Transesterification of vegetable oil with subcritical methanol using heterogeneous transition metal oxide catalysts†
Tomoharu
Oku
*,
Masanori
Nonoguchi
,
Toshimitsu
Moriguchi
,
Hiroko
Izumi
,
Atsushi
Tachibana
and
Takeo
Akatsuka
Strategic Technology Research Center, Nippon Shokubai Co. Ltd., 5-8, Nishi Otabi-cho, Suita, Japan. E-mail: tomoharu_oku@shokubai.co.jp; Fax: +81 6 6317 2992; Tel: +81 6 6317 2252
First published on 6th August 2012
Abstract
Transesterification of vegetable oil with subcritical methanol over newly-developed heterogeneous Mn catalysts for the production of biodiesel fuel is reported. The dense CH3OH can improve the catalytic performance as a result of the effective removal of the reaction products with methylation of the intermediate carboxylates on the catalyst surface.
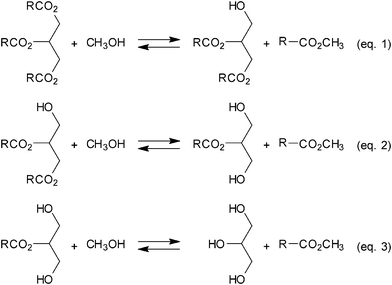
The utilization of biomass resources as a new energy source or chemical raw materials attracts much attention because they are not only alternatives for exhaustible resources but also support global warming policies. One of the most widespread practical applications of biomass is the production of biodiesel fuel (BDF), namely fatty acid methyl esters (FAME) from vegetable oils.1 BDF is conventionally produced by transesterification of vegetable oil with methanol using a homogenous alkaline catalyst, NaOH or NaOCH3etc., as shown in Scheme 1. Although the homogeneous catalyst systems provide reasonable productivity, the complicated catalyst removal process causes an increase in wastes and a serious decrease in the product yields and quality. In order to simplify the process, various heterogeneous metal-oxide based basic catalysts have been developed, however, most of them have serious drawbacks such as catalyst leaching and poor durability.2 In fact, during reactions large amounts of Mg and Al ions were leached from MgO/Al2O3 hydrotalcite catalysts. On the other hand, since ordinary solid acid catalysts1 required relatively harsh reaction conditions for a smooth reaction, the undesired decomposition of glycerin and methanol led to a serious decrease in the productivity and selectivity. Thus, the solid acid or base catalysts suffered from a dilemma of activity/selectivity and durability for BDF synthesis. Moreover, Yoo et al.3 has recently reported that unary metal oxide catalysts (SrO, CaO, ZnO, TiO2 and ZrO2) can promote the transesterification of rape seed oil with supercritical or subcritical methanol using a batch-type reactor; but even the optimal ZnO catalyst can dissolve slightly in FAME during a reaction. Recently, the Hillion group at the Institut Français du Petrole4 has reported that a mixed oxide of Zn and Al can promote the transesterification of triglycerides using a continuous-flow fixed-bed reactor under the subcritical condition of methanol, 200 °C (Tc = 239.5 °C), in a heterogeneous manner without serious catalyst leaching. These results prompted us to investigate the transesterification of vegetable oil with supercritical (SCF) or subcritical methanol (SubCF) as a reaction medium. Previously, Ikariya and Oku5 reported that the use of supercritical or subcritical methanol as a reaction medium and a reactant significantly improved the catalytic performance of solid catalysts and promoted methylation of organic compounds thanks to its unique properties such as high diffusivity and reasonable high solubility of organic compounds. Although the catalytic reactions over the solid catalysts6 in SCF with unique physicochemical properties are a promising approach to attain highly efficient catalytic reactions, it still remains relatively unexplored. In particular, the synthesis of BDF over solid catalysts in near-supercritical methanol conditions is not well understood. Here we report on a manganese heterogeneous catalyst with high activity and long catalyst lifetime for the transesterification of vegetable oil using subcritical methanol (subCH3OH) as a reactant and a compatible solvent. This unique solvent can dissolve both non-polar fatty acid esters (vegetable oil and FAME) and polar glycerin (by-product).
