the azomethine nitrogen to metal ions.31 Conclusive evidence of the azomethine nitrogen bonding is elucidated by the appearance of medium bands at 562, 569 and 452 cm−1 for (1), (2) and (3), respectively, assigned to νM–N. A broad band around 3400 cm−1 might be attributed to hydrated or coordinated water molecules. In the uranyl complex, a very strong band at 1376 cm−1 corresponds to free nitrates.32 Two intense sharp bands at 919, 757 cm−1 were attributed to asymmetric and symmetric stretching vibrations of O
N is shifted to lower frequencies and appears at 1600, 1598 and 1603 cm−1 for (1), (2) and (3), respectively, indicating coordination of the azomethine nitrogen to metal ions.31 Conclusive evidence of the azomethine nitrogen bonding is elucidated by the appearance of medium bands at 562, 569 and 452 cm−1 for (1), (2) and (3), respectively, assigned to νM–N. A broad band around 3400 cm−1 might be attributed to hydrated or coordinated water molecules. In the uranyl complex, a very strong band at 1376 cm−1 corresponds to free nitrates.32 Two intense sharp bands at 919, 757 cm−1 were attributed to asymmetric and symmetric stretching vibrations of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.33 In Co(II) and Fe(III) complexes, medium bands below 300 cm−1 are observed assigned to νM–Cl vibrations.34
O, respectively.33 In Co(II) and Fe(III) complexes, medium bands below 300 cm−1 are observed assigned to νM–Cl vibrations.34
The study of the electronic spectra in the ultraviolet and visible ranges for the salen Schiff base and its reported complexes were carried out in ethanol solution. Fig. 2 presents the absorption spectra of 2.5 × 10−4 M solutions of the compounds. The electronic spectrum of the free Schiff base exhibits three bands at 214, 252 and 321 nm, the two latter bands might be assigned to π → π* and n → π* transitions, respectively, of the azomethine group. Upon chelation through the azomethine nitrogen, these bands are slightly shifted to higher and/or to lower wavelength in the prepared complexes35 with a slight increase of intensity in case of (1) and decrease of intensity in case of (2) and (3).
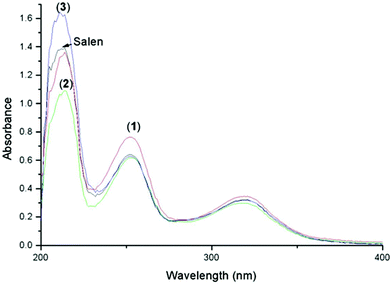 | ||
| Fig. 2 UV-Vis absorption spectra (2.5 × 10−4 M) of the salen Schiff base and its complexes. | ||
The effective magnetic moments, μeff, expressed in multiples of the Bohr Magneton μB, are calculated as 4.8 μB and 5.91 μB for (1) and (2), respectively, which indicate a high-spin octahedral configuration.36 The uranyl complex is diamagnetic.
In case of (1), three bands at 15.580–15.370, 17.610–18.050 and 21.980–22.110 cm−1 are displayed assigned to 4T1g(F) → 4T2g(F)(ν1), 4T1g(F) → 4A2g(F)(ν2) and 4T1g(F) → 4T2g(P)(ν3) transitions, respectively, confirming the octahedral geometry. Bands at 24.592–24.638 cm−1 correspond to charge transfer bands.36 In the case of (2), an absorption band is displayed in the range of 21.288–20.930 cm−1, assigned to the 6A1g → 6T2g (G) transition.36 The 6A1g → 6T1g transition is split into two bands in the ranges of 17.530–18.225 cm−1 and 14.980–12.121 cm−1. The reported bands are consistent with an octahedral geometry.36 The spectra showed bands in the range 30.658–31.673 cm−1 attributed to ligand to metal charge transfer.36
The ESR spectrum of the cobalt complex (3) was recorded in DMSO at room temperature, and exhibits a broad signal with an axial magnetic symmetry of the spin-Hamiltonian (g⊥ = 2.090; gII = 2.006), which indicates the cobalt ion is possesses a six coordinate structure.37 In case of iron complex (2), the Fe(III) environment is indicated by an ESR signal at g = 2.010, which is assigned to Fe(III) in a highly symmetric octahedral framework.38 The conclusion can be drawn that the cobalt and iron coordination compounds derived from the salen Schiff ligand are paramagnetic due to the central Fe(III) and Co(II) atoms which contain in the electronic superior layer five and seven odd electrons (3d5), (3d7), respectively.
