Self-assembly of a renewable nano-sized triterpenoid 18β-glycyrrhetinic acid†
Braja Gopal
Bag
* and
Rakhi
Majumdar
Department of Chemistry and Chemical Technology, Vidyasagar University, Midnapore 721102, West Bengal, India. E-mail: braja@mail.vidyasagar.ac.in; Fax: 00913222275297
First published on 2nd August 2012
Abstract
The nano-sized triterpenoid 18β-glycyrrhetinic acid extractable from Glycyrrhiza glabra self assembled in different liquids affording mostly nano to microsized spherical and flower like objects consisting of fibrillar networks yielding thermoreversible gels. The self-assemblies have been utilized for the templated growth of CdS nanoparticles.
There has been an immense interest in studying the self-assembly of molecules in aqueous and non-aqueous media for an improved understanding of the self-assembly process in a medium and also because of their tremendous technological and medicinal applications.1–3 Moreover, utilization of plant based chemicals in various facets of science and technology has gained renewed importance in recent years because of its renewable nature in contrast to the petroleum based chemicals that are depleting fast.4 Detailed studies on the self-assembly of different classes of compounds such as amino acids,3 fatty acids,5 sophorolipids,6 steroids etc.1 have been reported. However, there are only a few examples in the literature on the self-assembly of triterpenoids7,8 and examples of the self-assembly of molecules without functional modifications are rare.9,10 High natural abundance of the triterpenoids commonly having more than ten chiral centers render them significant for the study of their self-assembly properties in different liquids.11 Functional triterpenoids with varied rigid and flexible lengths with different functional groups at different positions and orientations might lead to interesting nano and micro-sized architectures. Hence, it occurred to us that the self-assembly property of 18β-glycyrrhetinic acid1, a nanosized 6-6-6-6-6 pentacyclic monohydroxy triterpenic acid can be studied in different liquids even without derivatization.
18β-glycyrrhetinic acid has a rigid 6-6-6-6-6 pentacyclic backbone with an enone moiety in the C-ring and the carboxyl group is attached at the E-ring. Glycyrrhiza glabra root, the main source of 18β-glycyrrhetinic acid,12 is used for sweetening and flavouring food and beverages13 and it has been shown that the sweetening agent glycyrrhizin is converted to 18β-glycyrrhetinic acid in the human intestine.1418β-glycyrrhetinic acid has been shown to be an anti-inflammatory, anticancer and antitumor drug as well as a nutraceutical for remedying lung diseases and its in vivo assays have also been carried out.13,15 According to our knowledge, self-assembly of 18β-glycyrrhetinic acid without derivatization has not been reported so far. Herein we report an improved method for its isolation from Glycyrrhiza glabra root and its self-assembly properties in different liquids along with the morphological characteristics of the self-assemblies. The spherical and flower-like self-assemblies of the triterpenoid consisting of fibrillar networks have been utilized for the templated growth of CdS nanoparticles
The compound 1 was isolated from the root of Glycyrrhiza glabra following an improved method developed in our laboratory (see Experimental section‡ and the Supporting Information, ESI†).16 For self assembly studies, 1–5 mg of 1 contained in a vial (capacity 4 mL, 1 cm inner diameter (i.d.)) was dissolved in a liquid by heating over a hot plate with magnetic stirring and the resulting solution was allowed to cool at room temperature. No gravitation flow of the material after waiting for several seconds indicated the formation of a gel. Semi-transparent gels were obtained (Fig. 1b) in 15 liquids among the 22 liquids tested (Table 1). All the aromatic liquids were gelled within 1 h and the gels were stable for several months when stored at room temperature under sealed conditions. Whereas compound 1 crystallized or precipitated out from solutions in methanol, ethanol and propanol, strong gels were formed in both ethylene glycol and glycerol. All the four aliphatic chloro solvents tested formed partial gels. Strong gels were obtained in DMSO–water and DMF–water mixtures (Table 1 and 2). With increasing percentage of water the Tgel values increased (Table 2).