 | ||
| Scheme 1 | ||
Firstly, we investigated the phase behavior of vegetable oil, FAME, and CH3OH under various conditions by using a sapphire cell. Visual inspection revealed that under subcritical CH3OH conditions, 200 °C at 5 MPa, all organic compounds were dissolved, resulting in a single liquid phase. Table 1 shows some results of screening tests of various binary transition metal oxide catalysts7 for the reaction of triolein with subCH3OH using a batch reactor at 200 °C and autogenous pressure. All catalysts tested exhibited good activity toward the transesterification without any serious leaching of the active species, except for the catalysts listed in entries 9, 10 and 13 in Table 1. Contrary to these metal oxide catalysts, the hydrotalcite catalyst suffered from a serious leaching of Mg and Al metal ions at milder conditions, 150 °C (entry 14, Table 1). Among them, the most efficient catalysts were the crystalline ilmenites, FeTiO3, MnTiO3, and the mixed oxide consisting of Mn and Al calcined at high temperature. The MnTiO3 catalyst can promote transesterification of variously vegetable oil, palm, rape-seed, canola and coconut oil as listed in Table 2. Also of note, short-chain triglycerides were convertible with a reasonably high rate to produce glycerin in good yield. The discrepancies between the yields of FAME and glycerin in Table 1 and 2 can be explained by the formation of mono- and diglycerides as intermediates in such incomplete reactions.
| Entry | Catalyst | Yield, mol (%) | Leaching conc.,b ppm | |
|---|---|---|---|---|
| FAME | Glycerin | |||
| a Conditions: under autogenous pressure at 200 °C for 24 h, 60 g of triolein, 20 g of CH3OH and 2.5 g of catalyst powder in a 200 ml stainless-steel vessel. b n.d. means ‘not detected’. c Exceptional temperature is 150 °C. | ||||
| 1 | ZnTiO3 | 83 | 36 | n.d. |
| 2 | NiTiO3 | 63 | 63 | n.d. |
| 3 | CoTiO3 | 77 | 41 | n.d. |
| 4 | FeTiO3 | 94 | 94 | n.d. |
| 5 | MnTiO3 | 87 | 49 | n.d. |
| 6 | Mn–Al mixed oxide | 94 | 61 | n.d. |
| 7 | TS-1 | 76 | 53 | n.d. |
| 8 | TiO2 on ZrO2 | 79 | 79 | Ti: <1 Zr: n.d. |
| 9 | Ti0.5Zr0.5O2 | 69 | 63 | Ti: <1 Zr: n.d. |
| 10 | HTiNbO5 | 80 | 56 | n.d. |
| 11 | CoV2O7 | 43 | 15 | n.d. |
| 12 | CeVO4 | 65 | 24 | Ce: n.d. V: 250 |
| 13 | Hydrotalcitec | 77 | 63 | Mg: 17800 Al: 6900 |
Based on the results obtained using the batch-type reactor, we then examined the reaction of refined palm oil with subCH3OH over the MnTiO3 catalyst using the continuous-flow fixed-bed reactor under the conditions of 200 °C at 5 MPa at various contact-times. Fig. 1 shows the contact-time course dependence of the product distributions of transesterification, indicating that the transesterification reaction proceeded consecutively and reversibly (Scheme 1). When the flow ratio of palm oil/methanol = 1/1 by weight and the liquid hourly space velocity, LHSV (ml-liquid ml-cat−1 h−1) is 1 h−1, which was calculated based on the liquid flow rate of reactants per volume of catalyst bed, the catalyst maintained its catalytic activity for more than 1000 h.
