CuOx nanotubes via an unusual complexation induced block copolymer-like self-assembly of poly(acrylic acid)†‡
Yen Nan
Liang
a,
Jinhua
Hu
a,
Michael Kam Chiu
Tam
b and
Xiao
Hu
*a
aSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798. E-mail: asxhu@ntu.edu.sg; Fax: (65) 6790 9081; Tel: (65)67904610
bDepartment of Chemical Engineering, Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Avenue West, Waterloo, Ontario, Canada N2L 3G1
First published on 9th August 2012
Abstract
Polyelectrolyte (PEL) mediated synthesis of functional inorganic nano-materials is attractive due to its versatility and compatibility inside aqueous media. Block copolymers are often employed to facilitate the formation of novel nanostructures. In this work, we report the synthesis of copper oxide (CuOx) crystals with an unexpected hollow nano-tubular morphology using only poly(acrylic acid) (PAA), i.e., a homopolymer. The quantification of the pH dependent binding limit of Cu2+ to PAA, which has often been given little emphasis previously, is found to be critical in understanding the formation mechanism of such nanotubes. Further quantification of the interdependent relationship of pH, α, and [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 using the pKa-α curves provided important insight. The formation of the CuOx nanotubes is believed to be due to an unusual copolymer-like self-assembly of PAA as a homopolymer in aqueous solution; caused by interactions between Cu2+ and charged PAA chains. Such a unique copolymer-like self-assembly behaviour of the PAA–Cu2+ complex manifests within a specific window of [Cu2+]
[COOH]0 using the pKa-α curves provided important insight. The formation of the CuOx nanotubes is believed to be due to an unusual copolymer-like self-assembly of PAA as a homopolymer in aqueous solution; caused by interactions between Cu2+ and charged PAA chains. Such a unique copolymer-like self-assembly behaviour of the PAA–Cu2+ complex manifests within a specific window of [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 ratio around the binding limit. Discussion is also carried out on the possibilities and potential limitations of applying this new concept to other PELs and metal ions.
[COOH]0 ratio around the binding limit. Discussion is also carried out on the possibilities and potential limitations of applying this new concept to other PELs and metal ions.
Introduction
Nano-sized metallic copper and its oxides possess good potential for catalytic,1 photo-catalytic,2 photovoltaic,3 and sensing4 applications. Their usage in bio-related fields including fouling control and nano-toxicology5,6 is also being explored. CuOx micro- and nano-crystals have been synthesized via different routes such as reduction under alkali conditions,7 double-jet precipitation,8 hydrothermal,2 micro-emulsion,9 and solvothermal10 methods; usually with the presence of ligands or surfactants to render the desired particle size and dispersion. Although well-defined CuOx crystallites with sizes of several nanometres can be readily prepared in organic media, aqueous medium synthesis is more desirable due to its biocompatibility and eco-friendliness. The synthesis of a wide range of CuOx nanostructures and their assemblies in aqueous medium have been attempted.11Hydrophilic polymers, especially polyelectrolytes (PELs), can be employed as versatile templating agents in the aqueous synthesis of inorganic nano-crystals by utilizing the specific interactions between polymer chains and metal ions. The functions of these polymers are multifold, e.g., (i) as self-assembled nano-reactors;12,13 (ii) facilitating anisotropic crystal growth via selective adsorption of polymer chains on crystal facets;14 and (iii) simply providing random confinement for the bio-mimetic growth of nano- or mesocrystals in a concentrated polymer solution or hydrogel.15
Due to its structural simplicity, biocompatibility, and availability in different molecular weights, poly(acrylic acid) (PAA), either as a homopolymer or in a block copolymer, represents one of the most widely employed PELs in inorganic nano-materials synthesis. The PAA hydrogel has been used as the medium for sol–gel synthesis of nano-particles (PAA in dispersed state)16 or bio-mineralization of mesocrystals (PAA in semi-solid state).17 PAA has also been employed for the random capping and confinement of synthesized nano-particles.15,18 PAA often serves as the model PEL for the investigation of PEL–counterions interactions. Interactions between PAA and alkali, alkali earth, or transition metal ions have been studied through the measurement of viscosity, turbidity, optical absorbance, osmotic pressure, and enthalpy.19–21
As another research direction, the theoretical and experimental studies of PEL–counterions interactions have been conducted for decades; focusing on counterions fraction, distribution and condensation based on principles of free energy or chemical potential.22–24 This fundamental knowledge could have been more effectively used as guidelines for PEL mediated synthesis, judging by the critical influence of the PEL–counterions interaction towards the morphologies of synthesized products. However, most reports on PEL mediated synthesis have adopted empirical approaches and focused on correlating the crystallite morphology with synthesis parameters. The reported nano-materials syntheses typically used PELs within a relatively concentrated regime, representing one of the conditions when the theoretical models deviate.15,25 Furthermore, many reports did not pay attention to the actual binding limits of Cu2+ onto available COO− on a charged PAA chain and the variation of pH.26,27 Therefore, we recognize that better bridging of the fundamental understanding on PEL–counterions interaction and nano-crystal formation mechanism can lead to more intelligent synthesis designs.