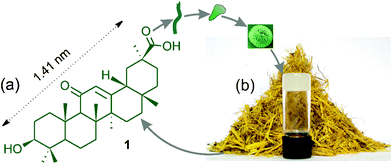 | ||
| Fig. 1 (a,b): Schematic representation of self-assembly of 18β-glycyrrhetinic acid1 affording spherical and flower-like objects yielding a gel. (b) A gel of 1 in bromobenzene with crushed Glycyrrhiza glabra root at the background. | ||
| Entry | Solvent | State | MGC | T gel (°C) |
|---|---|---|---|---|
| a MGC is the minimum gelator concentration (% w/v) at which the gel formed at room temperature. G = gel, PG = Partial gel. C = crystal, P = precipitate. Tgel values given here are at the corresponding MGC's. | ||||
| 1 | Benzene | G | 1.0 | 34 |
| 2 | Toluene | G | 1.7 | 60 |
| 3 | o-Xylene | G | 1.1 | 38 |
| 4 | m-Xylene | G | 0.71 | 45 |
| 5 | p-Xylene | G | 1.0 | 35 |
| 6 | Mesitylene | G | 0.71 | 37 |
| 7 | Bromobenzene | G | 3.0 | 37 |
| 8 | Chlorobenzene | G | 2.0 | 34 |
| 9 | o-Dichlorobenzene | G | 2.4 | 32 |
| 10 | Nitrobenzene | G | 5.0 | 35 |
| 11 | p-Methoxybenzaldehyde | G | 5.0 | 55 |
| 12 | Methanol | P | 0.36 | - |
| 13 | Ethanol | C | 0.87 | - |
| 14 | n-Propanol | P | 1.33 | - |
| 15 | Ethylene Glycol | G | 1.25 | 35 |
| 16 | Glycerol | G | 1.6 | 38 |
| 17 | Dichloromethane | PG | 1.0 | - |
| 18 | Chloroform | PG | 10.0 | - |
| 19 | 1,1,2,2-Tetrachloroethane | PG | 10.0 | - |
| 20 | Carbontetrachloride | PG | 0.66 | - |
| 21 | DMSO–Water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
G | 5.0 | 73 |
| 22 | DMF–Water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
G | 6.3 | 49 |
The thermoreversibility of gel to sol transitions, confirmed by repeated heating and cooling, allowed us to plot the gel to sol transition temperatures Tgelvs. % gelator concentration (Fig. 2). The Tgel value of the chlorobenzene gel was 34 °C at MGC (2.0% w/v) and increased to 62 °C at 5.0% w/v concentration. The Tgel values for the gels in bromobenzene and o-dichlorobenzene were comparable (32–37 °C) at their MGCs (2–3% w/v) and increased steadily with increasing concentrations of the gelator. The increase of the Tgel with increasing gelator concentration indicated stronger intermolecular associations at higher concentrations.
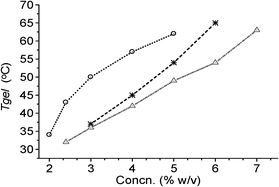 | ||
| Fig. 2 Plots of Tgelvs. concentration for 1 in: (a) chlorobenzene (-○-); (b) bromobenzene (-*-); (c) o-dichlorobenzene (-Δ-). | ||
The various thermodynamic parameters (ΔH°, ΔS° and ΔG°) during gel to sol phase transitions were calculated from the variation of Tgel with concentrations (Table 3).17 The free energy changes in chlorobenzene, bromobenzene and o-dichlorobenzene were identical though their enthalpy and entropy changes were different (Table 3). The positive free energy changes obtained in all the cases studied indicated the stability of the gels.
| Liquid | ΔH°/kJ mol−1 | ΔS°/J mol−1 °K−1 | ΔG°/kJ mol−1 |
|---|---|---|---|
| Chlorobenzene | 28.1 | 64.7 | 8.8 |
| Bromobenzene | 20.7 | 44.5 | 7.5 |
| o-Dichlorobenzene | 30.5 | 75.6 | 8.0 |
The scanning electron micrographs of the self-assemblies of 18β-glycyrrhetinic acid in o-dichlorobenzene and nitrobenzene at 0.25 % (w/v) concentration are shown in Fig. 3, 4, and 6. Flower-like spherical objects of 5–10 μm diameters were observed in o-dichlorobenzene (Fig. 3a–3c) and microsized flowers and petals were observed in nitrobenzene (Fig. 4). Petals of 33–88 nanometer thickness were observed in both the cases (Fig. 3, 4 and Fig. S2 and S3, ESI†). Nano and micro sized spherical objects were also observed in DMSO–water (Fig. S4, ESI†).