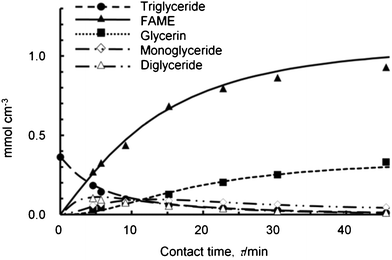 | ||
| Fig. 1 Contact-time course of the product distributions over the MnTiO3 catalyst. | ||
In order to gain further insights into the mechanism of the catalyst, the adsorbates to the surface of the MnTiO3 catalyst were evaluated by IR spectroscopy of the used catalyst which was washed with butanol at room temperature and dried under vacuum. As shown in Fig. 2, characteristic signals due to the metal carboxylate adsorbates were observed and then the signals disappeared after reuse in a batch reactor under otherwise identical conditions. Manganese diacetate as a model compound of the possible catalyst intermediates is known to readily react with alcohol to give manganese oxide which could further react with acetic acid to regenerate the diacetate complex.8 In fact, methylacetate was generated in a separate experiment of a reaction of manganese diacetate with excess methanol under atmospheric pressure and reflux for 8 h.
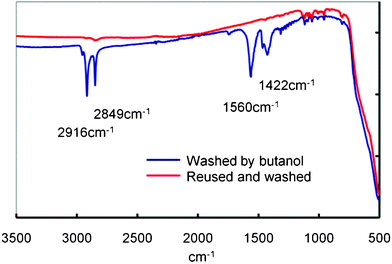 | ||
| Fig. 2 Infrared spectra of the MnTiO3 catalyst used. | ||
These experimental results strongly suggested the key role of the metal oxide catalyst during the catalysis. Firstly, the reaction of triglyceride with the metal hydroxide on the surface would afford diglyceride and the surface carboxylate species. The resulting carboxylate species bonded to the surface and released the desired esters, FAME, by a similar reaction to the model reaction, regenerating the metal hydroxide species (Scheme 2). Subcritical CH3OH promotes the transesterification with the help of the hydroxide units on the surface by effective removal of the surface carboxylate to simultaneously generate FAME. In a similar way, the diglyceride efficiently gives glycerin and two molecules of FAME through the monoglyceride as shown in Scheme 1, eqn (2) and (3). Notably, these reactions might proceed reversibly, and consequently a small amount of the mono-glycerides intermediates remains in the product FAME due to thermodynamic reasons.
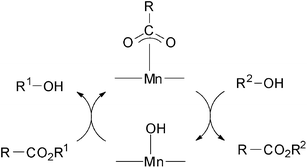 | ||
| Scheme 2 | ||
The residual monoglycerides were further converted into glycerin and FAME by using the bench-scale consecutive two-stage fixed-bed reactor,4 almost achieving a quantitative reaction over the MnTiO3 catalyst. The transesterification of refined palm oil with subCH3OH in the first reactor, under the conditions described in Table 3, proceeded smoothly to give a mixture of FAME (91.1 mol%) and glycerin (82.5 mol%) with 14.2 mol% of glycerides as the intermediates at 96.7% conversion. After the excess methanol was removed by the flash evaporator, the ester phase containing FAME and glycerides obtained from the simple separation of immiscible glycerin (see ESI†) reacted with fresh subCH3OH in the second reactor to afford the desired FAME and glycerin with 99.4 mol% and 98.4 mol% as the overall yield, respectively (Table 3).
| Reactor | Conditions | Result, mol (%) |
|---|---|---|
| a RPO = refined palm oil. b Yield of glycerides = the sum of monoglyceride and diglyceride intermediates. | ||
| 1st | RPOa/CH3OH = 1/1 by wt. | Conv. = 96.7 |
| 200 °C, 5.0 MPa, LHSV = 0.7 h−1 | Yield = 91.1 (FAME) | |
| 82.5 (Glycerin) | ||
| 14.2 (Glyceridesb) | ||
| 2nd | Ester phase/CH3OH = 1/1 by wt. | Conv. = 100 |
| 200 °C, 5.0 MPa, LHSV = 1.0 h−1 | Yield = 99.4 (FAME) | |
| 98.4 (Glycerin) | ||
| 1.6 (Glyceridesb) | ||
Practical advantages in the Mn–Al mixed oxide catalyst system were demonstrated by its high catalytic performance in terms of the activity and durability, compared to the MnTiO3 catalyst as shown in Fig. 3. Notably, no serious catalyst deactivation was observed even after 5000 h operation under 200 °C at 5 MPa and the 1.0 h−1 of LHSV at a flow ratio = 1/1 by weight of the refined palm oil and methanol. At the first reactor, the yields of FAME, glycerin and glycerides intermediates were 96.4 mol%, 96.6 mol% and 2.5 mol%, respectively, at 99.1% conversion, and a similar second reaction of the FAME mixtures with fresh methanol under the same conditions, except the 2.7 h−1 of LHSV, gave a 99.8 mol% yield of FAME and a 98.4 mol% yield of glycerin.
