Magnetoresistive field-effect transistors based on organic donor–acceptor blends†
Thomas
Reichert
,
Tobat P. I.
Saragi
* and
Josef
Salbeck
Macromolecular Chemistry and Molecular Materials (mmCmm), Department of Mathematics and Science and Center for Interdisciplinary Nanostructure Science and Technology, University of Kassel, Heinrich-Plett Strasse 40, D 34132 Kassel, Germany. E-mail: tobat.saragi@uni-kassel.de; Fax: +49 561 804 455
First published on 27th June 2012
Abstract
We present magnetoresistive field-effect transistors, which are sensitive to external magnetic fields as low as 1.7 mT. The magnetosensitivity is achieved through the mixture of electron acceptor 1,4,5,8,9,11-hexaazatriphenylene hexacarbonitrile (HAT-CN) with electron donor 2,2′,7,7′-tetrakis-(N,N-di-p-methylphenylamino)-9,9′-spirobifluorene (Spiro-TTB), which leads to magnetic-field-sensitive intermolecular radical pair states. These states are responsible for the magnetoresistance effect in Spiro-TTB–HAT-CN blends.
Organic semiconductors are intensively used as active thin films in optoelectronic devices like light-emitting diodes,1 solar cells,2 field-effect transistors,3 and sensors.4 Due to the high performance of these devices they are already available in the market or on the edge of commercialization. To take the next step towards magnetooptoelectronic devices, electron spin can be used as an (additional) control parameter. In organic semiconductors the spin correlation between quasiparticles can be influenced by low magnetic fields, which leads to large magnetoresistive effects at room temperature.5 This organic magnetoresistance (OMAR) is not only highly interesting for addressing fundamental questions about spin transport and spin manipulation in organic materials, but also for the realization of lightweight, flexible, and multifunctional magnetooptoelectronic applications. Until now OMAR is nearly always analyzed in two-terminal devices.6 For a deeper understanding of OMAR, it is of high importance to explore this phenomenon in organic field-effect transistors (OFETs), because this (three-terminal) device geometry allows the verification of the charge carrier sign in the conducting channel as well as the determination of the corresponding mobility.7 Surprisingly, until now only very few examples of magnetoresistive OFETs can be found, which all refer to a limited number of acene-based materials.8
In this communication, we report for the first time on magnetoresistive field-effect transistors, which are sensitive to ultrasmall magnetic fields (<5 mT) at room temperature and without any additional irradiation. The magnetosensitivity of the charge transport layer is achieved through the mixture of the strong acceptor molecule 1,4,5,8,9,11-hexaazatriphenylene hexacarbonitrile (HAT-CN)9 with the donor unit 2,2′,7,7′-tetrakis-(N,N-di-p-methylphenylamino)-9,9′-spirobifluorene (Spiro-TTB).10 Pristine p-type Spiro-TTB transistors as well as pristine n-type HAT-CN transistors do not possess any magnetic-field dependency. In contrast, devices with a mixture of Spiro-TTB and HAT-CN, which induced intermolecular radical pair states in blended systems, show a significant response to external magnetic fields (see Fig. 1).
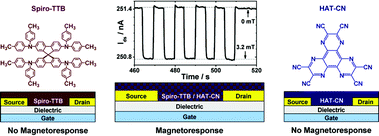 | ||
| Fig. 1 Schematic representation of our experimental schemes. On the left and right side non-magnetoresistive field-effect transistors based on p-type Spiro-TTB and n-type HAT-CN, as well as the corresponding chemical structures, are shown. Devices with a mixture of Spiro-TTB and HAT-CN show a significant response to an external magnetic field of 3.2 mT (depicted in the middle). | ||
Devices based on pristine compounds show transistor behavior with pure unipolar output (results are shown in Fig. S1 in the ESI†) and transfer (Fig. 2) characteristics. The transistors possess well-defined conducting and non-conducting channels and the gate-induced field effect leads to on/off ratios of ∼105 for both devices. Spiro-TTB exhibits a hole mobility of 3.2 × 10−4 cm2 V−1 s−1 while the electron mobility of HAT-CN is 5.6 × 10−5 cm2 V−1 s−1. Due to six strong electron-withdrawing carbonitrile groups, HAT-CN is a strong acceptor molecule. Its lowest unoccupied molecular orbital (LUMO) resides at a stable energy position of ∼5.1 eV and it can also be used for doping p-type organic semiconductors.11 The four substituted arylamine moieties that built up Spiro-TTB are known for stabilizing positively charged cationic states via mesomeric effects which cause a strong donor character. The resulting energy of the highest occupied molecular state (HOMO) is ∼4.9 eV.10 Due to this large difference in the electron affinities, an electron transfer from the donor Spiro-TTB to the acceptor HAT-CN appears to be highly favorable. Consequently, transistors with charge transport layers based on codeposited Spiro-TTB–HAT-CN blends exhibit extremely changed device characteristics. The fundamental differences of pristine Spiro-TTB and HAT-CN transistors as well as the corresponding mixed systems are summarized in Table S1 (ESI†) and will be discussed in the next paragraph.