As is generally accepted, PAA–Cu2+ interaction involves electrostatic attraction, complexation, and counterion condensation; leading to phenomena such as intra- or inter-chain bridging and change of hydration structure.23,28,29 PAA–Cu2+ interaction is known to be distinctly different from interactions between PAA and alkaline earth metal ions, e.g., Ca2+.30 The mechanism is affected by the degree of deprotonation (α) that is pH dependent: Cu2+ may bind with COO− either in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (low α) or 1
1 (low α) or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (high α) ratios28 and form a bridging bidentate structure.29 The chain conformation that changes depending on physicochemical conditions represents a unique characteristic of PELs. The change is reflected as measurable properties such as pKa, hydrodynamic radius, and solution conductivity. There exist different theories of PEL chain conformation;31,32 among them the necklace model for flexible PEL is useful in describing PAA. Precise morphological control requires not only the accurate quantification of both the binding limit of PEL–metal ions and pH variation, but also the understanding of their effects towards PEL chain conformation.
2 (high α) ratios28 and form a bridging bidentate structure.29 The chain conformation that changes depending on physicochemical conditions represents a unique characteristic of PELs. The change is reflected as measurable properties such as pKa, hydrodynamic radius, and solution conductivity. There exist different theories of PEL chain conformation;31,32 among them the necklace model for flexible PEL is useful in describing PAA. Precise morphological control requires not only the accurate quantification of both the binding limit of PEL–metal ions and pH variation, but also the understanding of their effects towards PEL chain conformation.
In this study, we firstly quantified the relationship among pH, α and [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 ratio. Furthermore, we correlated them to the chain conformation that determined CuOx nano-crystal morphologies obtained in our aqueous PAA-templated synthesis. The concentration of PAA in the slight semi-dilute regime we employed was critical in minimizing inter-chain interactions but still providing the chance for self-assembly and reasonable nano-crystals yield. In this study, we report a new phenomenon that, under carefully controlled conditions, the nano-crystals formed by the PAA–Cu2+ complex bear a striking resemblance to those formed via the self-assembly of an amphiphilic rod–coil–rod triblock copolymer. This has revealed one new dimension of nano-material synthesis by employing PEL as the morphology-directing template.
[COOH]0 ratio. Furthermore, we correlated them to the chain conformation that determined CuOx nano-crystal morphologies obtained in our aqueous PAA-templated synthesis. The concentration of PAA in the slight semi-dilute regime we employed was critical in minimizing inter-chain interactions but still providing the chance for self-assembly and reasonable nano-crystals yield. In this study, we report a new phenomenon that, under carefully controlled conditions, the nano-crystals formed by the PAA–Cu2+ complex bear a striking resemblance to those formed via the self-assembly of an amphiphilic rod–coil–rod triblock copolymer. This has revealed one new dimension of nano-material synthesis by employing PEL as the morphology-directing template.
Experimental section
Materials
CuCl2·2H2O (Fisher Scientific) and poly(acrylic acid) (Mn = 124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1, PDI = 1.25, Polymer Source) were purchased and used without further purification. Throughout the experiments of interaction studies and templated synthesis, the concentration of carboxylic acid groups (i.e. [COOH]0) was fixed at 1.25 mM and an ionic background of 0.01 M NaNO3 was used for all samples.
500 g mol−1, PDI = 1.25, Polymer Source) were purchased and used without further purification. Throughout the experiments of interaction studies and templated synthesis, the concentration of carboxylic acid groups (i.e. [COOH]0) was fixed at 1.25 mM and an ionic background of 0.01 M NaNO3 was used for all samples.
Titration experiments
Potentiometric titrations coupled with a cupric ion-selective electrode (denoted as ‘PT + Cu-ISE’ in future appearance) were carried out using a Metrohm Titrando 905 titration system connected with conductivity module 856. Cupric ion concentration (i.e. [Cu2+]) was measured using Cu-ISE (Orion 9629BNWP). All the titrations were done at 25 °C; temperature was regulated by linking the jacketed vessel to a water circulator. Two sets of titration experiments were designed and carried out:Experiment A: Using PT + Cu-ISE to measure the unbound [Cu2+], to determine the [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 binding limit of Cu2+ onto PAA at different initial pH. Titrating 20 mM CuCl2·2H2O (titrant, initial pH = 5) into 1.25 mM PAA of variable initial pH (titrand).
[COOH]0 binding limit of Cu2+ onto PAA at different initial pH. Titrating 20 mM CuCl2·2H2O (titrant, initial pH = 5) into 1.25 mM PAA of variable initial pH (titrand).
Conditions: pH 6.6, pH 6, pH 5.75, pH 5; only applied for PAA solution.
Experiment B: Studying the dissociation behaviour (pKaversus α) via pH and conductivity measurements. Titrating 50 mM NaOH (titrant) into 1.25 mM PAA with different [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 ratio (titrand).
[COOH]0 ratio (titrand).
Conditions: [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 ratios = 0, 0.05, 0.075, 0.125, 0.175, 0.25, 0.375; pH of titrand was adjusted to ∼3.3 prior to the start of titration.
[COOH]0 ratios = 0, 0.05, 0.075, 0.125, 0.175, 0.25, 0.375; pH of titrand was adjusted to ∼3.3 prior to the start of titration.
In all the titration experiments, 30 μL of titrant was titrated into 60 mL of titrand at 180 s interval.
