Shape-controlled synthesis of CdCO3 microcrystals and corresponding nanoporous CdO architectures†
Yong
Jia
ab,
Xin-Yao
Yu
a,
Tao
Luo
a,
Jin-Huai
Liu
a and
Xing-Jiu
Huang
*a
aResearch Center for Biomimetic Functional Materials and Sensing Devices, Institute of Intelligent Machines, Chinese Academy of Sciences, Hefei 230031, PR China. E-mail: xingjiuhuang@iim.ac.cn; Fax: 86 551 5592420; Tel: 86 551 5591142
bDepartment of Pharmacy, Anhui University of Traditional Chinese Medicine, Hefei 230031, PR China
First published on 31st August 2012
Abstract
In this paper, we have demonstrated a simple ethylene glycol (EG) mediated solution method for the controlled synthesis of cadmium carbonate (CdCO3) microcrystals. The volume ratios of EG and water have a decisive impact on the morphology of CdCO3. With a decreased water volume, CdCO3 microcrystals transformed from microcubes to nanobelts. Concave microcubes, pinecone-like, and dumbbell-shaped CdCO3 microcrystals were also synthesized with moderate volume ratios of EG and water. The types of cadmium source also has great influence on the formation of CdCO3 microcrystals. Cadmium nitrate and cadmium sulfate are two effective cadmium sources for the preparation of CdCO3 microcrystals. However, pure CdCO3 microcrystals can not be obtained when using cadmium chloride as the cadmium source. The as-prepared CdCO3 microcrystals were an excellent precursor for the fabrication of nanoporous CdO micro/nanostructures via a simple thermal transformation process. This simple and controllable solution method opens an important way to fabricate nanoporous CdO micro/nanostructures.
Introduction
Micro/nanostructures have attracted much attention owing to their unique properties.1–3 Among them, metal oxide micro/nanostructures present very important potential applications in the fields of photocatalysis,4 solar cells,5 water treatment,6 and sensors.7 CdO, which adopts the centrosymmetric rocksalt structure, has received much attention due to its narrow energy band gap (2.27 eV),8,9 and good sensing properies.10–12 For the preparation of CdO, the precursor (CdCO3 and Cd(OH)2) calcination strategy was a very important method, which has been widely reported.9,13–18 The structures of the obtained CdO greatly depended on the structure of the precursor. So, synthesis of precursors with different morphology plays a key roles in the preparation of CdO nanomaterials. However, to date, it is still a challenge to control the synthesis of CdCO3 using a simple and general method. In our previous work, porous CdO nanowires were prepared by calcining the hydroxy- and carbonate-containing cadmium compound precursor nanowires.17 Herein, using a simple solution method, the morphology of the CdCO3 precursors were successfully controlled by the volume ratios of ethylene glycol (EG) and water. After calcination, serials of porous CdO nanomaterials with different structures were synthesized. In addition, the types of cadmium source also has a great influence on the structure of the final precursors, which is discussed in detail.Experimental section
Preparation of CdCO3 precursors
Cadmium nitrate, cadmium sulfate, cadmium chloride, EG, ethanol, and urea were purchased from the Shanghai Chemical Reagents Company, and were of analytical grade and used without further purification. For the synthesis of CdCO3 microcubes, a certain amount of cadmium nitrate was dissolved in the mixture solution of EG and deionized water with volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 at room temperature. The concentration of the Cd2+ was 0.1 mol L−1. After that, urea was added in the above solution, and its concentration was 0.4 mol L−1. After stirring for 10 min, the above solution was transferred into a conical flask with a stopper, and then heated at 100 °C for 10 h and allowed to cool to room temperature naturally. The resulting precipitates were separated by centrifugation and thoroughly washed with distilled water and absolute alcohol several times, and finally dried in an oven at 80 °C.
9 at room temperature. The concentration of the Cd2+ was 0.1 mol L−1. After that, urea was added in the above solution, and its concentration was 0.4 mol L−1. After stirring for 10 min, the above solution was transferred into a conical flask with a stopper, and then heated at 100 °C for 10 h and allowed to cool to room temperature naturally. The resulting precipitates were separated by centrifugation and thoroughly washed with distilled water and absolute alcohol several times, and finally dried in an oven at 80 °C.