The 1H NMR spectrum of the salen Schiff base displays the azomethine proton (H–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) at 8.11 ppm, a singlet due to the symmetrical imine protons. The aromatic protons appear as a multiplet and are observed at 7.14–7.54 ppm. The C–H protons present in the ethylenediamine-bridged ligand are observed at 3.95 ppm as a triplet. The signal due to the methyl protons is observed at 3.8 ppm. In the case of (3), due to the electron density shift from the salen Schiff base to the uranium metal, the signal of the azomethine proton is shifted downfield to 8.31 ppm, inferring coordination through the azomethine nitrogen atom of the ligand.39 The signal around 3.3 ppm may be due to the proton resonance signal of coordinated water, which could be involved in a hydrogen bond to the sulfoxide oxygen.
N) at 8.11 ppm, a singlet due to the symmetrical imine protons. The aromatic protons appear as a multiplet and are observed at 7.14–7.54 ppm. The C–H protons present in the ethylenediamine-bridged ligand are observed at 3.95 ppm as a triplet. The signal due to the methyl protons is observed at 3.8 ppm. In the case of (3), due to the electron density shift from the salen Schiff base to the uranium metal, the signal of the azomethine proton is shifted downfield to 8.31 ppm, inferring coordination through the azomethine nitrogen atom of the ligand.39 The signal around 3.3 ppm may be due to the proton resonance signal of coordinated water, which could be involved in a hydrogen bond to the sulfoxide oxygen.
The thermal decomposition and stability of the salen Schiff base and complexes (1), (2) and (3) were studied up to 800 °C by simultaneous thermogravimetric (TG) and differential thermal (DTA) analyses. All the decomposition steps of all the compounds under study are plotted and provided in the ESI.†
The thermal decomposition of the salen Schiff base occurs in one decomposition step within the range 134–553 °C, a complete decomposition is observed with an estimated mass loss of 99.8% (calcd. 100%). DTA displays three successive endothermic signals at 117, 273 and 385 °C and one exothermic signal at 509 °C due to the large loss of the ligand.
Thermal decomposition of (1) comprises four successive decomposition steps within the temperature range 40–660 °C, it starts with the elimination of the hydrated water molecules between 40–126 °C with an estimated mass loss of 5.57% (calcd. 7.17%). DTA presents a small exothermic peak at 58 °C. The second decomposition step at 127–275 °C corresponds to loss of coordinated water, liberation of Cl2 with a mass loss of 21.99% (calcd. 21.49%). This step is connected with two endothermic effects at 193 and 252 °C. The third and fourth mass loss steps occur in the range of 276–660 °C due to the decomposition of the rest of organic ligand as C18H20N2O (56.29%, calcd. 56.22%) and formation of CoO as a residue (14.55%, calcd. 15.07%). These steps are accompanied by two exothermic peaks at 375 and 425 °C.
According to the TG profile of (2), the decomposition occurs in three well defined decomposition steps within the range of 60–650 °C, liberation of HCl and hydrated water may occur in the first step connected with endothermic and exothermic process at 188 and 227 °C, respectively, within the temperature range 60–250 °C with a mass loss of 11.60% (calcd. 11.02%). The second step, within the range 251–432 °C, can be assigned to loss of coordinated water and evolution of chlorine gas with an estimated mass loss of 17.62% (calcd. 17.99%). DTA presents two exothermic signals at 274 and 297 °C. The last step could be attributed to the decomposition of the remaining ligand as C18H19N2O in the range of 432–650 °C with an estimated loss of 54.69% (calcd. 54.80%). This step is connected with two exothermic effects at 539 and 639 °C. Fe2O3 remains as a final residue with a mass loss of 16.09% (calcd. 16.19%).