PAA templated synthesis
The aqueous PAA-templated synthesis of Cu based nano-crystals was carried out. The desired amount (i.e. [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 = 0.175) of CuCl2·2H2O solution (initial pH = 5) was mixed with PAA solution (initial pH = 6) and the mixture was stirred at room temperature for 14 h. After that, an excess amount of 1 M NaOH (32× higher than [COOH]0 in mol) was added into the solution to induce hydrolysis and it was stirred further at room temperature for 5 h. The samples were heated subsequently at 55 °C for 3 h.
[COOH]0 = 0.175) of CuCl2·2H2O solution (initial pH = 5) was mixed with PAA solution (initial pH = 6) and the mixture was stirred at room temperature for 14 h. After that, an excess amount of 1 M NaOH (32× higher than [COOH]0 in mol) was added into the solution to induce hydrolysis and it was stirred further at room temperature for 5 h. The samples were heated subsequently at 55 °C for 3 h.
Characterization
Transmission electron microscopy (TEM) and selective area electron diffraction (SAED) were done using a JEOL 2010 TEM. UV-Vis absorbance was measured using a UV-Vis spectrophotometer (UV 2501PC, Shimadzu).Results and discussion
Quantification of binding limit of Cu2+ onto PAA at different initial pH
In a PEL-templated synthesis, while it is necessary to understand the PEL–metal ions interaction mechanism, the importance of quantifying the binding limit of metal ions onto the PEL cannot be underestimated. Unfortunately, there are very few reports on the quantification of the actual binding limit of weak PEL, which is pH dependent and usually deviates considerably from the deduced binding limit which assumes 100% deprotonation. Therefore, on one hand, often only low nano-particle concentrations were obtained, indicating that the metal ion concentration was far below the binding limits.15,18 On the other hand, if the metal ion concentration exceeded the binding limits significantly, larger sized crystals with poor size dispersity would be obtained.26,33Our synthesis strategy involves employing the PAA homopolymer for complexation with Cu2+ and the templated formation of CuOx nano-crystals. This strategy relies on PAA with small molecular weight polydispersity (PDI). Our hypothesis stems from the notion that the state of metal ions (Cu2+ in this case) in the PEL solution (PAA in this case) considerably affects the morphological uniformity of the nano-crystals. This means that preventing the co-existence of bound and unbound Cu2+ ions on PAA chains can avoid non-uniform nano-crystal morphology. When Cu2+ ions are added into a PAA solution of a certain concentration and initial pH value, they interact with PAA via the well-known mechanism of rapid electrostatic interaction and complexation. The Cu2+ complexation onto PAA can be evidenced through changes in the absorbance spectra (see ESI, Fig. S1†). When the [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 binding limit was reached during the titration of Cu2+ into the PAA solutions, further Cu2+ added became detectable by Cu-ISE. The bound Cu2+ ions were non-detectable. Fig. 1(a) plots the [Cu2+] detected by the Cu-ISE versus [Cu2+]
[COOH]0 binding limit was reached during the titration of Cu2+ into the PAA solutions, further Cu2+ added became detectable by Cu-ISE. The bound Cu2+ ions were non-detectable. Fig. 1(a) plots the [Cu2+] detected by the Cu-ISE versus [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 ratio at different initial pH (titration experiment A). It should be mentioned that although it is understood that the binding limit increases with pH of the PAA solution due to increasing α, all analyses in this work were carried out at initial pH values below 6.6. This was to avoid the possibility of Cu(OH)2 formation due to hydrolysis, which adds complication to the particle morphological uniformity caused by divergence of the PAA–Cu2+ interaction manners.34 Within the [Cu2+] range used in this study, Cu(OH)2 is expected to only noticeably form at pH values above 7. The largest [Cu2+]
[COOH]0 ratio at different initial pH (titration experiment A). It should be mentioned that although it is understood that the binding limit increases with pH of the PAA solution due to increasing α, all analyses in this work were carried out at initial pH values below 6.6. This was to avoid the possibility of Cu(OH)2 formation due to hydrolysis, which adds complication to the particle morphological uniformity caused by divergence of the PAA–Cu2+ interaction manners.34 Within the [Cu2+] range used in this study, Cu(OH)2 is expected to only noticeably form at pH values above 7. The largest [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 binding limit (at initial pH value of 6.6) is only 0.18, far below the theoretical ratio of 0.50. This large shortfall is certainly due to a combination of many factors including incomplete deprotonation and steric effect associated with PAA chain conformation.
[COOH]0 binding limit (at initial pH value of 6.6) is only 0.18, far below the theoretical ratio of 0.50. This large shortfall is certainly due to a combination of many factors including incomplete deprotonation and steric effect associated with PAA chain conformation.