Synthesis of nanoporous CdO
For the preparation of nanoporous CdO micro/nanostructures, The as-synthesized CdCO3 microcrystals were heated in air (heating rate of 2 °C min−1) and kept at 500 °C for 1 h. The products were allowed to cool naturally to room temperature.Characterization
The as-prepared CdCO3 and CdO micro/nanostructures were characterized by field emission scanning electron microscopy (SEM, FEI Sirion 200 FEG, operated at 10 kV), transmission electron microscopy (TEM, JEOL-2010, operated at 200 kV) and X-ray diffraction (XRD, X′Pert ProMPD, Cu-Kα radiation, wavelength 1.5418 Å).Results and discussion
Fig. 1 presents the SEM images of the products synthesized using cadmium nitrate as cadmium resource, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 volume ratio of EG and water. It is clear that well-faceted cubelike and oblique cubelike microcrystals were obtained. The microcrystals with flat surfaces and sharp edges/corners are evidently shown in the high-magnification SEM image in Fig. 1b–d. The XRD pattern shown in Fig. S1† suggests the microcrystals were CdCO3.
9 volume ratio of EG and water. It is clear that well-faceted cubelike and oblique cubelike microcrystals were obtained. The microcrystals with flat surfaces and sharp edges/corners are evidently shown in the high-magnification SEM image in Fig. 1b–d. The XRD pattern shown in Fig. S1† suggests the microcrystals were CdCO3.
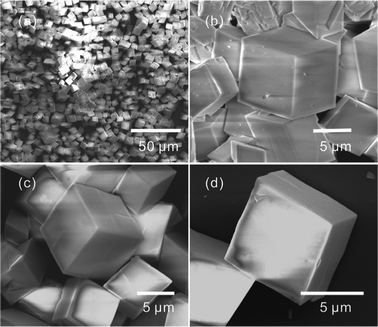 | ||
| Fig. 1 Low-magnification (a) and high-magnification (b–d) SEM images of the cubelike and oblique cubelike CdCO3 microcrystals. | ||
At high temperature, CO2 was given off owing to the hydrolysation of urea, and then CO32− was formed. So, the formation of CdCO3 resulted from the reaction between Cd2+ and CO32−. In formamide aqueous solution, Yu et al. reported an interesting top-down solid-phase approach for the fabrication of cubelike well-faceted CdCO3 microcrystals on cadmium foils.13 When using a liquid-phase precipitation method, cubelike CdCO3 microcrystals with flat surfaces and sharp edges/corners can not be obtained anymore. The proposed growth mechanism suggested that the concentration of carbon dioxide has a great influence on the structures of the products. The results of the present work demonstrate that in situ decomposition of urea is a promising strategy for the growth of CdCO3 microcrystals with flat surfaces and sharp edges/corners.
The obtained CdCO3 microcrystals were excellent precursors for the preparation of CdO. Fig. 2 shows the SEM images of the products after calcination of the CdCO3 microcrystals at 500 °C for 1 h in air. The obtained products still maintain the cubelike and oblique cubelike characters, as shown in Fig. 2a and b. Fig. 2c suggests that the flat surfaces and sharp edges/corners were also preserved. The obtained products were composed of nanoparticles, which resulted from the decomposition of CdCO3. At the same time, nanoporous structures were formed throughout the cube, as shown in Fig. 2c and d. The formation of the pores was due to the generated CO2, which resulted from the decomposition of CdCO3.13 The TEM image shown in the inset of Fig. 2d suggests that the nanoparticles were about 40–50 nm in diameter. The XRD pattern (Fig. S2†) confirmed that the obtained nanoporous cubelike structures were the cubic phase of CdO.