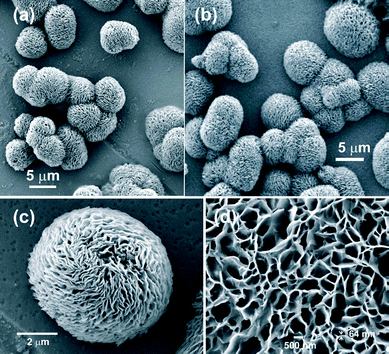 | ||
| Fig. 3 FESEM image of 18β-glycyrrhetinic acid xerogel at 0.25 % (w/v) concentration in: (a–c) o-dichlorobenzene at different magnifications, (d) nitrobenzene. | ||
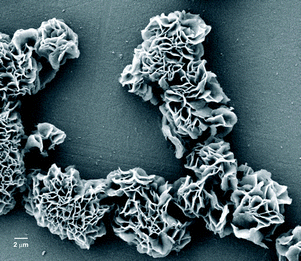 | ||
| Fig. 4 FESEM image of 18β-glycyrrhetinic acid xerogel in nitrobenzene (0.25 % w/v). | ||
The optical microscopy images of a solution of 18β-glycyrrhetinic acid in ethylene glycol (0.91 % w/v) revealed spherical microstructures (Fig. 5a, 5c and Fig. S5, ESI†) with an average diameter of 9.78 ± 0.13 μm (calculated from the individual diameters of 60 spheres). Such microstructures were also observed in p-methoxybenzaldehyde, nitrobenzene and o-dichlorobenzene (Fig. S5, ESI†). Atomic force microscopy (AFM) of a solution of 18β-glycyrrhetinic acid in p-methoxybenzaldehyde (1.25 % w/v) revealed nano-sized spherical objects of 20–75 nm diameters (Fig. 5b, 5d and Fig. S6, ESI†). Such spherical nano objects were also observed in nitrobenzene and DMSO-water (Fig. S7 and S8, ESI†).
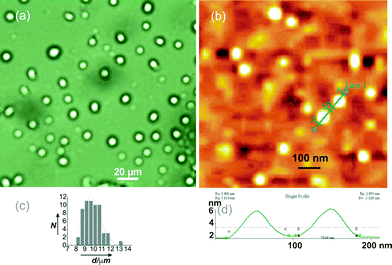 | ||
| Fig. 5 (a) OPM Image of 18β-glycyrrhetinic acid solution in ethylene glycol (0.91 % w/v) showing spherical objects, (b) AFM image of a dried sample prepared from a solution of 1 in p-methoxybenzaldehyde (1.25 %), (c) histogram of the diameters of the spherical objects observed in Fig. 5a, (d) height profile of the two spherical objects observed in Fig. 5b. | ||
The flower-like spherical objects (Fig. 3) and the flower and the petal-like objects (Fig. 4) observed in the xerogel from o-dichlorobenzene and nitrobenzene by field emission scanning electron microscopy (FESEM) consist of 2D-sheets of 33–88 nm thickness (Fig. 6a and Fig. S2 and S3, ESI†) and the 2D sheets consist of intertwined fibers of 30–40 nm cross-sections (Fig. 6b). The cross-sections of the fibers must contain several 18β-glycyrrhetinic acid molecules.
