Preparation and gas-sensing property of ultra-fine NiO/SnO2 nano-particles
Hao
Ding
,
Jianhui
Zhu
,
Jian
Jiang
,
Ruimin
Ding
,
Yamin
Feng
,
Guanming
Wei
and
Xintang
Huang
*
Institute of Nanoscience and Nanotechnology, Department of Physics, Central China Normal University, Wuhan 430079, P. R. China. E-mail: hding@phy.ccnu.edu.cn; xthuang@phy.ccnu.edu.cn; Fax: +86-027-67861185
First published on 4th September 2012
Abstract
Novel ultra-fine NiO/SnO2 nano-particles (NPs) have been fabricated by creatively using carbon black (CB) as a frame and dispersing agent and applied as gas-sensing materials. In a typical synthesis, a precursor composed of mixed NiO/SnO2 NPs and CB was initially prepared. Subsequently, the ultra-fine NiO/SnO2 NPs were obtained by a thermal treatment of the precursor. The final products were characterized by X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy, transmission electron microscopy, and Brunauer Emmett Teller N2 adsorption–desorption analysis. The ethanol-sensing tests revealed that the ultra-fine NiO/SnO2 NPs used as gas sensors have a quick response/recovery capability (response time: 2–40 s; recovery time: 3–20 s) within a detecting range of 5–100 ppm at an operating temperature of 300 °C; strikingly, the response/recovery times are quite short (2 s and 3 s, respectively) even under the detection limit of 5 ppm. As demonstrated, the ultra-fine NiO/SnO2 NPs are highly promising for real-time monitoring gas sensor applications.
Introduction
Tin oxide (SnO2) is a typical n-type semiconductor (Eg = 3.6 eV at 300 K) and is being studied as a potential material for detecting trace concentrations of toxic and flammable gases. At 200–400 °C, an electron depletion layer can be formed near the surface of n-type semiconductors due to oxygen adsorption creating negative charge, which establishes the core (semiconducting)–shell (resistive) structure and the potential barrier between the particles.1 If reducing gases are present in the atmosphere, they will be oxidized by reaction with negatively charged oxygen and the remnant electrons decrease the sensor resistance. In order to enhance the gas response, nanostructures with high surface area and full electron depletion are advantageous.2 Currently, researchers are mainly focusing on gas-sensing properties of various SnO2 nano-materials, such as nano-particles (NPs),3,4 single crystalline nanowires, nanobelts, nanorods,5,6 polycrystalline nanotubes or nanowires and hollow spheres7–10 due to their unique morphological structure and high surface-to-volume ratios. Nickel(II) oxide (NiO) is a significant p-type semiconductor with a band gap of 4.2 eV,11 and more importantly it can form useful heterojunctions with n-type semiconductors, particularly SnO2. The introduced NiO can be readily coupled with SnO2, leading to the formation of P–N junctions wherein electrical fields could be set up between the n-SnO2 and p-NiO grains. The yielded electrical fields would effectively increase the response of gas detectors by “band bending” in depletion layers on either side of physical interfaces.12High sensitivity, rapid response/recovery, selective detection, and linear range are the most important parameters in the design of oxide semiconductor-based gas sensors. Generally, researchers attempt to improve the gas sensitivity and selectivity parameters by the control of the nano-structures of oxides, sensing temperature, surface structure as well as the selection of catalyst.13–17 Nevertheless, the response/recovery performance of gas sensors has not yet received much attention. Therefore, designing a gas sensor with both high sensitivity and rapid response/recovery still remains challenging.
In this work, we report a facile and creative synthesis of ultra-fine NiO/SnO2 nano-particles (NPs), which are used as gas sensors. The SnCl2 and NiCl2 slats were initially filled into the carbon black (CB) “mold”. CB produced by the incomplete combustion of petroleum products is one type of useful amorphous carbon with good conductivity and large specific surface area.18–21 However, to the best of our knowledge, it has not been employed as a frame and disrupting agent for the synthesis of gas-sensor materials yet. After the calcinations that followed, the salts in the CB mold were successfully decomposed in air, leading to the formation of ultra-fine heterogeneous NiO/SnO2 NPs. The sensing properties of this ultra-fine product have been investigated. It is found that this material can exhibit both a high response towards ethanol within a detecting range of 5–100 ppm and a quick response/recovery behavior (response time: 2–40 s; recovery time: 3–20 s) at the operating temperature of 300 °C.
