Influence of the annealing temperatures on the photoluminescence of KCaBO3:Eu3+ phosphor†
A. Amarnath
Reddy
ab,
Subrata
Das
*ac,
Shahab
Ahmad
a,
S. Surendra
Babu
d,
José M. F.
Ferreira
b and
G. Vijaya
Prakash
a
aNanophotonics Laboratory, Department of Physics, Indian Institute of Technology Delhi, New Delhi, 110016, India. E-mail: prakash@physics.iitd.ac.in
bDepartment of Materials and Ceramic Engineering, CICECO, University of Aveiro, Aveiro 3810-193, Portugal
cDepartment of Chemical Engineering, National Taiwan University, Taipei, Taiwan, R.O.C.. E-mail: phy_subrata@yahoo.co.in; Fax: +91(11) 2658 1114; Tel: +91(11) 2659 1326
dDirectorate of Laser Systems, Research Centre Imarat, Hyderabad-500069, India
First published on 17th August 2012
Abstract
Novel red emitting KCaBO3:Eu phosphors have been synthesized by solid-state reaction at various temperatures. Systematic studies on annealing effects and consequent structural evolution and optical properties were investigated by various structural and photoluminescence studies. With an increase in annealing temperature (from 700 °C to 950 °C), these phosphors show a gradual change from a mixed low crystalline phase to a highly crystalline single phase, with minimized volatile impurities. Photoluminescence studies revealed that the low-temperature annealed phosphors showed distinct mixed emission composed of blue and red emissions upon UV excitation. Such dual emission is due to the coexistence of Eu3+ and Eu2+ ions, wherein the reduction of Eu3+ into Eu2+ was attributed to the presence of volatile impurities. Relatively high-temperature annealed phosphors exhibit strong red color photoluminescence due to homogeneously occupied Eu3+ ions in the host crystal charge-compensated (with K+ ions) sites of Ca2+ ions. The dominant red-to-orange emission intensity ratios and Judd–Ofelt parameters of Eu3+ ions support the strong covalent nature and site-occupation of higher asymmetry sites of K+ and Ca2+ ions. High emission life times and efficient and stable photoluminescence at different excitation wavelengths make these phosphors suitable for white LEDs and other display applications.
1. Introduction
White-light emitting diodes (W-LED) have been commendably obtained from two or three color phosphors coated onto blue/UV LEDs.1–4 In general, high color rendering index (CRI), long life time, high luminescence, high wall-plug efficiency, low power consumption and environmentally friendly behavior are among the key factors for LED technology development. However, such combinations of phosphors, without red components, exhibit a poor color rendering index (<80). While, the chemically durable red-emitting phosphors that have wide range transmission and excitation wavelengths matching those of available LED excitation are in current demand.1–4 Although Eu3+-doped sulfide phosphors (such as (Ca, Sr)S and Y2O2S) are commercially used,5,6 they are chemically unstable and the fabrication processing is environmentally unfriendly. During the quest for a stable alternative, single phase borates emerged as efficient hosts with advantages such as wide-range transparency, low-temperature processing and high chemical and physical durability.7–9 Borate phosphors are found to have potential applications in flat panel display devices, optical data storage, lasers and nonlinear optics.3–11 Rare-earth-doped alkali and alkaline earth mixed orthoborates are of special interest as phosphors10,12–14 for display devices and lamps.10,14The trivalent europium (Eu3+) ion is well-known for strong red and orange photoluminescence of f–f transitions (5D0 → 7F2 and 7F1) and is widely used in lighting and display fields. On the other hand, divalent europium (Eu2+) has also been used as an activator for purple color phosphors for many commercial applications. Generally, the Eu2+ doping is achieved from the reduction of Eu3+ to Eu2+ and such a valance change has been widely investigated, both in crystalline and glassy materials.15,16 The luminescent properties of Eu3+ (or Eu2+), hence, are strongly dependent on the host crystal structure and local environment,17 which can be conveniently controlled by crystal engineering and process conditions. Therefore, a deeper understanding of the affects of the local environment and process conditions on the luminescent properties of rare-earth ions in complex hosts, such as the orthoborate, is highly essential.
In this paper, we report the structural and photoluminescence properties of Eu3+-doped potassium calcium borate (KCaBO3) prepared by the solid state reaction method at different annealing temperatures. The aim is to assemble data to support the selection of the most favorable processing conditions for commercial applications. In this interesting composition, the PL arising from Eu2+ (UV–blue) and Eu3+ (orange–red) emission locations have been systematically monitored at various excitation energies and their intensity distribution dependence on the annealing temperature has been studied in detail. The potential application of Eu3+-doped KCaBO3 material as efficient red phosphor have been contemplated from a systematic study of their photoluminescence properties and their strong dependence on processing temperature.
2. Experimental details
The synthesis of Eu3+-doped KCaBO3 phosphors was carried out by solid state reaction followed by different annealing conditions. Stoichiometric amounts of analytical grade KCl, CaCl2, H3BO3 and Eu2O3 (2.0 wt%) were mixed and well ground in an agate mortar to obtain a fine and homogeneous powder. The mixture was then annealed in air atmosphere at 500, 700, 900, 950 °C and 1000 °C for 20 h in a silica crucible. Finally, the mixture was cooled to room temperature by natural cooling. Annealing above ∼1100 °C causes the mixture to melt and transforms into the glassy form. The samples annealed at 500 °C shows the highly moisture sensitive nature and the solid-state reaction was incomplete. The structural and spectral data is separately presented as supplementary data†.X-ray diffraction (XRD) data for these phosphor powders were collected using Cu-Kα radiation (λ = 1.54 Å) in the 2θ angle range 20°–80° with step size 0.02° s−1. The FTIR studies were carried out using the KBr pellet method. 11B MAS-NMR spectra were recorded at 128.36 MHz (9.4 T) using a 4 mm probe at a spinning rate of 12 kHz. A pulse length of 3.6 μs and delay time of 2 s time was used. For chemical shifts, standard H3BO3 is used as the reference. The compositional analysis was performed by an Energy Dispersive X-ray analysis (EDX) Analyzer operated at 30 keV.