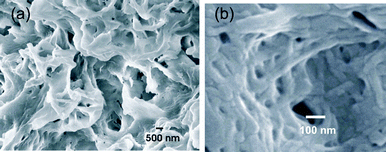 | ||
Fig. 6 FESEM image of 18β-glycyrrhetinic acid xerogel in o-dichlorobenzene (0.25 % w/v) at (a) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification, (b) 83 000× magnification, (b) 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. 000× magnification. | ||
Wide angle X-ray diffraction studies of xerogels from chlorobenzene, bromobenzene, nitrobenzene, o-dichlorobenzene and DMSO were carried out and their diffraction patterns were compared (Fig. S10 and Table S1, ESI†). Identical diffraction patterns in all the samples indicated identical self-assembled structures in the xerogels. An X-ray diffractogram of the o-dichlorobenzene gel (5 % w/v) of 18β-glycyrrhetinic acid revealed two broader peaks in the 2θ = 10–25° region due to heavy scattering by the solvent molecules. However, a sharp peak observed at 2θ = 8.19° (d = 10.8 Å) along with the broader peaks in the gel sample matched with that in the xerogels indicating similar order structures in the gel. The self assembly of the molecules (as represented schematically in Fig. 1) is believed to be due to the intermolecular forces such as H-bonding involving the carboxyl and hydroxyl groups in addition to the dispersive interactions by the triterpenoid backbones. The involvement of H-bonding by the carboxyl groups was evident by FTIR where a shift of the ‘C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O’ stretching frequency took place from 1705 cm−1 in a powder sample to 1695 cm−1 in the gel sample of 1 in o-dichlorobenzene (Fig. S11a, ESI†). The ‘C
O’ stretching frequency took place from 1705 cm−1 in a powder sample to 1695 cm−1 in the gel sample of 1 in o-dichlorobenzene (Fig. S11a, ESI†). The ‘C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O’ stretching frequencies of the xerogels prepared from chlorobenzene, bromobenzene, nitrobenzene, o-dichlorobenzene and DMSO appeared at 1702, 1702, 1702, 1703 and 1705 cm−1 respectively (Fig. S11b, ESI†) indicating a shift of 2–3 cm−1 (except the xerogel from DMSO) compared to that in the powder. The identical ‘C
O’ stretching frequencies of the xerogels prepared from chlorobenzene, bromobenzene, nitrobenzene, o-dichlorobenzene and DMSO appeared at 1702, 1702, 1702, 1703 and 1705 cm−1 respectively (Fig. S11b, ESI†) indicating a shift of 2–3 cm−1 (except the xerogel from DMSO) compared to that in the powder. The identical ‘C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O’ stretching frequencies in the xerogels indicated that the ‘C
O’ stretching frequencies in the xerogels indicated that the ‘C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O’ groups were in identical H-bonded environments.
O’ groups were in identical H-bonded environments.
Templated growth of CdS nanoparticles in the form of nano and micro sized spherical objects and fibers has become an area of intense research in recent years because of their applications in industry as well as advanced areas such as biological labelling, light emitting diodes, photoelectronic devices, etc.18–21 To examine whether the self-assembled nano and microstructures of the 18β-glycyrrhetinic acid can be used for the templated growth of semiconductor nano particles, we investigated the formation of CdS nanoparticles in a solution of 18β-glycyrrhetinic acid in ethylene glycol. When a solution of 18β-glycyrrhetinic acid contained in a vial (capacity 4 mL, 1 cm i.d.) in ethylene glycol (0.2 mL, 1 % w/v) containing cadmium acetate (5 mM) was kept under H2S atmosphere for 30 s, a light yellow color appeared almost instantly. High resolution transmission electron microscopy (HRTEM) images (Fig. 7), energy dispersive X-ray spectroscopy (EDX) and X-ray diffraction confirmed the formation of CdS nanoparticles on the self-assembled microstructures (Fig. S14 and S15, ESI†).
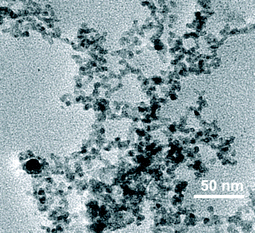 | ||
| Fig. 7 HRTEM image of CdS nanoparticles deposited on 18β-glycyrrhetinic acid self-assemblies. | ||
Examination of the microstructures under a polarized optical microscope with fluorescence attachment indicated the templated formation of greenish fluorescent spherical objects (Fig. S12, ESI†).22 Upon excitation at 395 nm, an intense emission peak at 447 nm also supported the formation of CdS nano particles (Fig. S13, ESI†).