Complex (3) is stable up to 114 °C, as a consequence the decomposition comprises three steps in the range of 114–650 °C. The first step, in the range 115–244 °C, can be assigned to liberation of coordinated water and loss of nitrate groups with an exothermic effect at 230 °C, the estimated mass loss is 19.52% (calcd. 19.08%). The second step, in the range 245–388 °C, may be due to loss of the remaining coordinated water molecules and loss of the ethylenediamine molecule accompanied with two exothermic effects at 311 and 350 °C with an estimated loss of 12.19% (calcd. 12.90%). The third decomposition step at 389–560 °C corresponds to loss of the remaining ligand as C16H12O2 with a loss of 31.48% (calcd. 31.72%) and formation of UO2 as a final residue 36.81% (calcd. 36.30%). DTA presented an exothermic signal at 463 °C.
On the basis of the used tools and techniques, the proposed structure of complexes (2) and (3) is six-coordinate with distorted octahedral geometry, whereas in case of (3), the uranium atom is at the centre of a pentagonal bipyramidal structure (Fig. 3).
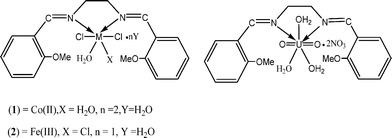 | ||
| Fig. 3 The proposed structures of the complexes. | ||
Antimicrobial studies
Herein, in this study, Staphylococcus aureus (Gram-positive) and Escherichia coli (Gram-negative) bacterial species and the fungi Aspergillus flavus and Candida albicans have been used as four representative models to examine the in vitro antimicrobial activity of the compounds under study, using the assay plates disc method on an appropriate nutrient medium. The results are displayed in Fig. 4. The inhibition zone diameters were measured for all the mentioned compounds. Standards (tetracycline, antibacterial and Amphotericin B, antifungal) were used to compare the biological activity of the salen Schiff base and its metal complexes, and upon examining the biological tests the following results are concluded.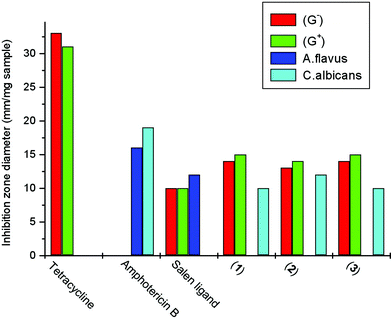 | ||
| Fig. 4 Biological activity of the tested samples against S. aureus (G+), E. coli (G−), A. flavus, C. albicans. | ||
The salen Schiff base exhibits antibacterial activity and some activity against Aspergillus flavus is displayed, the scanned antibacterial activity is lower than the standard tetracycline (Fig. 4). All the complexes exhibited higher antibacterial activity than the salen Schiff base but lower than the standard tetracycline. Higher antifungal activity of the complexes is recorded against Candida albicans; the salen Schiff base does not present any activity against this fungus, however it displayed remarkable activity against Aspergillus flavus. The uranyl complex (3) presents higher antimicrobial and antifungal activity than expected; the observed improvement in the in vitro biocompatibility profile of the uranyl complex is hypothesized to be an indication of not exuding any cytotoxic components at appreciable concentrations into the cellular space. This results led the author to perform cell viability tests to demonstrate how the chemical versatility can be used to influence biocompatibility profiles and cell–biomaterial interactions, which could be very useful and helpful for understanding their chemical metabolism in living beings, which gives substantial support for medical diagnosis to select the appropriate treatment to control poisoning by uranium or any similar metals .
In vitro cytotoxicity
It was of interest to investigate the cytotoxicity of the studied compounds. The utility of applying the salen Schiff base and its complexes for studying in vitro cell viability is based on their versatility in presenting low cytotoxic behaviour. Presenting low cytotoxicity is not directly advantageous, but may provide valuable insights into the cell culture translation system, which might be exploited into clinical and biomedical therapies.Fig. 5 presents the results of the in vitro MTT cytotoxicity assay showing the effect of varying concentrations of salen Schiff base and its reported complexes on growth inhibition of (a) L929 mouse fibroblasts and (b) HEK 293 cells derived from human embryonic kidney. The cytotoxicity was analyzed after two exposure time periods, 24 and 48 h. Error bars represent the standard deviation of the mean of five independent experimental values.