The steady-state and time-resolved photoluminescence (PL) measurements were carried out using home-built setups with 337 nm (N2 gas laser), 404 nm (diode laser) and 532 nm (DPSS laser) lasers as the excitation sources. The emission was dispersed into a monochromator coupled to a photo multiplier tube (PMT) through the appropriate lenses and filters. For time-resolved photoluminescence, a mechanical chopper (12 Hz), lock-in amplifier and digital storage oscilloscope were employed. Photoluminescence and excitation spectra were also measured using a commercial fluorimeter. The PL spectral scanning over a wide area was performed on a modified laser scanning confocal microscope equipped with XY-piezo stage coupled with the spectrometer using a 404 nm diode laser as the excitation source. The scan range is over an area of 200 μm × 200 μm with a step size of 4 μm. Both photoluminescence mapping and conventional confocal bright and dark field images and PL images were recorded using an ALP >410 nm filter.
3. Results and discussions
3.1 Powder X-ray diffraction analysis
The room temperature powder X-ray diffraction (PXRD) patterns of Eu3+-doped KCaBO3 powders annealed at different temperatures are shown in Fig. 1(a). The diffraction pattern of phosphor prepared at 700 °C shows mixed phase crystallinity with a prominent phase of monoclinic KCaBO3 (analogous to LiBaBO3; JCPDS card no. 81-1808).18 However, at such a temperature an incomplete reaction is possible and other diffraction peaks related to triclinic H3BO3 (JCPDS card no. 73-2158) and monoclinic Eu2O3 (JCPDS card no. 34-0072) are also visible. For annealing temperatures between 900–950 °C, the monoclinic KCaBO3 phosphor appears as the dominant phase and beyond 950 °C the sample shows a vitrified nature. As an example, the X-ray diffraction patterns in one of the samples was used to retrieve crystal structure information using the Le Bail19 fitting method from crystal structure refinement program ‘FULLPROF’.20Fig. 1(b) shows the corresponding XRD fitting of the Eu3+-doped KCaBO3 phosphors resulting in the P21/c space group of the monoclinic cell with Goodness of Fitting (GOF) equal to 2.26. The corresponding crystallographic information (listed in Table 1) confirms the monoclinic structure of the KCaBO3:Eu3+. The average crystallite sizes were estimated from Scherrer analysis for the samples synthesized at 700, 900 and 950 °C and found to be 75, 94 and 131 nm, respectively. Furthermore, the XRD results reveal that the host phosphor crystal structure is not changed with the Eu3+ doping. During the doping, the Eu3+ ions might have conveniently occupied the Ca2+ sites due to the similar ionic radius (Eu3+; 109 pm, Ca2+; 114 pm) by compensating the charge either as 2Ca2+ = Eu3+ + K+ or 3Ca2+ = 2Eu3+ + interstitial vacancies.21 This discussion will be further extended in the following sections.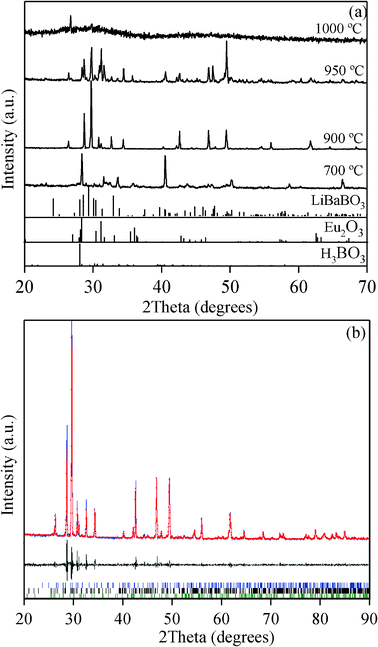 | ||
| Fig. 1 (a) Powder X-ray diffraction patterns of KCaBO3:Eu phosphors annealed at various temperatures. Standard JCPDS data for LiBaBO3, Eu2O3 and H3BO3 are also provided for comparison. (b) The powder X-ray diffraction pattern, along with the fitting, followed by Le Bail fitting procedure for the phosphor annealed at 900 °C (see text). | ||
| Chemical formula | KCaBO3 |
| Crystal system | monoclinic |
| a (Å) | 6.587 |
| b (Å) | 7.119 |
| c (Å) | 7.779 |
| Beta (°) | 118.41 |
| Cell volume (Å3) | 364.779 |
| Space group | P21/c |
| Crystallite size (nm) | 112.2 |
| Radiation type wavelength (Å) | Cu-Kα 1.5418 |
| Profile range (°2θ) | 20–80 |
| R-Bragg (°) | 2.208 |
| R exp | 6.68 |
| R wp | 15.51 |
| R p | 11.05 |
| GOF | 2.26 |
3.2 FTIR, NMR and EDX studies