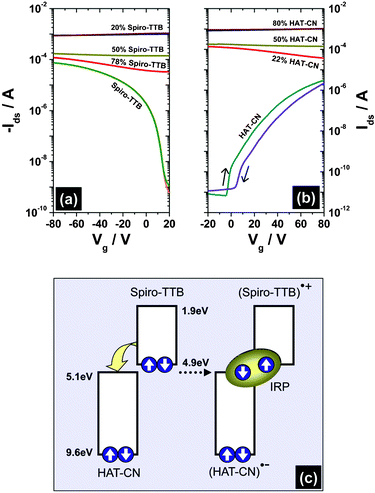 | ||
| Fig. 2 Transfer characteristics (Ids−Vg) of the OFETs (channel length = 2.5 μm, channel width = 10 mm) for Spiro-TTB, HAT-CN, and various blending concentrations, measured at a drain voltage of (a) Vds = −80 V and (b) Vds = +80 V. (c) Schematic energy level diagram of Spiro-TTB and HAT-CN and the corresponding formation process of IRP states via electron transfer. Spiro-TTB, HAT-CN, (Spiro-TTB)+, (HAT-CN)−, and IRP denote neutral Spiro-TTB, neutral HAT-CN, radical cation of Spiro-TTB, radical anion of HAT-CN, and intermolecular radical pair states. | ||
A remarkable increase in drain current (Ids) is obtained for various blending concentrations of Spiro-TTB and HAT-CN compared to devices with pristine compounds. Donor–acceptor blends possess Ids values as high as 1 mA (at Vds = ±80 V), and current flow is independent of gate voltage (Vg). There is no essential gate-induced current increase for all mixed systems, and no standard field-effect behavior can be observed either in the output (results are shown in Fig. S2 in the ESI†) or the transfer characteristics (see Fig. 2). The blended devices remain in a highly conducting state for all measured Vg and an on/off ratio as low as ∼1 can be achieved. This absence of a real field-effect behavior can be attributed to a high concentration of intrinsic charge carriers in the codeposited donor–acceptor blends. As schematically pictured in Fig. 2c, electron affinities of the isolated donor and acceptor molecules promote an electron transfer from the HOMO of Spiro-TTB to the LUMO of HAT-CN. While Spiro-TTB hosts the radical cation, the radical anion is localized on HAT-CN and together a coulomb-correlated intermolecular radical pair (IRP) is formed. The IRP state exists either in the singlet or triplet spin configuration and, therefore, can be influenced by external magnetic fields. Due to the large density of IRP states in the donor–acceptor films, a high magnetosensitivity could be achieved, which results in sensor-like magnetoresistive organic devices that respond to magnetic fields as low as 1.7 mT.
The magnetoresistive properties of our devices are shown in Fig. 3. As can be seen from the Ids–time curves (see Fig. 3a and b), the magnetoresistance effect only exists in devices based on donor-acceptor blends. During measurements an external magnetic field was switched on and off three times in a row, and the Ids–time curves were used to calculate the corresponding MR values (MR = {[R(B)-R(0)]/R(0)} × 100%, where R(B) and R(0) are the resistances with and without an external magnetic field, respectively). Results are shown in Fig. S3 in the ESI†. While the pristine Spiro-TTB and HAT-CN transistors do not show any magnetoresponse, a significant magnetic-field-induced current decrease can be obtained for the mixed system. The resistance of all donor–acceptor blended transport layers increases in an external magnetic field, which leads to positive organic magnetoresistance. The highest magnetoresponse can be obtained in the evenly mixed Spiro-TTB–HAT-CN system (see Fig. 3c). Changing the donor–acceptor composition to more unbalanced ratios leads to a decreasing magnetoresponse with smaller MR values. This can be attributed to a drop in the formation probability of IRP states. Hence, mixing concentration can be employed to adjust IRP density in the charge transport layer and the corresponding magnetoresistance of the device. To test the sensing performance of our systems we choose the 50 : 50 blend compositions with the highest density of IRP states and conducted measurements under the influence of ultrasmall magnetic fields (<5 mT). Fig. 3d depicts the experimental raw data of the corresponding Ids–time curves, where different ultrasmall magnetic fields were switched on and off five times in a row, each with a fixed magnetic field. A magnetoresistance of 0.380% could be achieved at 4.80 mT, and the device still shows a significant magnetoresponse of 0.125% with an external magnetic field as low as 1.70 mT. All our devices possess high stability and reproducibility as well as an excellent response to ultrasmall magnetic fields. Even the dynamically measured (and therefore often unstable, hysteretic and hardly reproducible) output and transfer characteristics feature significant magnetic-field dependence, and the influence of 1.65 mT can clearly be seen (results are shown in Fig. S2 and S4 in the ESI†). We therefore propose that the use of donor–acceptor blends and the corresponding IRP states are one promising opportunity to pave the way to multifunctional organic magnetooptoelectronic devices.