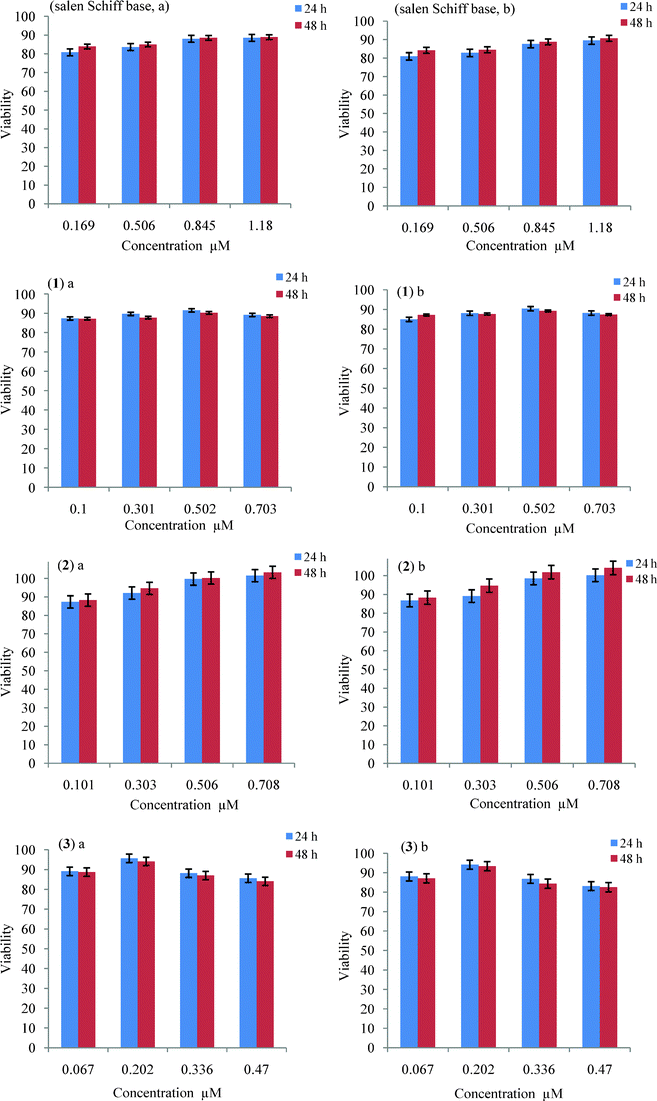 | ||
| Fig. 5 MTT cytotoxicity profiles for the salen Schiff base and its complexes on growth inhibition of (a) L929 mouse fibroblast and (b) HEK 293 cells, respectively after 24 and 48 h. | ||
Some significant decrease of the metabolic activity of L929 mouse fibroblasts and HEK 293 cells was observed at the tested salen Schiff base concentrations (0.169–1.18 μM) (Fig. 5, salen Schiff base a,b). With HEK 293, the cell proliferation rate was detected to be 90.75% with 1.18 μM of salen Schiff base at 48 h, and 89.54% at 24 h; whereas in the case of L929 mouse fibroblasts the cell proliferation rate was recorded to be 88.91% with 1.18 μM of salen Schiff base at 48 h.
In case of (1) at a concentration of 0.703 μM, the activity rate on both L929 mouse fibroblasts and HEK 293 cells is similar to what was observed in the case of the free ligand, whereas at a concentration of 0.502 μM, the maximum activity is displayed, about 91 and 90% on L929 mouse fibroblasts at 24 and 48 h, respectively. Similar behaviour was recorded in the case of HEK 293 cells, where the maximum activity was displayed at 0.502 μM of (1) (Fig. 5 (1) a,b).
It is noteworthy that the iron complex (2) displayed high activity, nearly as high as the cobalt complex at a concentration of 0.708 μM; it induced an increase of the metabolic activity to 103 and 104% after 48 h of incubation with L929 mouse fibroblasts and HEK 293 cells, respectively (Fig. 5 (2) a,b). This suggests that iron plays a key role in the entire process of viability.
The results for both L929 mouse fibroblasts and HEK 293 cells showed some dose-dependent cytotoxicity of the uranyl complex (3) (Fig. 5 (3) a,b). At the highest tested concentration (0.47 μM, 24 h, 48 h) the cell proliferation rates were found to be (85, 84%); (83, 82%) for L929 mouse fibroblasts and HEK 293 cells, respectively. The maximum proliferation was attained at 0.202 μM, the percentage of viable cells was in the range of 95 to 93% after incubation for 24 h and 48 h, respectively (Fig. 5 (3) a,b)). All the remaining complexes displayed higher cytotoxic behavior at the same concentration (0.202 μM).