![(a) Concentration of unbound Cu2+ measured by Cu-ISE during the titration of 20 mM CuCl2·2H2O (titrant, initial pH = 5) into 1.25 mM PAA (titrand) of different initial pH (i.e. 5, 5.75, 6, 6.6). The inset in the top-left corner is the 3D diagram showing the relationship among pH, α, and [Cu2+] : [COOH]0; plotted based on the results of titration experiment B. (b) By using the interpolated α, the re-plotted curves of concentration of unbound Cu2+versus [Cu2+] : [COO−] shows that the binding stoichiometry converge towards [Cu2+] : [COO−] = 0.5.](/image/article/2012/RA/c2ra20951a/c2ra20951a-f1.gif) | ||
Fig. 1 (a) Concentration of unbound Cu2+ measured by Cu-ISE during the titration of 20 mM CuCl2·2H2O (titrant, initial pH = 5) into 1.25 mM PAA (titrand) of different initial pH (i.e. 5, 5.75, 6, 6.6). The inset in the top-left corner is the 3D diagram showing the relationship among pH, α, and [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0; plotted based on the results of titration experiment B. (b) By using the interpolated α, the re-plotted curves of concentration of unbound Cu2+versus [Cu2+] [COOH]0; plotted based on the results of titration experiment B. (b) By using the interpolated α, the re-plotted curves of concentration of unbound Cu2+versus [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COO−] shows that the binding stoichiometry converge towards [Cu2+] [COO−] shows that the binding stoichiometry converge towards [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COO−] = 0.5. [COO−] = 0.5. | ||
It needs to be stressed again that the pH values discussed so far are the initial pH values of the PAA solutions prior to the addition of Cu2+; the pH is expected to decrease upon titration of Cu2+ into the PAA solution and this changes the α. α is a critical parameter in the PAA-templated synthesis because it is not only quantitatively linked to the bound metal ion concentration and the [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 binding limit, it also determines PAA chain conformation. For example, whether PAA chains adopt expanded (at higher pH) or collapsed (at lower pH) conformation affects the accessibility of COOH for the deprotonation and accessibility of COO− for Cu2+ complexation. Although these factors discussed above have significant effects on the morphology of the nano-crystals prepared, so far previous works on PEL-mediated synthesis of inorganic nano-materials have largely overlooked this important aspect.
[COOH]0 binding limit, it also determines PAA chain conformation. For example, whether PAA chains adopt expanded (at higher pH) or collapsed (at lower pH) conformation affects the accessibility of COOH for the deprotonation and accessibility of COO− for Cu2+ complexation. Although these factors discussed above have significant effects on the morphology of the nano-crystals prepared, so far previous works on PEL-mediated synthesis of inorganic nano-materials have largely overlooked this important aspect.
We further approached the binding limit issue by quantifying the relationship among pH, α, and [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0. Firstly, by titrating NaOH into a mixture of PAA with different fixed [Cu2+]
[COOH]0. Firstly, by titrating NaOH into a mixture of PAA with different fixed [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 ratios (titration experiments B), pKa-α curves could be plotted based on the modified Henderson–Hasselbalch equation: pKa = pH + log[(1 − α)/α] (the curves are included in the ESI, Fig. S3†). Furthermore, based on these pKa-α curves, we constructed a 3D diagram of pH, α, and [Cu2+]
[COOH]0 ratios (titration experiments B), pKa-α curves could be plotted based on the modified Henderson–Hasselbalch equation: pKa = pH + log[(1 − α)/α] (the curves are included in the ESI, Fig. S3†). Furthermore, based on these pKa-α curves, we constructed a 3D diagram of pH, α, and [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0, shown as the inset in Fig. 1(a) (the analysis details and high resolution 3D diagram are included in the ESI, under the section of ‘Construction of 3D diagram showing the relationship among pH, α, and [Cu2+]:[COOH]’†). Based on this 3D diagram, we could employ numerical interpolations of α to re-define the binding limits as real binding stoichiometry by plotting the measured [Cu2+] versus [Cu2+]
[COOH]0, shown as the inset in Fig. 1(a) (the analysis details and high resolution 3D diagram are included in the ESI, under the section of ‘Construction of 3D diagram showing the relationship among pH, α, and [Cu2+]:[COOH]’†). Based on this 3D diagram, we could employ numerical interpolations of α to re-define the binding limits as real binding stoichiometry by plotting the measured [Cu2+] versus [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COO−]; the results are shown in Fig. 1(b) (bear in mind that the pH is changing during the titration of Cu2+ into the PAA solution). Fig. 1(b) clearly shows that the binding stoichiometry in terms of [Cu2+]
[COO−]; the results are shown in Fig. 1(b) (bear in mind that the pH is changing during the titration of Cu2+ into the PAA solution). Fig. 1(b) clearly shows that the binding stoichiometry in terms of [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COO−] for the PAA solutions of different initial pH values converged towards 0.50. This is an important indication showing that within our experiment window of relatively low pH values, regardless of the α (and its variation) during Cu2+ titration, Cu2+ ions are quantitatively bound to COO− at the ratio of one Cu2+ to two COO− following rule of charge neutrality. This also indicates that Cu2+ hydrolysis is negligible.
[COO−] for the PAA solutions of different initial pH values converged towards 0.50. This is an important indication showing that within our experiment window of relatively low pH values, regardless of the α (and its variation) during Cu2+ titration, Cu2+ ions are quantitatively bound to COO− at the ratio of one Cu2+ to two COO− following rule of charge neutrality. This also indicates that Cu2+ hydrolysis is negligible.