FTIR, NMR and EDX spectral details were carefully studied to investigate the structural aspects of Eu3+-doped KCaBO3 phosphors obtained at various annealing temperatures. The FTIR spectra (Fig. 2(a)) show different vibrational energies with strong annealing temperature dependence. The broad transmission minima at 3430 cm−1 and 1630 cm−1 correspond to the O–H stretching vibrations, while the band at ∼2100 cm−1, appearing only at temperatures lower than 700 °C, could be attributed to symmetric hydrogen bonds.22–24 These broad bands show a sharp decrease in their intensities with the increase of the annealing temperature, due to a decrease in OH content, as well as a decrease in the residual KCl and CaCl2 contents.25 The important structural entities related to symmetric and asymmetric stretching vibrations of (BO3)−3 units appear in the region 1200–1613 cm−1 and at about 1149 cm−1, respectively. The sharp IR band at 707 cm−1 could be attributed to the bending vibration of triangular BO3 units and the band around 480 cm−1 is of the diborate group.22,23 Most importantly, with the increase of annealing temperature from 700 °C to 900 °C, the sharpness of BO3 stretching/bending vibrations increases with respect to tetrahedral BO4 units, indicating the formation of single phase KCaBO3. The vitreous state of borate always shows superimposed IR absorption peaks of BO3 and BO4 units (∼1000 cm−1) and, in the present case, the samples annealed at 1000 °C also show similar features.26 Overall, the FTIR results clearly indicate that these phosphors processed at various temperatures underwent a systematic transformation from a multi-phase to monophasic nature with the increase of annealing temperature, as confirmed by the X-ray diffraction analysis.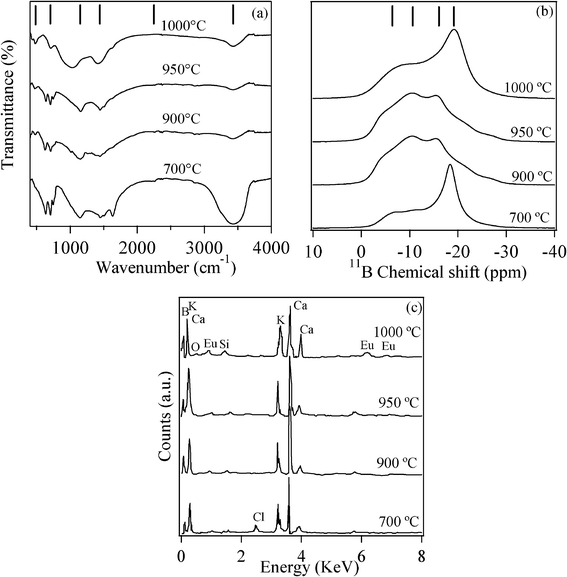 | ||
| Fig. 2 Room-temperature (a) FTIR (b) 11B-MAS NMR chemical shift and (c) EDX spectra of KCaBO3:Eu phosphors annealed at different temperatures. | ||
In order to monitor the local order around Boron atoms, high resolution 11B MAS-NMR spectra were also recorded for KCaBO3 phosphors annealed at various temperatures and are shown in Fig. 2(b). The basic pattern for all of the samples consists of a sharp peak (−18 ppm) superimposed over a broad peak (−6 ppm), which appear as shoulders symmetrically placed on either side of the sharp peak. The broad peak can be attributed to the trigonally coordinated BO3 structural units and the sharp peak to the tetrahedral coordinated BO4 structural units.27 Since 11B is a quadrupolar nuclei (I = 3/2), due to the lack of cubic symmetry, the boron nuclei in BO3 structural units are influenced by second order quadrupolar interactions, which results in NMR as a two-peaked broad resonance curve. On the other hand, boron in BO4 structural units has a highly symmetric cubic environment with negligible quadrupolar interaction and therefore gives rise to a sharp peak with an approximately Gaussian line shape. However, a distinct difference in peak position and shape can be seen in the spectra of phosphors annealed at a relatively high temperature (>900 °C). The relative integrated area of the NMR peak corresponding to BO3 (at ∼18 ppm) is increased with the increase of annealing temperature. These changes clearly indicate the transformation from the multi-phase to single phase orthoborate nature of the samples, as confirmed from the FTIR and XRD studies. The qualitative EDX microanalysis (Fig. 2(c)) is another indicator of the chemical composition of KCaBO3:Eu3+. As can be seen, the EDX spectra show all of the major components viz., K, Ca, B, O and Eu elements. The presence of trace element, Cl at 700 °C annealed phosphor indicate the existence of unreacted species, which was also evidenced from FTIR results.