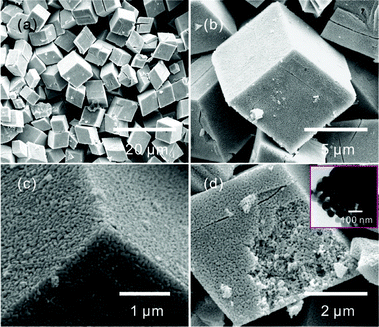 | ||
| Fig. 2 Low-magnification (a) and high-magnification (b–d) SEM images of CdO nanostructures after heating the CdCO3 microcrystals at 500 °C for 1 h in air. The inset of (d) is the TEM image of the CdO micro/nanostructures. | ||
It is well known that polyalcohols are a very important ligand for forming coordination complexes with metal cations.19,20 Polyalcohol based metal coordination complexes have been proven to be excellent precursors for the fabrication of metal oxide nanostructures.19–23 In the present work, the volume ratios of EG and water has a great influence on the structures of the CdCO3. The cubelike CdCO3 microcrystals prepared with pure water were non-uniform and defective (Fig. S3†). With volume ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, similar cubelike CdCO3 microcrystals were obtained. On further decreasing the volume of water, the structures of the products were clearly changed (Fig. S4†). The cubelike products were obviously decreased with the increased EG volume. Furthermore, a small amount of nanobelts were observed on increasing the volume ratios to 5
8, similar cubelike CdCO3 microcrystals were obtained. On further decreasing the volume of water, the structures of the products were clearly changed (Fig. S4†). The cubelike products were obviously decreased with the increased EG volume. Furthermore, a small amount of nanobelts were observed on increasing the volume ratios to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. On further increasing the volume of EG, more and more nanobelts were obtained (Fig. S5†). The results suggest that the CdCO3 microcrystals were transformed from microcubes to nanobelts with decreasing water volume. The intermediate products include concave cubelike, pinecone-like, and dumbbell-shaped CdCO3 microcrystals. Fig. 3 presents SEM images of the products obtained after calcining the CdCO3 microcrystals, which were prepared with a 3
5. On further increasing the volume of EG, more and more nanobelts were obtained (Fig. S5†). The results suggest that the CdCO3 microcrystals were transformed from microcubes to nanobelts with decreasing water volume. The intermediate products include concave cubelike, pinecone-like, and dumbbell-shaped CdCO3 microcrystals. Fig. 3 presents SEM images of the products obtained after calcining the CdCO3 microcrystals, which were prepared with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 volume ratio of EG and water. It is clear that, besides the cubelike products, some porous concave cubic, pinecone-like, and dumbbell-shaped CdO structures were obtained.
7 volume ratio of EG and water. It is clear that, besides the cubelike products, some porous concave cubic, pinecone-like, and dumbbell-shaped CdO structures were obtained.
 | ||
Fig. 3 SEM images of CdO micro/nanostructures after heating the CdCO3 microcrystals, synthesized with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 volume ratios of EG and water, at 500 °C for 1 h in air. 7 volume ratios of EG and water, at 500 °C for 1 h in air. | ||
On further increasing the volume ratio to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a large number of nanobelts that were tens of microns in length were obtained, as shown in Fig. 4 and Fig. S6†. The ringlike structure resulted from the curved nanobelts shown in Fig. 4c and d suggest the obtained nanobelts were very soft. No cubelike products were observed. So, the results mean that the morphology of CdCO3 was well controlled by the change in the volume ratio of EG and water. The XRD pattern (Fig. S6c†) confirmed that the obtained products were CdCO3. Fig. 5 presents the SEM image and XRD pattern of the products after calcination of the CdCO3 nanobelts. It is clear that one-dimensional CdO nanowires were obtained.
1, a large number of nanobelts that were tens of microns in length were obtained, as shown in Fig. 4 and Fig. S6†. The ringlike structure resulted from the curved nanobelts shown in Fig. 4c and d suggest the obtained nanobelts were very soft. No cubelike products were observed. So, the results mean that the morphology of CdCO3 was well controlled by the change in the volume ratio of EG and water. The XRD pattern (Fig. S6c†) confirmed that the obtained products were CdCO3. Fig. 5 presents the SEM image and XRD pattern of the products after calcination of the CdCO3 nanobelts. It is clear that one-dimensional CdO nanowires were obtained.
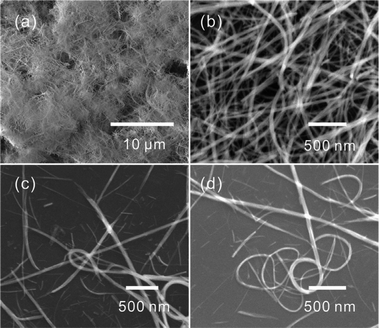 | ||
Fig. 4 Low-magnification (a) and high-magnification (b–d) SEM images of the CdCO3 nanobelts synthesized with a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of EG and water. 1 volume ratio of EG and water. | ||
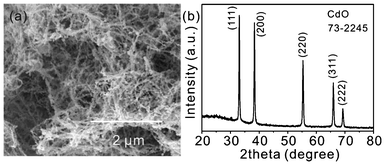 | ||
| Fig. 5 SEM image (a) and XRD pattern (b) of CdO nanowires after heating the CdCO3 nanobelts at 500 °C for 1 h in air. | ||
The results discussed above suggest that the transformtion of CdCO3 products from cubelike to nanobelt resulted from the presence of EG. It is well known that EG can react with metal cations to form linear complexes upon heating.19,24 So, herein, EG could serve as a ligand to form long chain-like coordination complexes with Cd2+ (Fig. S7†). At the same time, with the hydrolysation of urea, the concentration of CO32− was increased. Then, Cd2+ in chain-like complexes reacted with CO32− to form CdCO3 products, which then precipitated out from the reaction medium in the form of uniform nanobelts.