PAA templated synthesis – a new mechanism
Fig. 2 shows the TEM images of CuOx nano-crystals obtained via our PAA-templated synthesis, in which the Cu2+ loading was fixed at just below the binding limit. At first glance, it seemed that nanorod-like structures were obtained. However, closer examination revealed that they were in fact well-crystallized hollow tubular nanostructures. There are two categories of nanotubes: one is slender with a diameter of 10–30 nm and a length of 80–100 nm, having a typical wall thickness of ∼5 nm, the other is wider with a diameter of 40–60 nm and a length of 30–40 nm, having a typical wall thickness of ∼10 nm. Although the assembled structures are not perfectly mono-dispersed in size, the formation mechanism is same for them. The PAA with slightly larger molecular weight PDI that we used could be one of the reasons affecting the structure regularity. As shown in the inset images, the SAED pattern proved that the nanotubes consist of both Cu and Cu2O crystal phases, and this fact is further supported by the lattice spacings measured from high resolution TEM images. As in the previously reported synthesis, it is not unusual that both Cu and CuOx coexist as products.35,36 We believe that pure Cu nanotubes formed first and they were slowly oxidized into Cu2O. The absorbance spectra of the synthesized materials were obtained and are included in the ESI, Fig. S2.†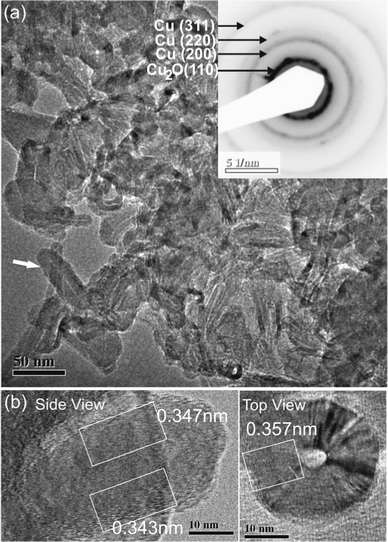 | ||
| Fig. 2 (a) TEM images of the obtained hollow nano-tubular structures. It is proved by SAED (inset image) that the synthesis product is a mixture of Cu and Cu2O phases. (b) Lattice fringes at the edge of the nano-tubes (side view) have a d-spacing of ∼0.34 nm, corresponding to Cu2O (211), while those at the central portion of the nano-tubes have a d-spacing of ∼3.6 nm, corresponding to the Cu (200) plane (top view). | ||
A more intriguing finding is that such nano-tubular structures were not observed in the usual homopolymer mediated synthesis and cannot be simply explained by known mechanisms, which normally involve selective polymer capping or confinement of crystal growth. In fact, the nano-structure as observed in Fig. 2 resembles those obtained via the self-assembly of block copolymers, which is the most plausible scenario.37,38 This finding prompted us to hypothesize that a unique interaction between Cu2+ with PAA is responsible for the obtained regular self-assembled nano-structures. A search of the literature revealed that there are detailed theoretical studies on the charge distribution in PELs based on a single chain using density functional theory (DFT) and Monte Carlo simulations.39–41 The essence of these works is that the charge distribution is non-uniform along a PEL chain in solution: charges (COO− in our case) are concentrated at end segments of the PEL chain due to charge repulsion along the backbone and energy minimization. The charge distribution was reported to be affected by a range of factors including temperature, solvent condition, charge density, Debye length and chain flexibility. In our study, as all these conditions are fixed, we therefore believe the addition of Cu2+ is the critical reason for the change of PAA chain structure. Shown in Fig. 3 are schematic illustrations of the mechanism of how Cu2+ affects the PAA chain conformation, together with the self-assembly of the PAA–Cu2+ complexes into the nano-tubular structure.
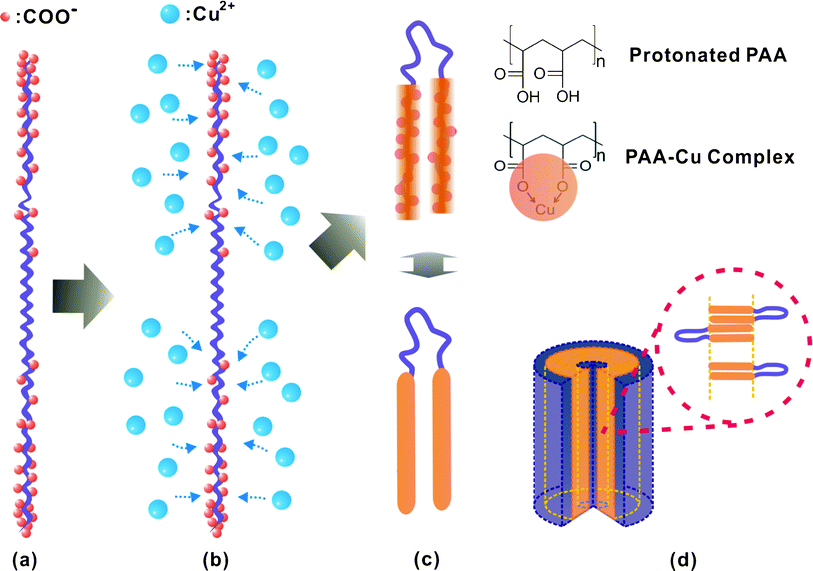 | ||
| Fig. 3 Schematic illustrations of (a) non-uniform charge distribution on a PAA chain, (b) preferential binding of Cu2+ onto the more deprotonated PAA chain ends via electrostatic attraction, (c) formation of a triblock copolymer-like PAA–Cu2+ complex due to change in chain stiffness and hydrophilicity (top right inset images show the PAA–Cu complex forms by complexation of one Cu2+ with two COO−, restricting the rotation), and (d) self-assembly of PAA–Cu2+ into a nano-tubular structure. | ||
Fig. 3(a) illustrates the charged chain structure of a PAA chain in solution at a certain pH value. According to earlier theoretical studies, the equilibrium charge distribution is inhomogeneous and the charge density is higher near the chain ends than that in the central segment, i.e., the PAA chain ends are more deprotonated.39–41 We believe that this non-homogeneous charge distribution and change of the PAA chain conformation during the addition of Cu2+ are ultimately responsible for the nano-tubular structure. Fig. 3(a) illustrates an ideal situation with negligible charge distribution at the centre portion of PAA. This is believed to be a good representation of the actual conditions considering (i) the low charge fraction, which is estimated to be ∼0.36 (i.e. 36% of the COOH are deprotonated at pH 6.6), (ii) aggravated charged non-uniformity found on more flexible polyelectrolyte, (iii) fast complexation of the Cu2+ with the