3.3 Photoluminescence studies
Photoluminescence (PL) spectra of KCaBO3:Eu3+ phosphors processed at various temperatures were recorded using 532 nm laser excitation (Fig. 3(a)). The prominent photoluminescence lines are at 578, 588, 612, 650 and 704 nm corresponding to 5D0 → 7FJ (J = 0, 1, 2, 3, 4) transitions of Eu3+ ions. The photoluminescence intensities of different 5D0 → 7FJ transitions and the splitting is strongly dependant on the local symmetry of the crystal field of the Eu3+ ion. As can be seen, the red color hypersensitive electric dipole transition observed at 612 nm (5D0 → 7F2) is found to be the strongest among all other emission lines. The second strongest is the orange emission line at 588 nm, corresponding to the magnetic dipole transition 5D0 → 7F1 and the less intense photoluminescence peak at 578 nm originates from the forbidden 5D0 → 7F0 transition. The 5D0 → 7F0 transition has considerable intensity in all KCaBO3 host matrices, suggesting the low point symmetry of the Eu3+ sites.28 All of the 5D0 → 7FJ transitions are well-resolved into a number of lines due to the crystal field effects, however, the phosphor annealed above 1000 °C shows featureless photoluminescence peaks for individual transitions. These spectra are similar to those of other Eu3+-doped borate and oxide vitreous systems,29–31 indicating the glass nature, which was also evidenced by XRD and FTIR results. In general, the transition probability of the magnetic-dipole transition 5D0 → 7F1 is nearly independent of the host matrix and other electric-dipoles allowed for 5D0 → 7FJ (J = 2, 4 and 6) transitions are strongly influenced by the local structure and site asymmetry around the Eu3+ ion.28 Therefore, the photoluminescence intensity ratio of 5D0 → 7F2 to 5D0 → 7F1 (Red to Orange, R/O), widely known as the asymmetric ratio, provides valuable information about the symmetry at the site occupied by Eu3+ ions and covalent nature of the host matrix.31,32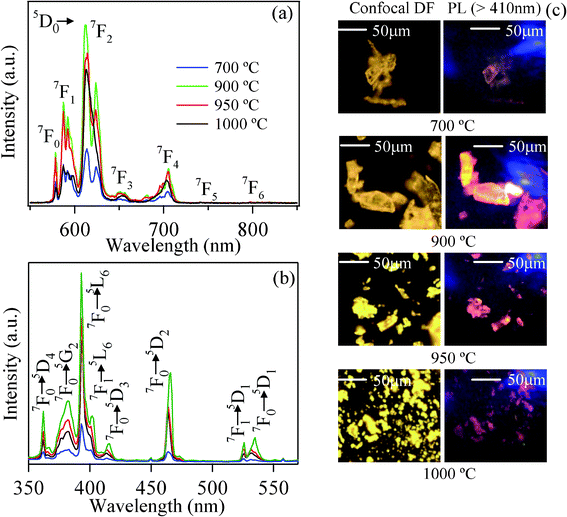 | ||
| Fig. 3 Room-temperature (a) photoluminescence spectra (λex = 532 nm) and (b) excitation (λem = 616 nm) spectra, and (c) confocal white light (dark field) and corresponding PL microscopic images (λex = 404 nm) of KCaBO3:Eu phosphors annealed at different temperatures | ||
The photoluminescence transition probabilities of electric-dipole and magnetic-dipole transitions can be expressed in terms of intensity parameters, famously known as Judd–Ofelt parameters Ωλ (λ = 2,4,6)33,34 using the following equation
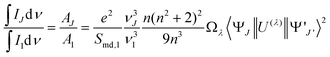 | (1) |
| T/°C | CIE coordinates | R/O ratio | Ω 2 | Ω 4 | Ω 6 | τ f (in ms) | τ rad (in ms) | η (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 404 nm | 532 nm | ||||||||||
| X | Y | X | Y | ||||||||
| 700 | 0.31 | 0.15 | 0.62 | 0.38 | 1.84 | 2.43 | 1.52 | 2.16 | 2.26 | 5.55 | 41 |
| 900 | 0.59 | 0.31 | 0.63 | 0.37 | 2.17 | 3.17 | 1.22 | 1.52 | 2.93 | 4.96 | 59 |
| 950 | 0.59 | 0.30 | 0.62 | 0.38 | 2.50 | 3.12 | 1.37 | 1.42 | 2.50 | 4.93 | 51 |
| 1000 | 0.57 | 0.30 | 0.61 | 0.38 | 4.65 | 4.62 | 2.08 | 1.77 | 2.46 | 3.66 | 67 |
Fig. 3(b) illustrates the excitation spectrum of KCaBO3:Eu3+ samples monitored at 612 nm emissions of Eu3+ ion. The excitation spectra show rich spectral lines spanning 350–600 nm, covering all commercially available UV/blue/violet LEDs to improve the color rendering property for W-LEDs.40 These characteristic transitions are from the ground 7F0 and 7F1 (thermally populated) states to various excited states of Eu3+, as indicated in Fig. 3(b). Both the photoluminescence and excitation spectra show strong temperature dependence, further confirming that the photoluminescence centers of Eu3+ ions increase greatly with the annealing temperature reaching a maximum at 900 °C. To demonstrate the photoluminescence characteristics of these microcrystalline phosphors, we have also recorded the high-resolution conventional confocal microscope images (white light illumination, dark field mode), as well as the PL (excited with 404 nm low-power laser) images. The PL images (Fig. 3(c)), as visible to the naked eye, show dominant red color photoluminescence. The critical analysis and PL spectral information from the macroscopic areas of the phosphors will be presented in the following sections. To further illustrate the excitation wavelength dependence of emission characteristics, several commercially available excitations (337, 393, 404 and 532 nm) were used to record PL spectra for both 700 and 900 °C annealed phosphors (Fig. 4(a) and (b)). The spectra show the characteristic features of Eu3+ emission without any spectral shifts under different excitations. The photoluminescence spectra of 900 °C annealed phosphors, excited with 393 nm, show relatively low intense higher order emission peaks at 430, 451, 469, 475, 492 and 536 nm, corresponding to 5D3 → 7F2, 5D3 → 7F3, 5D2 → 7F0, 5D2 → 7F1, 5D2 → 7F2 and 5D1 → 7F1 transitions of Eu3+ ion, respectively (Fig. 4(b)). Generally, multi-phonon relaxation, concentration quenching and the cross-relaxation between neighboring Eu3+ ions, substantially quench the higher-order photoluminescence. Therefore, the presence of such higher level emission may be an indication for minimum concentration quenching in the present phosphor system.41
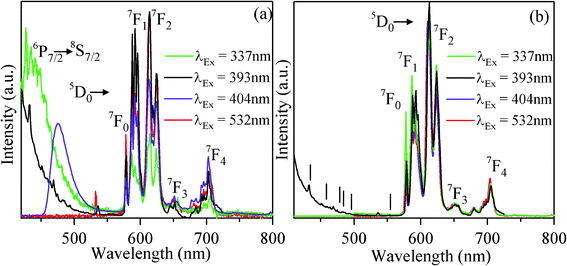 | ||
| Fig. 4 Room-temperature photoluminescence spectra excited by various excitation wavelengths (dashed lines indicate higher order photoluminescence transitions) of KCaBO3:Eu phosphors annealed at (a) 700 °C and (b) 900 °C temperatures. | ||
It is interesting to note that, besides the characteristic Eu3+ emission lines (spectral region 550–800 nm), the PL spectra of the KCaBO3:Eu3+ phosphor annealed at 700 °C shows additional broad emission (below 550 nm spectral region) for the excitation wavelengths 337 and 404 nm (Fig. 4(a)). Whereas, for the phosphor annealed at 900 °C, the spectra contain only Eu3+ signature emission lines in the desired spectral region, irrespective of the excitation wavelength (Fig. 4(b)).