The cytotoxicity of the complexes was found to be concentration-dependent, the cell viability of (3) decreased with increasing concentration, however by increasing the concentration the cytotoxicity of salen Schiff base, (1) and (2) decreased substantially.
Conclusion
The methodology used for the synthesis of these complexes is simple and efficient. The variation of metal ions seems to influence their biological activity towards different organisms. Both the salen Schiff base and its complexes represent promising candidates when set in the context of biocompounds for medical applications. The aim of this study was to assess the cytotoxicity of the salen Schiff base and some of its complexes. In this work, the interaction of the salen Schiff base containing the N,N chelating motif with Co(II), Fe(III) and UO2(IV) was characterized and analyzed. Their antimicrobial and biochemical effects on different organisms and cells were tested and evaluated. All the prepared complexes can be used as potent antimicrobial and antifungal agents; they displayed higher activity compared with the Schiff base. The cytotoxicity of these selected compounds was investigated on L929 mouse fibroblasts and HEK 293 cells using the MTT in vitro assay.Cytotoxic evaluation reveals these complexes to be potent and selective in vitro growth inhibitors of the tested cell lines. Uranyl complex (3) recorded higher antimicrobial and antifungal activity than expected; moreover the cell viability is higher than the remaining complexes at 0.202 μM. The enhanced activity of the metal complexes may be ascribed to the increased lipophilic nature of the complexes arising due to chelation. It is probably due to faster diffusion of the chelates through the cell membrane or due to the chelation effect. In conclusion, complexes with different metals and a similar ligand showed different trends of cytotoxicity which may imply that the metals have a major effect in the cytotoxic action of complexes. This perspective explores structure–function relationships, which may help to modify the complex structure in order to achieve more motivated compounds with higher potential use in biomedical applications. This study demonstrates that the replacement of the metal with different ones may give more promising results, which may help to assess human topical risk exposure to selected diseases. Such considerations have to be taken into account in the design and formulation of the complexes. The rapidly increasing functionality of salen Schiff base and its complexes allows this class of compounds to be used more broadly in clinical applications. This study may increase the utility of Schiff base complexes in order to solve critical issues in health to alleviate suffering and prolong life with a glance at future trends and challenges in biomedical fields. This perspective explores structure–property relationships associated with these compounds and their potential use in medical diagnostics.
Experimental
General procedures and materials
All chemicals used were of analytical reagent grade (AR) and of the highest purity available. They included 2-methoxybenzaldehyde, ethylenediamine, CoCl2·6H2O, UO2(NO3)·6H2O (Sigma), FeCl3·6H2O (BDH), absolute ethyl alcohol, diethyl ether (Acros), yeast extract and agar (Sigma), and MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (Carl Roth). De-ionized water collected from all glass equipments was usually used in all preparations.FTIR spectra were obtained from dispersions in KBr using a Perkin–Elmer FT-IR type 1650 spectrophotometer. The spectra were collected in the range of 200 to 4000 cm−1 with a resolution of 2 cm−1. UV-Vis spectrophotometric measurements were carried out using automated spectrophotometer UV-Vis Thermo Fisher Scientific Model Evolution 60 ranging from 200 to 900 nm. Molar conductivity was measured on a ELICO(CM82T) conductivity bridge. The molar magnetic susceptibility was measured on powdered samples using the Faraday method. The diamagnetic corrections were made by Pascal's constant and Hg[Co(SCN)4] was used as a calibrant. The solid reflectance spectra were performed on a Shimadzu 3101pc spectrophotometer. 1H-NMR spectra were recorded on a BRUKER DPX 400 instrument at room temperature (d6-DMSO solution with TMS as internal standard). The mass spectra were recorded by the EI technique at 70 eV using MS-5988 GS-MS Hewlett–Packard instrument. The ESR spectrum was taken on a Varian-E109 operating at the X-band frequency and at room temperature; tetracyanoquinodimethane was used as an external standard. Thermal analyses of the complexes were carried out using a Shimadzu TGA-50H and DTA-50H thermogravimetric analyzer in a dynamic nitrogen atmosphere (flow rate 20 ml min−1) with a heating rate of 10 °C min−1. The percentage weight loss was measured from ambient temperature to 1000 °C, highly sintered α-Al2O3 was used as reference.