deprotonated COO− acting to quench the non-uniform structure, (iv) Cu2+ lowering the deprotonation energy barrier of its nearby COOH. Furthermore, the negligible Cu2+ binding in the centre might not take place in the non-classical crystallization, simply because of low [Cu2+] in the vicinity. It can be reasonably deduced that, when Cu2+ is added into the PAA solution, preferential binding of Cu2+ to the PAA chain ends occurs rapidly via electrostatic attraction (Fig. 3(b)). The distance between two carboxylic acid functionalities (∼0.3 nm) represents the length scale that determines the strong complexation between the Cu2+ and COO−, which is smaller than the Bjerrum length in aqueous medium (∼0.7 nm). Due to the inter-chain correlated assembly phenomenon, the non-classical crystallization took place within a confined spatial volume defined by the PAA pseudo chain segments lengths. The Cu2+ ions are concentrated near the chain ends, while the central segments of the chains would comparatively have less Cu2+. This site-preferential Cu2+ complexation not only gives rise to a unique structural inhomogeneity of the PAA–Cu2+ complex, it also leads to (i) chain stiffening due to steric hindrance of rotation (note that PEL stiffening effect due to metal ions, in term of persistence length, was discussed in earlier theoretical and experimental studies42,43) and (ii) a change in hydrophilicity (note that the order of hydrophilicity is: COO− > COOH > COO2Cu). Therefore, such unique interactions of Cu2+ with the PAA homopolymer have effectively rendered the homo-PAA chain to possess characteristics of an amphiphilic rod–coil–rod block copolymer, as depicted in Fig. 3(c). As a result of the relatively rigid and more hydrophobic nature of the PAA–Cu2+ complex at the chain ends, and based on the knowledge of block copolymer self-assembly, it is logical that such triblock copolymer-like PAA–Cu2+ complexes could self-assemble into the nano-tubular structure through the mechanism depicted in Fig. 3(d). The central segments folded and the Cu2+ was ‘locked-in’, forming a bi-layered uni-lamellar hollow tubular assembly.44 This type of self-assembly behaviour is unlike those reported for DNA molecules possessing complicated molecular architecture.45 This is because PAA possess simplistic structures, and such assembly is induced by segmental counterion complexation. During the subsequent hydrolysis and heating steps, the nano-tubular crystals formed via a non-classical crystallization route (similar to biomimetic crystallization) since the diffusion of Cu2+ ions is inhibited due to their strong complexation with COO− inside the assembly. The molecular weight of PAA represents an important parameter. When a shorter PAA (10 K g mol−1) was used for the synthesis, spindle-like aggregates formed instead of a nano-tubular CuOx structure (results not shown). This is believed to be due a much higher thermodynamic penalty for chain folding. Furthermore, a long enough PAA chain is required for the triblock copolymer-like behaviour to manifest.
In fact, the mechanism and concept discussed above are of universal nature in terms of types of (i) PELs and metal ions and (ii) complexed assemblies; in other words, they are not restricted to the PAA–Cu2+ system only. However, in order to apply this new mechanism of metal ion binding induced self-assembly, the following prerequisites and conditions need to be considered. Firstly, the molecular weight distribution of the PEL should be sufficiently narrow for the self-assembly of the pseudo block copolymer to occur (PDI of the PAA used in this study is 1.25). Besides that, a suitable PEL concentration needs to be used because non-desirable inter-chain bridging caused by metal ions may take place (before assembly) when the PEL concentration is too high. The initial pH value is another important consideration as it not only determines the metal ion binding limit, high pH also alters or complicates the interaction mechanism due to hydrolysis. Furthermore, the number of metal ions should be sufficient for nano-crystals formation (above the concentration needed for embryo nucleation) while remaining still below the binding limit. While the concept of metal ion induced copolymer-like self-assembly could be extended to other transition metals, each metal ion has its own unique characteristics and interaction behaviour with the PEL.46 Finally, we need to bear in mind that copolymer self-assembly itself is highly sensitive to many intrinsic and extrinsic factors. For example, it is not uncommon that products with multiple morphologies are observed in copolymer self-assembly, e.g., rod-like with spherical micelles.47 In fact, in this work, a small amount of Cu nanoparticles were observed to coexist with the CuOx nanotubes (see ESI, Fig. S4†). Therefore, a detailed systematic study is required each time for a different set of PEL and metal ion.