To signify the color purity, the PL spectra were converted to the Commission International de I'Eclairage (CIE) 1931 chromaticity values (Fig. 5(a)). These CIE coordinate values of the phosphors annealed above 900 °C are close to that of the standard phosphors YAG:Eu and Y2O3:Eu,42,43 indicating the reddish orange color of KCaBO3:Eu3+ phosphor upon green excitation. Whereas for the phosphors annealed at relatively lower temperatures (<700 °C), the emission color is significantly away from the red and close to the blue region.
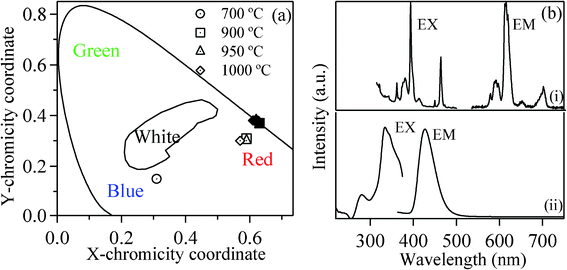 | ||
| Fig. 5 (a) The emission CIE coordinate diagram of KCaBO3:Eu phosphors annealed at different temperatures. The open symbols correspond to 404 nm excitation and filled symbols are for 532 nm excitation. (b) Room-temperature excitation (EX) and photoluminescence (EM) spectra of (i) Eu3+ (λem=612 nm and λex=532 nm) and (ii) Eu2+ ion (λem=429 nm and λex=334 nm) of KCaBO3:Eu phosphor annealed at 700 °C temperature. | ||
In order to confirm the origin of this special blue-end photoluminescence, the excitation spectra for the emission line of Eu3+ (at 612 nm) and the broad blue-end emission were separately monitored (Fig. 5(b)). While the characteristic red (612 nm) emission originates from the 7FJ → 5L6 (J = 1, 2) excitation transitions of Eu3+ ions (Fig. 3(b)), the intense broad emission, centered at 429 nm, resulted from strong and broad excitation lines below 350 nm. However, upon 404 nm, the broad emission shifted to 476 nm (Fig. 4(a)). Recently, Wang et al.45 reported emission at 475 nm upon 404 nm excitation for BaZrSi3O9:Eu2+, as well as for commercial BaMgAl10O17:Eu2+. Zhang et al.46 also reported the emission band at 475 nm for Sr6BP5O20:Eu2+ phosphor, having broad excitation bands at about 290 nm, 365 nm and a shoulder around 390 nm. Whereas Chang et al.47 reported broad-band yellow–orange emission band centered at 574 nm for Sr3B2O6:Eu2+ phosphor, attributed to the strong crystal field and nephelauxetic effected 4f65d1 → 4f7 (8S7/2) transition of Eu2+ and the excitation spectrum shows a broad absorption from UV to the blue region with a maximum at 377 nm. Furthermore, Salah et al.48 and Pandey et al.49 independently reported the emission at ∼436 nm for Eu2+-doped K2Ca2(SO4)3 phosphor excited at 332 nm. Hence, it is widely accepted that the Eu2+ broad emission positions differ from host to host and spans to a wide spectral domain (UV to green). The emission, depending on the Eu2+ host lattice site occupation, is excitation selective and strongly influenced by the local crystal field and nephelauxetic effects.50 From the present study, the observed excitation bands (Fig. 5(b) (ii)) at 334 nm accompanied by a minor peak at 280 nm can be identified as transitions corresponding to the divalent europium (Eu2+) ions from the ground state 8S7/2 with 4f7 configuration to the different crystal field splitting components with (4f6)5d configuration in the excited states.44 The broad blue-end photoluminescence can be attributed to the crystal field effected and site-selective transition from broad (4f6)5d excited state 6P7/2 to the 8S7/2 ground state of Eu2+ ion. As explained in the XRD analysis previously, the most possible site occupation of Eu3+ ion is the charge-compensated (by K+) Ca2+ sites. However, the reduced Eu2+ ions also have the possibility of occupying the sites of equal sized Ca2+ ions without any charge-compensation (ionic radii Eu2+ = 117 pm and Ca2+ = 114 pm). Since the Eu3+ to Eu2+ reduction is partial and uneven, the Eu2+ ions also have the possibility to occupy several other positions of varied crystal field strengths. Therefore, the emission observed at 429 nm (excited at 337 nm) is possibly from the Eu2+ ions situated in a weaker crystal field environment, whereas the emission at 476 nm (excited at 404 nm) is from the Eu2+ ions situated in a much stronger crystal field (such as Ca2+) sites.