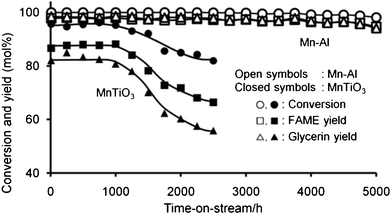 | ||
Fig. 3 Reaction of palm oil and subCH3OH over the MnTiO3 and the Mn–Al mixed oxide catalysts. Conditions: 15 ml of catalyst, 200 °C, 5.0 MPa, the flow ratio of palm oil to CH3OH was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight, and LHSV of the mixed solution of reactants was 1.0 h−1. 1 by weight, and LHSV of the mixed solution of reactants was 1.0 h−1. | ||
Conclusions
In conclusion, we have reported a practical synthetic process for BDF and glycerin from the transesterification of vegetable oils using subCH3OH as a reactant and a reaction medium over the newly-developed heterogeneous Mn catalysts which have high activity and a long lifetime. The quality of the co-product glycerin from this process is high enough to use directly as a raw material for various useful chemicals without further purification. The use of the successive continuous-flow fixed-bed reaction system causes quantitative conversion of the transesterification without any catalyst removal processes. The present process is notable since the operation is simple, waste-free, and produces high purity glycerin. The use of subCH3OH contributes not only to improving the limitations of contact and diffusion concerns by forming a single phase system, but also to promoting the effective desorption of the carboxylate species bonded to the catalyst surface. Further investigations of the detailed mechanism of the transesterification of the triglycerides over the Mn-Al mixed oxide catalyst as well as development into a commercial scale process are now in progress.Acknowledgements
We acknowledge the valuable discussions with Prof. Dr Takao Ikariya, Tokyo Institute of Technology, Tokyo, and part of this work was supported by the RITE Joint Program to Promote Technological Development.References
- (a) As reviews: A. Srivastava and R. Prasad, Renewable Sustainable Energy Rev., 2000, 4, 111 CrossRef CAS; (b) J. J. Spivey and K. M. Dooley, Catalysis, 2006, 19, 41 Search PubMed; (c) M. D. Serio, R. Tesser, L. Pengmei and E. Santacesaria, Energy Fuels, 2008, 22, 207 CrossRef; (d) J. A. Melero, J. Iglesias and G. Morales, Green Chem., 2009, 11, 1285 RSC.
- (a) M. Verziu, B. Cojocaru, J. Hu, R. Richards, C. Ciuculescu, P. Fillip and V. I. Parvulescu, Green Chem., 2008, 10, 373 RSC; (b) E. Akbar, N. Binitha, Z. Yaakob, S. K. Kamarudin and J. Salimon, Green Chem., 2009, 11, 1862 RSC; (c) G. R. Peterson and W. P. Scarrah, J. Am. Oil Chem. Soc., 1984, 61, 1593 CrossRef CAS; (d) V. S-Y. Lin and D. R. Radu, US Pat., US 7,122,688, 2006 Search PubMed; (e) V. S-Y. Lin, J. A. Nieweg, J. G. Verkade, C. R. V. Reddy and C. Kern, US Pat., US 7,790,651, 2010 Search PubMed; (f) V. S-Y. Lin, Y. Cai, C. Kern, J. I. Dulebohn and J. A. Nieweg, US Pat., US 7,906,665, 2011 Search PubMed; (g) T. Oku, M. Nonoguchi, T. Moriguchi, A. Tachibana and T. Akatsuka, PCT Pat., WO 2007/029851, 2007 Search PubMed.