Synthesis of complexes
The salen Schiff base was prepared by dissolving 2.0 mmol of 2-methoxybenzaldehyde in a minimum amount of ethyl alcohol at 60–70 °C, and adding to 1.0 mmol of ethylenediamine. The mixture was stirred at 50 °C for several hours. A white precipitate was obtained and was filtered, washed with ethanol and ether and dried over P4010 to obtain the Schiff base as a white powder. The reported complexes were prepared according to the following procedure by addition of a hot water-ethanolic solution (60 °C) of the metal salts for Co(II), Fe(III) and UO2(IV) (25 mL, 0.1 mmol) to a hot ethanolic solution of salen Schiff base (25 mL, 0.1 mmol). The resulting mixture was stirred under reflux for 2 h and left to cool, whereby the complexes precipitated as fine powders. The solid complexes were filtered, washed with ethanol, then with diethyl ether, and dried in a vacuum desiccator over P4010 and obtained as solid powders. All the compounds were obtained in yields greater than 92%. Mp. 117, 254, 188, 228 °C for Schiff base, (1), (2) and (3), respectively.The elemental analysis data with the proposed molecular structures are the following: Anal. Calcd for C18H20N2O2: (m/z = 296 (M+)); C, 72.95; H, 6.75; N, 9.45 . Found: C, 72.97; H, 6.76; N, 9.46%, (1) C18H28Cl2CoN2O6: (m/z = 499 (M+1)+); C, 43.37; H, 5.62; N, 5.62. Found: C, 43.31; H, 5.64; N, 5.61%, (2) C18H24Cl3FeN2O2: (m/z = 495.5 (M+1)+); C, 43.68; H, 4.85; N, 5.66. Found: C, 43.31; H, 4.87; N, 5.62%, (3) C18H26N4O13U: (m/z = 745 (M+1)+); C, 29.03; H, 3.49; N, 7.52. Found: C, 29.05; H, 3.46; N, 7.51%.
Antimicrobial activity
Antimicrobial activity of the tested samples was determined using a modified Kirby-Bauer disc diffusion method.40,41 100 μL of the test bacteria/fungi were grown in 10 mL of fresh media until they reached a count of approximately 108 cells mL−1 for bacteria or 105 cells mL−1 for fungi.42 100 μL of microbial suspension (20 mg mL−1) was spread onto agar plates. Plates were inoculated with filamentous fungi Aspergillus flavus, Candida albicans at 25 °C for 48 h; Gram-positive bacteria as Staphylococcus aureus and Gram-negative bacteria as Escherichia coli; were incubated at 35–37 °C for 24–48 h and yeast as Candida albicans incubated at 30 °C for 24–48 h and the diameters of the inhibition zones were measured in millimeters.43 Standard discs of Tetracycline (antibacterial agent) and Amphotericin B (antifungal agent) served as positive controls, while filter discs impregnated with 10 μL of solvent (distilled water, chloroform, DMSO) were used as a negative control.In vitro cytotoxicity tests
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) is absorbed by mitochondria, where it is transformed into formazan (purple crystals) by the enzyme succinic dehydrogenase. By assessing the activity of the mitochondrial dehydrogenases, the activity of viable cells in a cell population after treatment with the prepared complexes for different time periods is measured, after that the sensitivity of the cells to the concentrations and incubations period is determined. Briefly, growing HEK 293 and L929 cells are initially seeded at 150 μg mL−1 of cells mL−1 were treated with 1 mg ml−1 of salen Schiff base, (1), (2) and (3), respectively for 24 and 48 h at 37 °C, in an atmosphere supplemented with 5% CO2. MTT was added to the final concentration of 5 mg mL−1 and they were further incubated for 1 h at 37 °C, 5% CO2 in order for MTT (yellow) to be transferred into formazan crystals (purple) by the viable cells. The formazan crystals were solubilized upon addition of solution containing DMSO. The results from the MTT assay are expressed as the means of the absorptions at 630 nm, the measured absorbance is correlated with the number of viable cells. Cells incubated with media alone were employed as control and this was considered as 100% cell viability. The IC50 values are the concentrations at which the cell growth inhibition was 50% compared to untreated controls.