The co-existence of Eu2+ and Eu3+ emission has been previously reported by Howe and co-workers.51 Similarly, the combined emission from Eu3+ and Eu2+ has been observed from low-temperature (500 °C) processed CaF2:Eu and MgF2:Eu phosphors.52 Thomas Baby et al.44 studied the host lattice dependent emission properties of Eu2+ in mixed fluoride crystals. Pei and coworkers extensively investigated reduction of Re3+ to Re2+ (Re = Sm, Eu and Yb) alkaline earth borates.16,53 Such reduction in solid state materials generally requires reduction agents, such as H2–N2 or N2 atmosphere. In the present KCaBO3:Eu3+ phosphor, such reduction could be possible due to the presence of OH− bonds in phosphors annealed at less than 700 °C.41,54,55 The presence of water-related OH− bonds44,55 could possibly induce such partial reduction under an air atmosphere, by the following reaction. 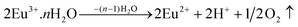 In our present synthesis, many of the reactants (CaCl, KCl, H3BO3etc.) are essentially hydroscopic in nature at relatively low temperature annealing conditions, therefore an excess of OH− contents is more likely to be trapped in the resultant host. FTIR results (Fig. 2(a)) reported in the previous section also demonstrate the dominant OH-related bonds in the phosphors processed at relatively low temperatures. Furthermore, the interaction of Eu3+ ions with the nearby halogen (Br, Cl) ions can also provide the necessary electrons for such reduction.56 In such cases, the reduction could be expressed as Eu3+ + Cl− = Eu2+ + Cl. Eventually, this reaction leaves the Cl atoms as interstitial defects in the host. The presence of native Cl ions is quite possible due to unreacted initial components CaCl2 and KCl, even in 700 °C annealing conditions. Indeed, the low intensity XRD patterns (Fig. 1(a)) and the EDX results (Fig. 2(c)) support the presence of Cl atoms in the phosphors annealed at temperatures below 700 °C. Furthermore, the CIE chromaticity coordinates, reported in Fig. 5(a), also suggest the whitish blue photoluminescence of the phosphor annealed at 700 °C or below.
In our present synthesis, many of the reactants (CaCl, KCl, H3BO3etc.) are essentially hydroscopic in nature at relatively low temperature annealing conditions, therefore an excess of OH− contents is more likely to be trapped in the resultant host. FTIR results (Fig. 2(a)) reported in the previous section also demonstrate the dominant OH-related bonds in the phosphors processed at relatively low temperatures. Furthermore, the interaction of Eu3+ ions with the nearby halogen (Br, Cl) ions can also provide the necessary electrons for such reduction.56 In such cases, the reduction could be expressed as Eu3+ + Cl− = Eu2+ + Cl. Eventually, this reaction leaves the Cl atoms as interstitial defects in the host. The presence of native Cl ions is quite possible due to unreacted initial components CaCl2 and KCl, even in 700 °C annealing conditions. Indeed, the low intensity XRD patterns (Fig. 1(a)) and the EDX results (Fig. 2(c)) support the presence of Cl atoms in the phosphors annealed at temperatures below 700 °C. Furthermore, the CIE chromaticity coordinates, reported in Fig. 5(a), also suggest the whitish blue photoluminescence of the phosphor annealed at 700 °C or below.
For a better understanding of the coexistence of Eu3+/Eu2+ photoluminescence and its microscopic distribution within the phosphor, the PL spectral mapping of the phosphor over a 200 × 200 μm2 was performed for the samples annealed at 500 °C and 700 °C (500 °C data is shown in supplementary data†). Fig. 6(a) shows the conventional confocal dark-field image of phosphor powder annealed at 700 °C. Fig. 6(b) shows the PL image recorded by exciting with a 404 nm laser and imaged using a 410 nm long-pass filter. A portion of the area (indicated by the blue square) has been spectrally scanned at each XY position at 4 μm step size. The intensity spatial mappings at 476 (of Eu2+) and 612 nm (of Eu3+) emission wavelengths are represented in Fig. 6(c) and (d), respectively. From these PL mapping images, it has been observed that for the 700 °C annealed sample (Fig. 6(d)) the red photoluminescence (612 nm) related to Eu3+ is found to be more intense compared to the photoluminescence related to Eu2+ (476 nm). While Eu3+ emission is uniform throughout the sample, the emission locations of Eu2+ are entirely different and random (Fig. 6(c), (d) and (e)). It is further interesting to note that at much lower annealing temperatures (500 °C), the present phosphor shows more uniformly distributed Eu2+/Eu3+ emission locations throughout the powders (shown in supplementary data†). For the phosphors annealed at temperatures higher than 700 °C, the blue emission from Eu2+ has completely disappeared. As discussed in the earlier sections, the OH and other volatile contents are predominant in low-temperature annealed phosphors. Therefore, the microscopic PL analysis of KCaBO3:Eu3+ phosphors suggest that the optimized annealing conditions not only increase the crystallinity and particle sizes, but also lessen the partial reduction of Eu3+, which is due to the presence of volatile impurities, like OH− and Cl−. Site-selective occupation of rare-earth ions, compatible ionic radii and appropriate host structure could also be other influencing factors.43 Nevertheless, Eu3+ to Eu2+ reduction can be further explored for strong blue color photoluminescence by appropriately adjusting the volatile impurities, aiming at applications such as lamp phosphors and blue components for television phosphors.43,57