- S. J. Yoo, H-s. Lee, B. Veriansyah, J. Kim, J-D. Kim and Y-W. Lee, Bioresour. Technol., 2010, 101, 8686 CrossRef CAS.
- (a) G. Hillion, B. Delfort, D. le Pennec, L. Bournay and J. A. Chodorge, Prepr. Pap.-Am. Chem. Soc., Div. Fuel Chem., 2003, 48, 636 CAS; (b) L. Bournay, D. Casanave, B. Delfort, G. Hillion and J. A. Chodorge, Catal. Today, 2005, 106, 190 CrossRef CAS; (c) M. Bloch, L. Bournay, D. Casanave, J. A. Chodorge, V. Coupard, G. Hillion and D. Lorne, Oil Gas Sci. Technol., 2008, 63, 405 CrossRef CAS.
- (a) T. Oku and T. Ikariya, Angew. Chem., Int. Ed., 2002, 41, 3476 CrossRef CAS; (b) T. Oku, Y. Arita, H. Tsuneki and T. Ikariya, J. Am. Chem. Soc., 2004, 126, 7368 CrossRef CAS; (c) T. Oku, Y. Arita and T. Ikariya, Adv. Synth. Catal., 2005, 347, 1553 CrossRef CAS.
- (a) As reviews: B. Sumramaniam and M. A. McHugh, Ind. Eng. Chem. Process Des. Dev., 1986, 25, 1 CrossRef; (b) P. E. Savage, S. Gopalan, T. I. Mizan, C. J. Martino and E. E. Brock, AIChE J., 1995, 41, 1723 CrossRef CAS; (c) P. E. Savage, in Handbook of Heterogeneous Catalysis, Vol. 4 (ed.: G. Ertl, H. Knözinger and J. Weitkamp), Wiley-VCH, Weinheim, 1997, pp1339 Search PubMed; (d) A. Baiker, Chem. Rev., 1999, 99, 453 CrossRef CAS; (e) L. Fan and K. Fujimoto, in Chemical Synthesis Using Supercritical Fluids (ed.: P. G. Jessop and W. Leitner), Wiley-VCH, Weinheim, 1999, pp388 Search PubMed; (f) R. Wandeler and A. Baiker, CATTECH, 2000, 4, 34 CAS; (g) J. R. Hyde, P. Licence, D. Carter and M. Poliakoff, Appl. Catal., A, 2001, 222, 119 CrossRef CAS; (h) B. Subramaniam, C. J. Lyon and V. Arunajatesan, Appl. Catal., B, 2002, 37, 279 CrossRef CAS; (i) J.-D. Grünwaldt, R. Wandeler and A. Baiker, Catal. Rev. Sci. Eng., 2003, 45, 1 CrossRef; (j) T. Seki and A. Baiker, Chem. Rev., 2009, 109, 2409 CrossRef CAS.
- (a) T. Oku, Catalysts & Catalysis, 2008, 50, 397 CAS; (b) T. Oku, M. Nonoguchi and T. Moriguchi, PCT Pat., WO 2005/021697, 2005 Search PubMed; (c) T. Oku, T. Moriguchi, T. Akatsuka and M. Nonoguchi, PCT Pat., WO 2006/088254, 2006 Search PubMed; (d) T. Akatsuka, M. Nonoguchi and T. Oku, PCT Pat., WO 2008/113189, 2008 Search PubMed; (e) T. Akatsuka, M. Nonoguchi and T. Oku, PCT Pat., WO 2009/06653, 2009 Search PubMed.
- (a) P. Patnaik, in Handbook of Inorganic Chemicals, McGraw-Hill, New York, 2003, pp538 Search PubMed; (b) K. C. Barick and D. Bahadur, J. Nanosci. Nanotechnol., 2007, 7, 1935 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental details about the preparation of typical catalysts and the reaction methods using the continuous-flow fixed-bed reactor under subcritical conditions. See DOI: 10.1039/c2ra21666c/ |
| This journal is © The Royal Society of Chemistry 2012 |