Acknowledgements
The author would like to thank Bianka Müller, University of Cologne, for her thorough technical assistance in performing the cytotoxicity tests.References
- S. J. Coles, M. B. Hursthouse, D. G. Kelly, A. J. Toner and N. M. Walker, J. Chem. Soc., Dalton Trans., 1998, 3489–3494 RSC.
- J. P. Costes, F. Dahan, B. Donnadieu, M. I. Fernandez-Garcia and M. J. Rodriguez-Douton, J. Chem. Soc., Dalton Trans., 2003, 19, 3776–3779 RSC.
- C. G. Zhang, D. Wu, C. X. Zhao, J. Sun and X. F. Kong, Transition Met. Chem., 1999, 24, 718–721 CrossRef CAS.
- M. Matsumoto and K. Kuroda, Tetrahedron Lett., 1981, 22, 4437–4440 CrossRef CAS.
- E. Tas, M. Aslanoglee, M. Ulusoy and M. Guler, Polish J. Chem., 2004, 78, 903–909 CAS.
- J. Estrela dos Santos, E. R. Dockal and É. T. G. Cavalheiro, J. Therm. Anal. Calorim., 2005, 79, 243–248 CrossRef CAS.
- Y.-W. Ren, H. Guo, C. Wang, J.-J. Liu and H. Jiao, Transition Met. Chem., 2006, 31, 611–615 CrossRef CAS.
- M. Masteri-Farahani, F. Farzaneh and M. Ghandi, J. Mol. Catal. A: Chem., 2006, 248, 53–60 CrossRef CAS.
- M. Salavati-Niasari and S. H. Banitaba, J. Mol. Catal. A: Chem., 2003, 201, 43–54 CrossRef CAS.
- J. Costamagna, J. Vargas, R. Latorre, A. Alvarado and G. Mena, Coord. Chem. Rev., 1992, 119, 67–88 CrossRef CAS.
- R. H. Grubbs, S. Chang, L. R. Jones and C. Wang, US Patent 5977393 (1999) Search PubMed.
- (a) B. D. Clercq and F. Verpoort, J. Organomet. Chem., 2003, 672, 11–16 CrossRef; (b) R. Drozdzak, B. Allaert, N. Ledoux, I. Dragutan, V. Dragutan and F. Verpoort, Coord. Chem. Rev., 2005, 249, 3055–3074 CrossRef CAS.
- R. Drozdzak, N. Ledoux, B. Allaert, I. Dragutan, V. Dragutan and F. Verpoort, Cent. Eur. J. Chem., 2005, 3, 404–416 CrossRef CAS.
- P. Viswanathamurthi, R. Karvembu, V. Tharaneeswaran and K. Natarajan, J. Chem. Sci., 2005, 117, 235–238 CrossRef CAS.
- L. Shi, W.-J. Mao, Y. Yang and H.-L. Zhu, J. Coord. Chem., 2009, 62, 3471–3477 CrossRef CAS.
- M. A. Phaniband and S. D. Dhumwad, Transition Met. Chem., 2007, 32, 1117–1125 CrossRef CAS.
- M. T. Miller, P. K. Gantzel and T. B. Karpishin, Inorg. Chem., 1999, 38, 3414–3422 CrossRef CAS.
- A. Puia and J.-P. Mahy, Polyhedron, 2007, 26, 3143–3152 CrossRef.
- A. Prakash, B. K. Singh, N. Bhojak and D. Adhikari, Spectrochim. Acta, Part A, 2010, 76, 356–362 CrossRef.
- M. Amirnasr, K. J. Schenk, A. Gorji and R. Vafazadeh, Polyhedron, 2001, 20, 695–702 CrossRef CAS.
- E. N. Jacobsen, M. L. Güler and W. Zhang, J. Am. Chem. Soc., 1991, 113, 6703–6704 CrossRef CAS.
- R. Irie, K. Noda, Y. Ito, N. Matsumoto and T. Katsuki, Tetrahedron Lett., 1990, 31, 7345–7348 CrossRef CAS.
- L. Canali and D. C. Sherrington, Chem. Soc. Rev., 1999, 28, 85–93 RSC.