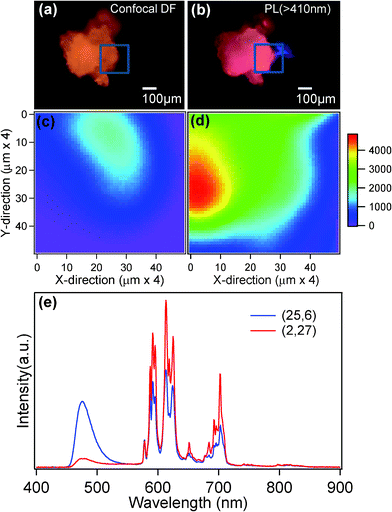 | ||
| Fig. 6 (a) Confocal image light (white light illuminated dark-field mode) and (b) PL (λex = 404 nm) images recorded using high-resolution images for KCaBO3:Eu phosphors annealed at 700 °C (colors are not to be scaled). The PL intensity spatial scans (4 μm step) monitored at (c) 476 nm and (d) 612 nm PL peaks, respectively (see text). (e) PL spectra recorded at different XY positions (25,6) and (2,27) (× 4 μm). | ||
4. Conclusions
In conclusion, we developed intense red emitting KCaBO3:Eu3+ phosphors by solid-state reaction and studied the annealing temperature dependence of the structural and photoluminescence characteristics. These phosphors annealed above 700 °C show a single-phased monoclinic structure and the Eu3+ ions have conveniently occupied the Ca2+ sites in the host lattice by charge-compensation. The phosphors developed in this work show strong and stable red photoluminescence (612 nm) of dominant hypersensitive electric dipole transition 5D0 → 7F2 when excited by a wide range of excitation wavelengths that covers many commercially available UV/NUV/blue LEDs, a favorable color rendering property for W-LED applications. For relatively low-temperature annealed phosphors, the photoluminescence show distinct mixed emissions composed of blue and red emissions upon UV excitation. The dual emission is attributed to the coexistence of Eu3+ and Eu2+ ions, wherein the reduction of Eu3+ into Eu2+ was attributed to the presence of volatile impurities, like OH− and Cl−. Annealing temperature at around 900 °C is the suitable condition to obtain better crystallinity. The dominant hypersensitive to non-hypersensitive photoluminescence intensity ratios of Eu3+ (5D0 → 7F2 to 5D0 → 7F1, R/O) and Judd-Ofelt parameters suggesting strong covalent nature of the Eu3+ bonding with the surroundings and Eu3+ occupation of higher asymmetry sites of different valency (K+, Ca2+). With the optimized conditions, these charge-compensated phosphors show high photoluminescence intensities with wide choice of excitations. Therefore, the investigated KCaBO3:Eu3+ could be a potential candidate as a red phosphor for white-LEDs.Acknowledgements
The authors acknowledge the financial support from Department of Information Technology (DIT), Govt. of India, under Photonics Development Program (ref. 12(1)/2008-PDD). This is part of the High-Impact Research Scheme of IIT Delhi. The authors are highly thankful to Prof. Jeremy Baumberg, Cavendish Laboratory, University of Cambridge, UK for allowing us to record PL mapping studies. The support from CICECO, University of Aveiro is also acknowledged.References
- F. A. Ponce and D. P. Bour, Nature, 1997, 386, 351 CrossRef CAS
.
- Y. Cong, B. Li, S. Yue, Y. Liu and W. Li, J. Phys. Chem. C, 2009, 113, 493 CAS
.
- G. Jia, P. A. Tanner, C. K. Duan and J. D. Ghys, J. Phys. Chem. C, 2010, 114, 2769 CAS
.
- D. S. Thakare, S. K. Omanwar, S. V. Moharil, S. M. Dhopte, P. L. Muthal and V. K. Kondawar, Opt. Mater., 2007, 29, 1731 CrossRef CAS
.
- M. Han, S. J. Oh, J. H. Park and H. L. Park, J. Appl. Phys., 1993, 73, 4546 CrossRef CAS
.
- C. Yongjie, Q. Guanming, G. Xiujuan, Y. Ying, S. Shuang, W. Hui and Z. Zhongqiu, J. Rare Earths, 2007, 25, 113 CrossRef
.
- W. R. Liu, C. H. Huang, C. P. Wu, Y. C. Chiu, Y. T. Yeh and T. M. Chen, J. Mater. Chem., 2011, 21, 6869 RSC
.
- H. Liang, H. Lin, G. Zhang, P. Dorenbos and Q. Su, J. Lumin., 2011, 131, 194 CrossRef CAS
.
- X. Zhang, X. Qiao and H. J. Seo, Curr. Appl. Phys., 2011, 11, 442 CrossRef
.
- G. Jia, H. You, M. Yang, L. Zhang and H. Zhang, J. Phys. Chem. C, 2009, 113, 16638 CAS
.
- G. M. Cai, X. L. Chen, W. Y. Wang, Y. F. Lou, J. Liu, J. T. Zhao and H. H. Chen, J. Solid State Chem., 2008, 181, 646 CrossRef CAS
.
- S. Das, A. A. Reddy, S. S. Babu and G. V. Prakash, J. Mater. Sci., 2011, 46, 7770 CrossRef CAS
.
- S. Das, A. A. Reddy, S. Ahmad, R. Nagarajan and G. V. Prakash, Chem. Phys. Lett., 2011, 508, 117 CrossRef CAS
.
- C. K. Chang and T. M. Chen, Appl. Phys. Lett., 2007, 91, 081902 CrossRef
.
- Z. Lian, J. Wang, Y. Lv, S. Wang and Q. Su, J. Alloys Compd., 2007, 430, 257 CrossRef CAS
.
- Z. Pei, Q. Su and J. Zhang, J. Alloys Compd., 1993, 198, 51 CrossRef CAS
.
- J. D. Ghys, R. Mauricot, B. Caillier, P. Guillot, T. Beaudette, G. Jia, P. A. Tanner and B. M. Cheng, J. Phys. Chem. C, 2010, 114, 6681 Search PubMed
.
- M. Schlager and R. Z. Hoppe, Z. Anorg. Allg. Chem., 1993, 619, 976 CrossRef
.
- A. Le Bail, H. Duroy and J. L. Fourquet, Mater. Res. Bull., 1988, 23, 447 CrossRef CAS
.
- J. Rodriquez-Carvajal, M. T. Fernadez-Diaz and J. L. Martinez, J. Phys.: Condens. Matter, 1991, 3, 3215 CrossRef
.
- J. Liu, H. Lian and C. Shi, Opt. Mater., 2007, 29, 1591 CrossRef CAS
.