This study demonstrates that the 1.41 nm long, 18β-glycyrrhetinic acid, having a rigid, 6-6-6-6-6 pentacyclic backbone with the hydroxyl and the carboxyl groups separated by 1.09 nm rigid spacer (Fig. S1, ESI†) self-assembles in different liquids forming nano to microsized spherical and flower-like objects having fibers of nano-meter diameters affording strong gels in 15 liquids and partial gels in 4 liquids. The self assembled nano to microstructures of the triterpenoid have been utilized for the templated growth of CdS nano particles. To the best of our knowledge, this is the first report of the self-assembly of a 6-6-6-6-6 pentacyclic monohydroxy triterpenoid, without structural modification, in different liquids and the results described here will be useful for its applications in biomedicine as well as nanoscience and nanotechnology. Minute structural variations in the triterpenoids leading to different morphologies and nanoarchitechtures by self-assembly in different liquids can be tested on many such functional nano entities, some of which are under investigation in our laboratory and will be reported in due course.
We gratefully thank CSIR for financial support. RM thanks UGC for a research fellowship.
References
- (a) E. Carretti, M. Bonini, L. Dei, B.H. Berrie, L.V. Angelova, P. Baglioni and R.G. Weiss, Acc. Chem. Res., 2010, 43, 751–760 CrossRef CAS; (b) M. Suzuki and K. Hanabusa, Chem. Soc. Rev., 2009, 38, 967–975 RSC; (c) M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489–497 CrossRef CAS; (d) R.G. Weiss, P. Terech, in : Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, 2006 Search PubMed; (e) J. H. van Esch and B. L. Feringa, Angew. Chem., Int. Ed., 2000, 39, 2263–2266 CrossRef CAS.
- (a) S. Dutta and S. Bhattacharya, Chem. Commun., 2012, 48, 877–879 RSC; (b) S. Bhattacharya and J. Biswas, Langmuir, 2011, 27, 1581–1591 CrossRef CAS; (c) S. Bhattacharya, A. Srivastava and A. Pal, Angew Chem. Int. Ed., 2006, 45, 2934 CrossRef CAS.
- (a) D. Das, T. Kar and P. K. Das, Soft Matter, 2012, 8, 2348–2365 RSC; (b) P. Koley and A. Pramanik, Adv. Funct. Mater., 2011, 21, 4126–4136 CrossRef CAS.
- (a) P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS; (b) P. K. Vemula and G. John, Acc. Chem. Res., 2008, 41, 769 CrossRef CAS; (c) G. John, B.V. Shankar, S.R. Jadhav and P.K. Vemula, Langmuir, 2010, 26, 17843–17851 CrossRef CAS.
- (a) M. Delample, F. Jerome, J. Barrault and J.-P. Douliez, Green Chem., 2011, 13, 64 RSC; (b) B. Novales, L. Navailles, M. Axelos, F. Nallet and J.-P. Douliez, Langmuir, 2008, 24, 62–68 CrossRef CAS; (c) J.-P. Douliez, J. Am. Chem. Soc., 2005, 127, 15694–15695 CrossRef CAS.
- (a) N. Baccile, N. Nassif, L. Malfatti, I.N.A. Van Bogaert, W. Soetaert, G. Pehau-Arnaudet and F. Babonneau, Green Chem., 2010, 12, 1564–1567 RSC; (b) S. Zhou, C. Xu, J. Wang, W. Gao, R. khverdiyeva, V. Shah and R. Gross, Langmuir, 2004, 20, 7926–7932 CrossRef CAS.
- (a) B.G. Bag, S.K. Dinda, P.P. Dey, V.A. Mallia and R.G. Weiss, Langmuir, 2009, 25(15), 8663–8671 CrossRef CAS; (b) B.G. Bag and S.K. Dinda, Pure Appl Chem, 2007, 79, 2031–2038 CrossRef CAS; (c) B.G. Bag, G.C. Maity and S.K. Dinda, Org. Lett., 2006, 8, 5457 CrossRef CAS.
- (a) J. Hu, J. Lu, R. Lia and Y. Ju, Soft Matter, 2011, 7, 891 RSC; (b) J. Lu, J. Hu, Y. Song and Y. Ju, Org. Lett., 2011, 13, 3372 CrossRef CAS; (c) J. Hu, M. Zhanga and Y. Ju, Soft Matter, 2009, 5, 4971–4974 RSC.