Conclusions
In this work, unique nano-tubular CuOx crystals were synthesized by employing the PAA homopolymer templated synthesis in aqueous medium. The formation of nano-crystals with this unique morphology is believed to be due to a triblock copolymer-like self-assembly of the PAA–Cu2+ complexes. This complexation induced self-assembly behaviour arised from the combination of the following sequential phenomena; non-uniform charge distribution along the PAA chains, preferential Cu2+ binding on deprotonated carboxyl groups, which concentrate at the chain ends, and copper complexation induced changes in the chain conformation (via chain-stiffening) and hydrophilicity at the chain ends. The initially flexible coil-like PAA chain essentially became a pseudo rod–coil–rod triblock copolymer with rigid rod-like chain ends, while the central segments remained flexible. This is found to be affected by several factors such as pH and PAA concentration, but critically relied on quantitative control of [Cu2+] in the PAA solution in relation to the [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0 binding limit. With caution, the application of this proposed new concept of metal ion induced copolymer-like PEL assembly can be extendable to other PELs and transition metal ions.
[COOH]0 binding limit. With caution, the application of this proposed new concept of metal ion induced copolymer-like PEL assembly can be extendable to other PELs and transition metal ions.
Acknowledgements
This project is funded by Agency for Science, Technology and Research (A-star, Singapore) under MIMO Program and scholarship from Nanyang Technological University (NTU). One of the authors, Y.N. Liang, would like to thank K.C. Tam for his guidance during 6-month exchange in Canada under Canadian Commonwealth Exchange Program. The electron microscopy works were performed at the Facility for Analysis, Characterization, Testing and Simulation (FACTS) in Nanyang Technological University, Singapore.References
- Q. Sun, Y.-L. Zhang, H.-Y. Chen, J.-F. Deng, D. Wu and S.-Y. Chen, J. Catal., 1997, 167, 92–105 CrossRef CAS.
- Z. Zheng, B. Huang, Z. Wang, M. Guo, X. Qin, X. Zhang, P. Wang and Y. Dai, J. Phys. Chem. C, 2009, 113, 14448–14453 CAS.
- C. M. McShane and K.-S. Choi, J. Am. Chem. Soc., 2009, 131, 2561–2569 CrossRef CAS.
- J. Zhang, J. Liu, Q. Peng, X. Wang and Y. Li, Chem. Mater., 2006, 18, 867–871 CrossRef CAS.
- G. Ren, D. Hu, E. W. C. Cheng, M. A. Vargas-Reus, P. Reip and R. P. Allaker, Int. J. Antimicrob. Agents, 2009, 33, 587–590 CrossRef CAS.
- W.-M. Lee, Y.-J. An, H. Yoon and H.-S. Kweon, Environ. Toxicol. Chem., 2008, 27, 1915–1921 CrossRef CAS.
- C.-H. Kuo and M. H. Huang, Nano Today, 2010, 5, 106–116 CrossRef CAS.
- S.-H. Lee, Y.-S. Her and E. Matijevic, J. Colloid Interface Sci., 1997, 186, 193–202 CrossRef CAS.
- Q. Chen, X. Shen and H. Gao, J. Colloid Interface Sci., 2007, 308, 491–499 CrossRef CAS.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc., 2004, 126, 8124–8125 CrossRef CAS.
- Y. Chang and H. C. Zeng, Cryst. Growth Des., 2004, 4, 397–402 CAS.
- T. Wiliana, P. Ravi, J. Yao and K. C. Tam, Nanotechnology, 2006, 17, 5988–5994 CrossRef.
- V. Kozlovskaya, E. Kharlampieva, S. Chang, R. Muhlbauer and V. V. Tsukruk, Chem. Mater., 2009, 21, 2158–2167 CrossRef CAS.
- J. Zhang, H. Liu, Z. Wang, N. Ming, Z. Li and A. S. Biris, Adv. Funct. Mater., 2007, 17, 3897–3905 CrossRef CAS.
- Y. Gotoh, R. Igarashi, Y. Ohkoshi, M. Nagura, K. Akamatsu and S. Deki, J. Mater. Chem., 2000, 10, 2548–2552 RSC.
- Y. Wan and S.-H. Yu, J. Phys. Chem. C, 2008, 112, 3641–3647 CAS.
- B. J. McKenna, J. H. Waite and G. D. Stucky, Cryst. Growth Des., 2009, 9, 4335–4343 CAS.
- E. Falletta, M. Bonini, E. Fratini, A. Lo Nostro, G. Pesavento, A. Becheri, P. Lo Nostro, P. Canton and P. Baglioni, J. Phys. Chem. C, 2008, 112, 11758–11766 CAS.
- I. Pochard, A. Foissy and P. Couchot, Colloid Polym. Sci., 1999, 277, 818–826 CAS.
- C. G. Sinn, R. Dimova and M. Antonietti, Macromolecules, 2004, 37, 3444–3450 CrossRef CAS.