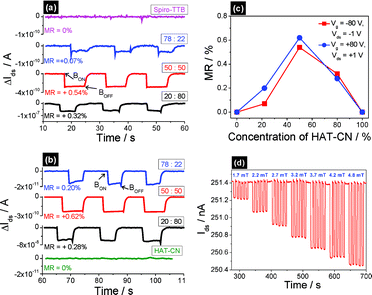 | ||
| Fig. 3 I ds–time curves of devices based on pristine compounds as well as various blending concentrations measured at (a) Vds = −1 V and Vg = −80 V and (b) Vds = +1 V and Vg = +80 V. An external magnetic field of 60 mT was switched on and off three times in a row and the corresponding magnetic-field-induced current decrease is shown. (c) MR values obtained from different compositions of Spiro-TTB and HAT-CN. (d) Ids–time curve of device based on evenly blended Spiro-TTB and HAT-CN, measured at Vds = −2.5 V and Vg = 0 V. The device shows a significant magnetosensitivity to ultrasmall magnetic fields (<5 mT). | ||
All our results show that magnetosensitivity can only be achieved in charge transport layers based on donor–acceptor blends. If pristine p-type Spiro-TTB or pristine n-type HAT-CN are used, the corresponding field-effect transistors possess well-defined unipolar output and transfer characteristics. In Spiro-TTB (HAT-CN) only holes (electrons) can be observed in the conducting channel, and the charge transport in these strictly unipolar devices does not depend on low external magnetic fields (<100 mT). Therefore, no magnetosensitive states are present in charge transport layers based on pristine Spiro-TTB or pristine HAT-CN. But if both materials are mixed, an electron can be transferred from the HOMO of Spiro-TTB to the LUMO of HAT-CN, and a radical cation [(Spiro-TTB)•+] as well as a radical anion [(HAT-CN)•−] are generated. This redox process results in coulomb-correlated intermolecular radical pairs, which implement a magnetosensitivity into the mixed systems. This clearly indicates that the existence of IRP states is a fundamental requirement for organic magnetoresistance in our devices. The IRP states can be considered as quasiparticles, which exist in magnetic-field-dependent singlet and triplet spin configurations. Due to the relatively large intermolecular separation distance and the corresponding small coulomb correlation, the behavior of the IRPs can be discussed in a similar manner to weakly coupled polaron pairs. After formation, the IRP states can undergo dissociation or recombination as well as interaction with other quasiparticles. Dissociation and collision-induced release of trapped charges lowers the resistance, while recombination or scattering of free charges increases the resistance of the blended transport layers. It is known that all these processes can be magnetic-field dependent either in a direct way or indirectly due to a magnetic-field-dependent singlet/triplet ratio.5b,12 Hence, we suggest that the magnetic-field-induced current decrease in our donor–acceptor compositions can be attributed to the following reasons: i) decrease of the IRP formation rate in a magnetic field; ii) magnetic-field-induced reduction of IRP dissociation; iii) magnetic-field-dependent interaction rate of IRP states with other quasiparticles. Thereby, either one or a combination of more processes is responsible for the observed positive magnetoresistance in our devices. The basic requirements for all these processes and the corresponding magnetoresponse are the IRP states. However, we cannot exclude the bipolaron model as possible reasons for the magnetoresponse of our devices, because bipolarons are normally formed at high doping levels and, subsequently, bipolarons can be stabilized by oppositely charged polarons.5b,13–16 However, further work is needed to elucidate the fundamental processes that are responsible for magnetic-field effects in OFETs. In particular special theoretical simulations for magnetoresistive OFETs have to be carried out. Detailed investigations like electron spin resonance and pulsed electrically detected magnetic resonance experiments of the donor/acceptor blends must be conducted as well.
In summary, we have presented magnetoresistive organic field-effect transistors, which are sensitive even to ultrasmall (<5 mT) magnetic fields at room temperature and without any additional irradiation. The magnetosensitivity of our devices is achieved through the mixture of the strong acceptor molecule HAT-CN with the donor unit Spiro-TTB, which leads to a high density of IRP states with magnetic-field-dependent formation, dissociation and interaction rates. The amount of IRP states can be tuned by changing the blending composition and, as a result, the magnetoresponse of the devices can be adjusted as well. The usage of donor–acceptor blends as active thin films in organic semiconductor devices is therefore a powerful tool to fabricate magnetoresistive devices without ferromagnetic materials.