- S. Grassi, E. Carretti, L. Dei, C.W. Branham, B. Kahr and R.G. Weiss, New J. Chem., 2011, 35, 445–452 RSC.
- B.G. Bag and S.S. Dash, Nanoscale, 2011, 3, 4564–4566 RSC.
- B.G. Bag, C. Garai, R. Majumdar and M. Laguerre, Struct. Chem., 2012, 23, 393–398 CrossRef CAS.
- L. Rackova, V. Jancinova, M. Petrikova, K. Drabikova, R. Nosal, M. Stefek, D. Kostalova, N. Pronayova and M. Kovacova, Nat Prod Res, 2007, 21, 1234–1241 CrossRef CAS.
- T.-C. Kao, M.-H. Shyu and G.-C. Yen, J. Agric. Food Chem., 2010, 58, 8623–8629 CrossRef CAS.
- D.H. Kim, S.W. Hong, B.T. Kim, E.A. Bae, H.Y. Park and M.J. Han, Arch Pharm Res, 2000, 23, 172–177 CrossRef CAS.
- T. Ikeda, K. Yokomizo, M. Okawa, R. Tsuchihashi, J. Kinjo, T. Nohara and M. Uyeda, Biol Pharm Bull, 2005, 28, 1779–1781 CAS.
- M. Amani, R. Sotudeh-Gharebagh, N. Mostouti and H. A. Motahhari Kashani, J. Food. Tech., 2005, 3, 576–580 Search PubMed.
- D. Rizkov, J. Gun, O. Lev, R. Sicsic and A. Melman, Langmuir, 2005, 21, 12130 CrossRef CAS.
- S. Rengaraj, S. Venkataraj, S.H. Jee, Y. Kim, C.-W. Tai, E. Repo, A. Koistinen, A. Ferancova and M. Sillanp, Langmuir, 2011, 27, 352–358 CrossRef CAS.
- Y. Ma, L. Qi, J. Ma, H. Cheng and W. Shen, Langmuir, 2003, 19, 9079–9085 CrossRef CAS.
- D. Wu, X. Ge, Z. Zhang, M. Wang and S. Zhang, Langmuir, 2004, 20, 5192–5195 CrossRef CAS.
- J.-S. Hu, Y.-G. Guo, H.-P. Liang, L.-J. Wan, C.-L. Bai and Y.-G. Wang, J. Phys. Chem. B, 2004, 108, 9734–9738 CrossRef CAS.
- K.-S. Ock, E.-O. Ganbold, S. Jeong, J.-H. Seo and S.-W. Joo, Bull. Korean Chem. Soc., 2011, 32, 3610–3613 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Energy minimized structure and detailed characterizations of 1, additional SEM, AFM, OPM images of the self-assemblies 1 in different liquids, calculation of the thermodynamic parameters, X-ray diffractograms, FTIR, epifluorescence images and absorption and emission spectra of templated CdS, HRTEM and EDX spectrum of templated CdS nanoparticles. See DOI: 10.1039/c2ra21051g/ |
‡ Experimental Section 18β-Glycyrrhetinic acid1. The crushed and dried root of Glycyrrhizia glabra (300 g) was extracted with distilled water (700 mL × 3) and the water extract was cooled in ice and acidified with conc. H2SO4 (20 mL). The resulting brown precipitate was washed with distilled water (25 mL × 3) and dried under reduced pressure. The crude glycyrrhezinic acid (obtained as a light brown solid, 11.64 g) was hydrolyzed by stirring at room temperature (30 °C – 35 °C) with hydrochloric acid (4 N) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–water mixture (80 mL) for 48 hours. The volatiles were removed under reduced pressure and the crude product, separated from the aqueous layer by centrifugal force, was purified by column chromatography (twice) using 15 % ethyl acetate–dichloromethane as the eluant and decolorized with charcoal to afford the desired product as a white fibrous crystalline solid (1.60 g, 0.53 %). HRMS: calculated for C30H46NaO4 493.3288, obtained 493.3275, 1H-NMR (600 MHz, DMSO-d6) δ: 12.19 (s, 1H), 5.40 (s, 1H), 4.31 (d, J = 4.2 Hz, 1H), 2.99-3.03 (m, 1H), 2.59-2.56 (m, 1H), 2.32 (s, 1H), 1.34 (s, 3H), 1.10 (s, 3H), 1.03 (s, 3H), 1.02 (s, 3H), 0.90 (s, 3H), 0.75 (s, 3H), 0.68 (s, 3H), 2.10 – 0.66 (m, triterpenoid protons, 19 H’s). 