- J. F. Larrow and E. N. Jacobsen, Topics Organomet. Chem., 2004, 6, 123–152 CAS.
- T. P. Yoon and E. N. Jacobsen, Science, 2003, 299, 1691–1693 CrossRef CAS.
- P. G. Cozzi, Chem. Soc. Rev., 2004, 33, 410–421 RSC.
- M. Alvaro, C. Baleizao, E. Carbonell, M. El Ghoul, H. García and B. Gigante, Tetrahedron, 2005, 61, 12131–12139 CrossRef CAS.
- S. J. Wezemberg and A. W. Kleij, Angew. Chem., Int. Ed., 2008, 47, 2354–2364 CrossRef.
- (a) J. A. Dean, Lange's Handbook of Chemistry, 14th Edn, Table 8.35, McGraw-Hill, New York, 1992 Search PubMed; (b) G. G. Mohamed and N. E. A. El-Gamel, J. Therm. Anal. Calorim., 2005, 81, 111–118 CrossRef.
- R. Ramesh and S. Maheshwaram, J. Inorg. Biochem., 2003, 96, 457–461 CrossRef CAS.
- S. A. Ali, A. A. Soliman, M. M. Aboaly and R. M. Ramadan, J. Coord. Chem., 2002, 55, 1161–1170 CrossRef CAS.
- (a) K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Third edition, Wiley, New York, 1978 Search PubMed; (b) M. Tiliakos, P. Cordopatis, A. Terzis, C. P. Raptopoulou, S. P. Perlepes and E. M. Zoupa, Polyhedron, 2001, 20, 2203–2214 CrossRef CAS.
- N. E. A. El-Gamel and D. Gerlach, J. Coord. Chem., 2008, 61, 2246–2265 CrossRef CAS.
- N. E. A. El-Gamel and M. A. Zayed, Spectrochim. Acta, Part A, 2011, 82, 414–423 CrossRef CAS.
- A. Garg and J. P. Tondon, Transition Met. Chem., 1987, 12, 212–214 CrossRef CAS.
- (a) F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, Sixth ed. Wiley, New York, 1999 Search PubMed; (b) G. G. Mohamed and N. E. A. El-Gamel, Spectrochim. Acta, Part A, 2005, 61, 1089–1096 CrossRef; (c) G. G. Mohamed and N. E. A. El-Gamel, Vib. Spectrosc., 2004, 36, 97–104 CrossRef; (d) N. E. A. El-Gamel, R. R. Mohamed and M. A. Zayed, Dalton Trans., 2012, 41, 1824–1831 RSC.
- S. Bandyopadhyaya, G. N. Mukherjeed and M. G. B. Drewb, Inorg. Chim. Acta, 2006, 359, 3243–3251 CrossRef.
- (a) R. Szostak, V. Nair and T. L. Thomas, J. Chem. Soc., Faraday Trans., 1987, 1, 487–494 Search PubMed; (b) D. Goldfarb, M. Bernardo, K. G. Strohmaier, D. E. W. Vaughan and H. Thomann, J. Am. Chem. Soc., 1994, 116, 6344–6353 CrossRef CAS.
- (a) K. Singh, M. S. Barwa and P. Tyagi, Eur. J. Med. Chem., 2006, 41, 147–153 CrossRef CAS; (b) A. Majumder, G. M. Rosair, A. Mallick, N. Chattopadhyay and S. Mitra, Polyhedron, 2006, 25, 1753–1762 CrossRef CAS.
- A. W. Bauer, W. M. Kirby, C. Sherris and M. Turck, Am. J. Clin. Pathol., 1966, 45, 493–496 CAS.
- National Committee for Clinical Laboratory Standards. ( 2002). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Conidium-Forming Filamentous Fungi: Proposed Standard M38-A. NCCLS, Wayne, PA, USA Search PubMed.
- M. V. N. de Souza, Mini-Rev. Med. Chem., 2005, 5, 1009–1017 CrossRef CAS.
- M. A. Pfaller, L. Burmeister, M. A. Bartlett and M. G. Rinaldi, J. Clin. Microbiol., 1988, 26, 1437–1441 CAS.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20733h/ |
| This journal is © The Royal Society of Chemistry 2012 |