A different cadmium source, cadmium sulfate rather than cadmium nitrate, was used to prepare CdCO3 using the same solution method with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 volume ratio of EG and water. To our surprise, CdCO3 microspheres rather than cubelike products were obtained (Fig. S8†). The diameter of the microspheres was about 10–20 μm. Their surfaces were obviously coarser than those of the CdCO3 microcubes. The results suggest that the morphology of the CdCO3 microcrystals also depended on the anions of the cadmium salt. In addition, most of the obtained CdCO3 microspheres were not perfect. Some concaves were clearly observed. The formation of the concaves was due to the growth of other small spheres (Fig. S8b†). After calcination, CdO microspheres were obtained, as shown in Fig. 6. The surface and the cross-sectional images shown in Fig. 6c and d confirm that the obtained CdO were porous throughout the sphere.
9 volume ratio of EG and water. To our surprise, CdCO3 microspheres rather than cubelike products were obtained (Fig. S8†). The diameter of the microspheres was about 10–20 μm. Their surfaces were obviously coarser than those of the CdCO3 microcubes. The results suggest that the morphology of the CdCO3 microcrystals also depended on the anions of the cadmium salt. In addition, most of the obtained CdCO3 microspheres were not perfect. Some concaves were clearly observed. The formation of the concaves was due to the growth of other small spheres (Fig. S8b†). After calcination, CdO microspheres were obtained, as shown in Fig. 6. The surface and the cross-sectional images shown in Fig. 6c and d confirm that the obtained CdO were porous throughout the sphere.
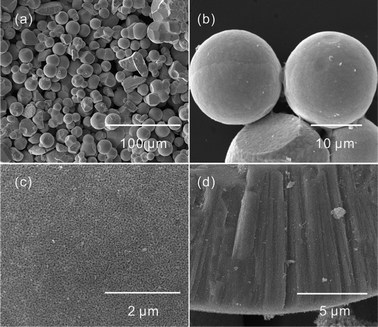 | ||
Fig. 6 Low-magnification (a, b) and high-magnification (c, d) SEM images of the CdO nanostructures after heating the CdCO3 spheres, prepared with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 volume ratio of EG and water, at 500 °C for 1 h in air. 9 volume ratio of EG and water, at 500 °C for 1 h in air. | ||
To further investigate the role of the anions of the cadmium salt, cadmium chloride was chosen as the cadmium source. The volume ratio of EG and water also has a great influence on the morphology of the products. With the volume ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, sphere like products composited with nanoplates were obtained. Rod-like products were prepared with the volume ratio of 1
1, sphere like products composited with nanoplates were obtained. Rod-like products were prepared with the volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S9†). When the volume of water was increased, cube-like and pinecone-like products were synthesized. Furthermore, only some irregular particles were formed in pure water (Fig. S10†). The sphere-like products were calcined at 500 °C for 1 h in air, and the obtained products are shown in Fig. 7. It is clear that porous spheres were obtained. However, the XRD pattern suggests that the above porous spheres were composed of CdO and Cd3O2Cl2 (Fig. S11b†). In addition, the corresponding precursor was composed of CdCO3, CdOHCl, and Cd4Cl3(OH)5 (Fig. S11a†). The results suggest that cadmium chloride was not an effective candidate for the synthesis of pure CdCO3 precursors.
1 (Fig. S9†). When the volume of water was increased, cube-like and pinecone-like products were synthesized. Furthermore, only some irregular particles were formed in pure water (Fig. S10†). The sphere-like products were calcined at 500 °C for 1 h in air, and the obtained products are shown in Fig. 7. It is clear that porous spheres were obtained. However, the XRD pattern suggests that the above porous spheres were composed of CdO and Cd3O2Cl2 (Fig. S11b†). In addition, the corresponding precursor was composed of CdCO3, CdOHCl, and Cd4Cl3(OH)5 (Fig. S11a†). The results suggest that cadmium chloride was not an effective candidate for the synthesis of pure CdCO3 precursors.