- M. Menaka, E. Samuel, S. E. Lofland, K. V. Ramanujachary and A. K. Ganguli, J. Organomet. Chem., 2010, 695, 1002 CrossRef
.
- A. Kumar, S. B. Rai and D. K. Rai, Mater. Res. Bull., 2003, 38, 333 CrossRef CAS
.
- V. D. Maiorov and N.
B. Librovich, Russian. Chem. Bull., 1991, 40, 1203 CrossRef
.
- C. Sekar and R. Parimaladevi, Spectrochim. Acta, Part A, 2009, 74, 1160 CrossRef CAS
.
- B. Karthikeyan and S. Mohan, Phys. B, 2003, 334, 298 CrossRef CAS
.
- M. Yang, K. Li, J. Su, Q. Huang, W. Bao, L. You, Z. Li, Y. Wang, Y. Jiang, F. Liao and J. Lin, J. Alloys Compd., 2011, 509, 4707 CrossRef CAS
.
-
C. G. Walrand and K. Binnemans, Handbook on the Physics and Chemistry of Rare-Earthsvol. 23, Elsevier, Amsterdam, 1996 Search PubMed
.
- B. Padlyak, M. Grinberg, B. Kukliński, Y. Oseledchik, O. Smyrnov, D. Kudryavtcev and A. Prosvirnin, Opt. Appl., 2010, XL, 413 Search PubMed
.
- H. Li, S. Zhang, S. Zhou, X. Cao and Y. Zheng, J. Phys. Chem. C, 2009, 113, 13115 CAS
.
- G. V. Prakash and R. Jagannathan, Spectrochim. Acta, Part A, 1999, 55, 1799 CrossRef
.
- R. Reisfeld, E. Zigansky and M. Gaft, Mol. Phys., 2004, 102, 1319 CrossRef CAS
.
- B. R. Judd, Phys. Rev., 1962, 127, 750 CrossRef CAS
.
- G. S. Ofelt, J. Chem. Phys., 1962, 37, 511 CrossRef CAS
.
- Q. Su, Z. Pei, L. Chi, H. Zhang, Z. Zhang and F. Zou, J. Alloys Compd., 1993, 192, 25 CrossRef CAS
.
- V. R. Bandi, M. Jayasimhadri, J. Jeong, K. Jang, H. S. Lee, S. S. Yi and J. H. Jeong, J. Phys. D: Appl. Phys., 2010, 43, 395103 CrossRef
.
- W. R. Liu, C. C. Lin, Y. C. Chiu, Y. T. Yeh, S. M. Jang and R. S. Liu, Opt. Express, 2010, 18, 2946 CrossRef CAS
.
- Y. Li, J. Zhang, X. Zhang, Y. Luo, S. Lu, Z. Hao and X. Wang, J. Phys. Chem. C, 2009, 113, 17705 CAS
.
- N. Xie, Y. Huang, X. Qiao, L. Shi and H. J. Seo, Mater. Lett., 2010, 64, 1000 CrossRef CAS
.
- G. Feng, W. Jiang, Y. Chen and R. Zeng, Mater. Lett., 2011, 65, 110 CrossRef CAS
.
- X. Liu, C. Li, Z. Quan, Z. Cheng and J. Lin, J. Phys. Chem. C, 2007, 111, 16601 CAS
.
- A. G. Chakhovskoi, C. E. Hun, M. E. Malinowski, T. E. Felter and A. A. Talin, J. Vac. Sci. Technol., B, 1997, 15, 507 CAS
.
- W. Wei-Ning, W. Widiyastuti, T. Ogi, I. W. Lenggoro and K. Okuyama, Chem. Mater., 2007, 19, 1723 CrossRef
.
- T. Baby and V. P. N. Nampoori, Solid State Commun., 1992, 81, 367 CrossRef CAS
.
- D. Wang, C. Huang, Y. Wu and T. Chen, J. Mater. Chem., 2011, 21, 10818 RSC
.
- M. Zhang, J. Wang, W. Ding, Q. Zhang and Q. Su, Appl. Phys. B, 2007, 86, 647 CrossRef CAS
.
- C. Chang and T. Chen, Appl. Phys. Lett., 2007, 91, 081902 CrossRef
.
- N. Salah, S. S. Habib and Z. H. Khan, J. Fluoresc., 2010, 20, 1009 CrossRef CAS
.
- A. Pandey, R. G. Sonkawade and P. D. Sahare, J. Phys. D: Appl. Phys., 2002, 35, 2744 CrossRef CAS
.
-
G. Blasse, Luminescence of Inorganic Solids, ed. B. O. Bartolo, p. 463, Plenum Press, New York, 1978 Search PubMed
.
- B. Howe and A. L. Diaz, J. Lumin., 2004, 109, 51–59 CrossRef CAS
.
- B. C. Hong and K. Kawano, J. Alloys Compd., 2006, 408, 838 CrossRef
.
- Q. Zeng, Z. Pei, S. Wang, Q. Su and S. Lu, Mater. Res. Bull., 1999, 34, 1837 CrossRef CAS
.
- Q. Zhang, X. Liu, Y. Qiao, B. Qian, G. Dong, J. Ruan, Q. Zhou, J. Qiu and D. Chen, Opt. Mater., 2010, 32, 427 CrossRef CAS
.
- S. M. Dhopte, P. L. Muthal, V. K. Kondawar and S. N. Moharil, J. Lumin., 1991, 50, 187 CrossRef CAS
.
- W. J. Chung and J. J. Heo, J. Appl. Phys., 2002, 92, 1274 CrossRef CAS
.
- F. Tao, Z. Wang, L. Yao, W. Cai and X. Li, J. Phys. Chem. C, 2007, 111, 3241 CAS
.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20866k/ |
| This journal is © The Royal Society of Chemistry 2012 |