- Z. Iatridi, G. Bokias and J. K. Kallitsis, J. Appl. Polym. Sci., 2008, 108, 769–776 CrossRef CAS.
- A. V. Dobrynin and M. Rubinstein, Prog. Polym. Sci., 2005, 30, 1049–1118 CrossRef CAS.
- R. Kumar, A. Kundagrami and M. Muthukumar, Macromolecules, 2009, 42, 1370–1379 CrossRef CAS.
- K. Huber and U. Scheler, Curr. Opin. Colloid Interface Sci., 2012, 17, 64–73 CrossRef CAS.
- P. Schuetz and F. Caruso, Chem. Mater., 2004, 16, 3066–3073 CrossRef CAS.
- C. Lu, L. Qi, J. Yang, D. Zhang, N. Wu and J. Ma, J. Phys. Chem. B, 2004, 108, 17825–17831 CrossRef CAS.
- M. S. Mo, S. H. Lim, Y. W. Mai, R. K. Zheng and S. P. Ringer, Adv. Mater., 2008, 20, 339–342 CrossRef CAS.
- J. Francois, C. Heitz and M. M. Mestdagh, Polymer, 1997, 38, 5321–5332 CrossRef CAS.
- H. Yokoi, S. Kawata and M. Iwaizumi, J. Am. Chem. Soc., 1986, 108, 3361–3365 CrossRef CAS.
- S. Lages, R. Michels and K. Huber, Macromolecules, 2010, 43, 3027–3035 CrossRef CAS.
- P. G. De Gennes, P. Pincus, R. M. Velasco and F. Brochard, J. Phys., 1976, 37, 1461–1473 CAS.
- A. V. Dobrynin, R. H. Colby and M. Rubinstein, Macromolecules, 1995, 28, 1859–1871 CrossRef CAS.
- M. Moffitt, H. Vali and A. Eisenberg, Chem. Mater., 1998, 10, 1021–1028 CrossRef CAS.
- C. F. Baes and R. E. Mesmer, The hydrolysis of cations, Wiley, New York, 1976 Search PubMed.
- H. Yu, J. Yu, S. Liu and S. Mann, Chem. Mater., 2007, 19, 4327–4334 CrossRef CAS.
- X. Liu, B. Geng, Q. Du, J. Ma and X. Liu, Mater. Sci. Eng., A, 2007, 448, 7–14 CrossRef.
- H.-D. Koh, S. Park and T. P. Russell, ACS Nano, 2010, 4, 1124–1130 CrossRef CAS.
- G.-e. Yu and A. Eisenberg, Macromolecules, 1998, 31, 5546–5549 CrossRef CAS.
- G. Berghold, P. van der Schoot and C. Seidel, J. Chem. Phys., 1997, 107, 8083–8088 CrossRef CAS.
- M. Castelnovo, P. Sens and J. F. Joanny, Eur. Phys. J. E: Soft Matter Biol. Phys., 2000, 1, 115–125 CrossRef CAS.
- T. Zito and C. Seidel, Eur. Phys. J. E, 2002, 8, 339–346 CrossRef CAS.
- E. Nordmeier and W. Dauwe, Polym. J., 1992, 24, 229–238 CrossRef CAS.
- M. N. Spiteri, F. Boué, A. Lapp and J. P. Cotton, Phys. B, 1997, 234–236, 303–305 CrossRef CAS.
- J. Hu, H. Yu, L. H. Gan and X. Hu, Soft Matter, 2011, 7, 11345–11350 RSC.
- M. J. Stevens, Biophysical Journal, 2011, 80, 130–139 CrossRef.
- R. Roma-Luciow, L. Sarraf and M. Morcellet, Eur. Polym. J., 2001, 37, 1741–1745 CrossRef CAS.
- P. R. Majhi, P. L. Dubin, X. Feng, X. Guo, F. A. M. Leermakers and C. Tribet, J. Phys. Chem. B, 2004, 108, 5980–5988 CrossRef CAS.
Footnotes |
† Electronic Supplementary Information (ESI) available: Supporting documents of (i) absorbance spectra of PAA–Cu2+ complex at different fixed [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0; (ii) absorbance spectra of solutions at different stages of synthesis; (iii) pKa-α curves at different fixed [Cu2+] [COOH]0; (ii) absorbance spectra of solutions at different stages of synthesis; (iii) pKa-α curves at different fixed [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0; (iv) analysis details on construction of 3D diagram showing relationship among pH, α, and [Cu2+] [COOH]0; (iv) analysis details on construction of 3D diagram showing relationship among pH, α, and [Cu2+]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [COOH]0; (v) TEM image of spherical Cu nano-crystals formed; had been included. See DOI: 10.1039/c2ra20951a [COOH]0; (v) TEM image of spherical Cu nano-crystals formed; had been included. See DOI: 10.1039/c2ra20951a |
| ‡ Author Contributions: Yen Nan Liang is a PhD student who contributed primarily in terms of the experimental works and writing. Jinhua Hu is a PhD student involved in the project. Xiao Hu is the PhD supervisor of above two PhD students. Kam Chiu Tam is the overseas project collaborator. |
| This journal is © The Royal Society of Chemistry 2012 |