Acknowledgements
The Otto-Braun scholarship is gratefully acknowledged for support of T. Reichert. We thank Dr. Manfred Kussler for synthesizing and purifying HAT-CN.References
- K. Müllen and U. Scherf, Organic Light-Emitting Devices, Wiley-VCH, Weinheim, 2006 Search PubMed.
- C. Brabec, U. Scherf and V. Dyakonov, Organic Photovoltaics: Materials, Device Physics, and Manufacturing Technologies, Wiley-VCH, Weinheim, 2008 Search PubMed.
- Z. Bao and J. Locklin, Organic Field-Effect Transistors, CRC Press, Florida, 2007 Search PubMed.
- B. Crone, A. Dodabalapur, A. Galperin, L. Torsi, H. E. Katz, A. J. Lovinger and Z. Bao, Appl. Phys. Lett., 2001, 78, 2229 CrossRef CAS.
- (a) Ö. Mermer, G. Veeraraghavan, T. L. Francis, Y. Sheng, D. T. Nguyen, M. Wohlgenannt, A. Köhler, M. K. Al-Suti and M. S. Khan, Phys. Rev. B, 2005, 72, 205202 CrossRef; (b) W. Wagemans and B. Koopmans, Phys. Status Solidi B, 2011, 248, 766 CrossRef.
- V. Vardeny, Organic Spintronics, CRC Press, Florida, 2010 Search PubMed.
- G. Szulczewski, S. Sanvito and M. Coey, Nat. Mater., 2009, 8, 693 CrossRef CAS.
- (a) M. Nishioka, Y. B. Lee, A. M. Goldman, Y. Xia and C. D. Frisbie, Appl. Phys. Lett., 2007, 91, 092117 CrossRef; (b) T. Reichert and T. P. I. Saragi, Appl. Phys. Lett., 2011, 98, 063307 CrossRef; (c) T. Reichert and T. P. I. Saragi, Org. Electron., 2012, 13, 377 CrossRef CAS; (d) T. P. I. Saragi and T. Reichert, Appl. Phys. Lett., 2012, 100, 073304 CrossRef.
- (a) K. Kanakarajan and A. W. Czarnik, J. Org. Chem., 1986, 51, 5241 CrossRef CAS; (b) P. S. Szalay, J. R. Galan-Mascaros, R. Clerac and K. R. Dunbar, Synth. Met., 2001, 122, 535 CrossRef CAS.
- (a) T. P. I. Saragi, T. Fuhrmann-Lieker and J. Salbeck, Adv. Funct. Mater., 2006, 16, 966 CrossRef CAS; (b) T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011 CrossRef CAS.
- (a) C. Falkenberg, S. Olthof, R. Rieger, M. Baumgarten, K. Muellen, K. Leo and M. Riede, Sol. Energy Mater. Sol. Cells, 2011, 95, 927 CrossRef CAS; (b) K. S. Yook, S. O. Jeon and J. Y. Lee, Thin Solid Films, 2009, 517, 6109 CrossRef CAS; (c) Y. Kim, J. W. Kim and Y. Park, Appl. Phys. Lett., 2009, 94, 063305 CrossRef.
- (a) N. E. Geacintov, M. Binder, C. E. Swenberg and M. Pope, Phys. Rev. B, 1975, 12, 4113 CrossRef CAS; (b) N. E. Geacintov, M. Pope and S. Fox, J. Phys. Chem. Solids, 1970, 31, 1375 CrossRef CAS; (c) V. Ern and R. E. Merrifield, Phys. Rev. Lett., 1968, 21, 609 CrossRef CAS; (d) B. Hu and Y. Wu, Nat. Mater., 2007, 6, 985 CrossRef CAS; (e) B. Hu, L. Yan and M. Shao, Adv. Mater., 2009, 21, 1500 CrossRef CAS.
- T. H. Lee, J. H. Li, W. S. Huang, B. Hu, J. C. A. Huang, T. F. Guo and T. C. Wen, Appl. Phys. Lett., 2011, 99, 073307 CrossRef.
- Z. Sun, Y. Li, K. Gao, D. Liu, Z. An and S. Xie, Org. Electron., 2010, 11, 279 CrossRef CAS.
- W. R. Salaneck, R. H. Friend and J. L. Bredas, Phys. Rep., 1999, 319, 231 CrossRef CAS.
- A. Kadashchuk, V. I. Arkhipov, C. H. Kim, J. Shinar, D. W. Lee, Y. R. Hong, J. I. Jin, P. Heremans and H. Bässler, Phys. Rev. B, 2007, 76, 235205 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20901b/ |
| This journal is © The Royal Society of Chemistry 2012 |