 | ||
Fig. 7 Low-magnification (a, b) and high-magnification (c, d) SEM images of nanostructures after heating the microcrystals at 500 °C for 1 h in air. The microcrystals were synthesized with a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of EG and water using CdCl2 as the cadmium source. 1 volume ratio of EG and water using CdCl2 as the cadmium source. | ||
Conclusions
In summary, this work demonstrated a simple EG assisted controlled synthesis for fabricating CdCO3 microcrystals. The morphology and structures of the CdCO3 microcrystals were controlled well by the volume of EG and water, and by the types of cadmium sources. Using the obtained CdCO3 microcrystals as precursors, nanoporous CdO micro/nanostructures with different morphologies were successfully prepared by thermal treatment.Acknowledgements
This work was supported by a grant from the Natural Science Foundation of Education Committee of Anhui Province (KJ2012A179), the National Key Scientific Program, Nanoscience and Nanotechnology (Grant No. 2011CB933700), the China Postdoctoral Science Foundation (2011M501073 and 20110490386), the Anhui University of Traditional Chinese Medicine (2011zr017B), and the National Natural Science Foundation of China (21103198 and 21073197).References
- S. J. Guo and E. K. Wang, Acc. Chem. Res., 2011, 44, 491–500 CrossRef CAS.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- Z. R. Dai, Z. W. Pan and Z. L. Wang, Adv. Funct. Mater., 2003, 13, 9–24 CrossRef CAS.
- Z. F. Bian, J. Zhu, J. G. Wang, S. X. Xiao, C. Nuckolls and H. X. Li, J. Am. Chem. Soc., 2012, 134, 2325–2331 CrossRef CAS.
- S. H. Ko, D. Lee, H. W. Kang, K. H. Nam, J. Y. Yeo, S. J. Hong, C. P. Grigoropoulos and H. J. Sung, Nano Lett., 2011, 11, 666–671 CrossRef CAS.
- W. Q. Cai, J. G. Yu and M. Jaroniec, J. Mater. Chem., 2010, 20, 4587–4594 RSC.
- H. G. Zhang, Q. S. Zhu, Y. Zhang, Y. Wang, L. Zhao and B. Yu, Adv. Funct. Mater., 2007, 17, 2766–2771 CrossRef CAS.
- A. Gulino, F. Castelli, P. Dapporto, P. Rossi and I. Fragala, Chem. Mater., 2002, 14, 704–709 CrossRef CAS.
- Z. Y. Jia, Y. W. Tang, L. J. Luo and B. H. Li, Cryst. Growth Des., 2008, 8, 2116–2120 CAS.
- A. S. Kamble, R. C. Pawar, J. Y. Patil, S. S. Suryavanshi and P. S. Patil, J. Alloys Compd., 2011, 509, 1035–1039 CrossRef CAS.
- T. Krishnakumar, R. Jayaprakash, T. Prakash, D. Sathyaraj, N. Donato, S. Licoccia, M. Latino, A. Stassi and G. Neri, Nanotechnology, 2011, 22, 325501 CrossRef CAS.
- R. R. Salunkhe, V. R. Shinde and C. D. Lokhande, Sens. Actuators, B, 2008, 133, 296–301 CrossRef.
- H. D. Yu, D. S. Wang and M. Y. Han, J. Am. Chem. Soc., 2007, 129, 2333–2337 CrossRef CAS.
- M. F. Ye, H. Z. Zhong, W. J. Zheng, R. Li and Y. F. Li, Langmuir, 2007, 23, 9064–9068 CrossRef CAS.
- A. Askarinejad and A. Morsali, Chem. Eng. J., 2009, 150, 569–571 CrossRef CAS.
- S. Ashoka, P. Chithaiah and G. T. Chandrappa, Mater. Lett., 2010, 64, 173–176 CrossRef CAS.
- Z. Guo, M. Q. Li and J. H. Liu, Nanotechnology, 2008, 19, 245611 CrossRef.
- W. S. Wang, L. Zhen, C. Y. Xu and W. Z. Shao, J. Phys. Chem. C, 2008, 112, 14360–14366 CAS.
- X. C. Jiang, Y. L. Wang, T. Herricks and Y. N. Xia, J. Mater. Chem., 2004, 14, 695–703 RSC.
- D. Larcher, G. Sudant, R. Patrice and J. M. Tarascon, Chem. Mater., 2003, 15, 3543–3551 CrossRef CAS.
- S. W. Cao and Y. J. Zhu, J. Phys. Chem. C, 2008, 112, 6253–6257 CAS.
- S. W. Cao, Y. J. Zhu, M. Y. Ma, L. Li and L. Zhang, J. Phys. Chem. C, 2008, 112, 1851–1856 CAS.
- T. Zhu, J. S. Chen and X. W. Lou, J. Phys. Chem. C, 2012, 116, 6873–6878 CAS.
- Y. L. Wang, X. C. Jiang and Y. N. Xia, J. Am. Chem. Soc., 2003, 125, 16176–16177 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: SEM, TEM images and XRD patterns. See DOI: 10.1039/c2ra21103c |
| This journal is © The Royal Society of Chemistry 2012 |