13C-NMR (150 MHz, DMSO-d6) δ: 199.0, 177.7, 169.7, 127.3, 76.6, 61.1, 54.1, 48.1, 44.9, 43.1, 42.9, 40.6, 38.8, 38.5, 37.5, 36.7, 32.1, 31.5, 30.4, 28.4, 28.1, 27.8, 27.0, 26.1, 25.8, 23.0, 18.4, 17.2, 16.2, 16.0. (see Supporting Information for additional data). Templated growth of CdS nanoparticles: When a solution of 18β-Glycyrrhetinic acid contained in a vial (capacity 4 mL, 1 cm i.d.) in ethylene glycol (0.2 mL, 1 % w/v) containing cadmium acetate (5 mM) was kept under H2S atmosphere for 30 s, a light yellow color appeared almost instantly. Examination of the microstructures under polarized optical microscope with fluorescence attachment indicated the templated formation of greenish fluorescent spherical objects (Fig. S12, ESI†). Upon excitation at 395 nm, a strong emission at spectrum at 447 nm indicated the formation of the templated CdS nanoparticles (Fig. S13). HRTEM, EDX and XRD studies confirmed the templated growth of CdS nanoparticles (Fig. S14 and S15, ESI†). 1 ethanol–water mixture (80 mL) for 48 hours. The volatiles were removed under reduced pressure and the crude product, separated from the aqueous layer by centrifugal force, was purified by column chromatography (twice) using 15 % ethyl acetate–dichloromethane as the eluant and decolorized with charcoal to afford the desired product as a white fibrous crystalline solid (1.60 g, 0.53 %). HRMS: calculated for C30H46NaO4 493.3288, obtained 493.3275, 1H-NMR (600 MHz, DMSO-d6) δ: 12.19 (s, 1H), 5.40 (s, 1H), 4.31 (d, J = 4.2 Hz, 1H), 2.99-3.03 (m, 1H), 2.59-2.56 (m, 1H), 2.32 (s, 1H), 1.34 (s, 3H), 1.10 (s, 3H), 1.03 (s, 3H), 1.02 (s, 3H), 0.90 (s, 3H), 0.75 (s, 3H), 0.68 (s, 3H), 2.10 – 0.66 (m, triterpenoid protons, 19 H’s). 13C-NMR (150 MHz, DMSO-d6) δ: 199.0, 177.7, 169.7, 127.3, 76.6, 61.1, 54.1, 48.1, 44.9, 43.1, 42.9, 40.6, 38.8, 38.5, 37.5, 36.7, 32.1, 31.5, 30.4, 28.4, 28.1, 27.8, 27.0, 26.1, 25.8, 23.0, 18.4, 17.2, 16.2, 16.0. (see Supporting Information for additional data). Templated growth of CdS nanoparticles: When a solution of 18β-Glycyrrhetinic acid contained in a vial (capacity 4 mL, 1 cm i.d.) in ethylene glycol (0.2 mL, 1 % w/v) containing cadmium acetate (5 mM) was kept under H2S atmosphere for 30 s, a light yellow color appeared almost instantly. Examination of the microstructures under polarized optical microscope with fluorescence attachment indicated the templated formation of greenish fluorescent spherical objects (Fig. S12, ESI†). Upon excitation at 395 nm, a strong emission at spectrum at 447 nm indicated the formation of the templated CdS nanoparticles (Fig. S13). HRTEM, EDX and XRD studies confirmed the templated growth of CdS nanoparticles (Fig. S14 and S15, ESI†). |
| This journal is © The Royal Society of Chemistry 2012 |