Experimental
Synthesis
All chemicals in this work were analytically pure and used without further purification. The average diameter of the CB particles is about 70 nm. The CB usually has unfavorable oxygen groups (e.g., carboxylic, lactonic, phenolic groups, etc.) chemisorbed on its surface.20 In order to change its surface chemistry, the CB needs to be pre-treated in concentrated HNO3 by sonication. In detail, CB powders (∼1 g) were dispersed into a 100 mL of 7.0 M HNO3 solution by ultra-sonication treatment. After 10 min, the mixture was stirred and treated by reflux at 120 °C for 12 h. The mud-like product was washed by distilled water three times and finally dried at 60 °C in air.In a typical synthesis of ultra-fine NiO/SnO2 NPs, 1 g of tin(II) chloride (SnCl2·2H2O) and 0.1 g nickel chloride (NiCl2·6H2O) were dissolved in 40 mL distilled water. Then, 0.25 mL of concentrated HCl (38%) and 0.1 g of treated CB were successively added into the solution. After the mixture was ultra-sonicated and stirred for 2 h at room temperature, the solution was separated by centrifugation and washed with distilled water several times until its pH was neutral. Finally, the white ultra-fine NiO/SnO2 NPs were obtained by calcination treatment of the separated solid products at 350 °C for 2 h (for crystalline), and then at 600 °C for 20 min in air (for the removal of CB). For the comparative study, pure ultra-fine SnO2 NPs were prepared by following the same process as for the ultra-fine NiO/SnO2 NPs but without adding NiCl2·6H2O into the solution. In the absence of the CB frame, normal-sized NiO/SnO2 NPs can be also made.
Characterization and gas-sensing measurement
The products were characterized by X-ray diffractometer (XRD, Cu-Kα radiation, λ = 1.5418 Å), Brunauer Emmett Teller (BET, Belsorp Mini) N2 adsorption–desorption analyses, field-emission scanning electron microscopy (SEM, JSM-6700F; 5 kV), transmission electron microscopy and high-resolution transmission electron microscopy (TEM and HRTEM, JEM-2010FEF; 200 kV). X-ray photoelectron spectroscopy (XPS) was performed on a VG-ESCALAB LKII instrument with a Mg-Kα ADES (hν = 1253.6 eV) source at a residual gas pressure of below 10−8 Pa. The weight content of NiO and SnO2 in the precursor was measured by thermal gravimetric analysis in air flow (TGA, Pyris 1; heating rate: 2.5 deg min−1). The gas sensors were fabricated by coating an aqueous paste of NiO/SnO2 NPs onto a ceramic tube, which was composed of a pair of Au electrodes and Ni–Cr heating coil in the tube to control the operating temperature. The gas sensing properties were detected by a Navigation4000-NMDOG instrument.The sensor response is defined as S = Ig/Ia, where Ia and Ig are the currents measured in air and in ethanol–air mixed gas, respectively. In addition, the response time (tres) is defined as the time required for the current value to reach 90% of the equilibrium value after the testing gas was injected, and the recovery time (trecov) is the time necessary for a sensor to attain a current value 10% above its original value in air.
Results and discussions
SEM images of purified CB powders, NiO/SnO2/CB NPs calcined at 350 °C for 2 h and ultra-fine NiO/SnO2 NPs calcined at 600 °C for 30 min are shown in Fig. 1. As is observed from Fig. 1 (a), the diameters of CB particles are in the range of 50–80 nm. Fig. 1 (b) displays the morphology of NiO/SnO2/CB nanocomposites prepared at 350 °C. It can be noticed that the as-formed hybrid products have the same diameter as the CB NPs. However, after calcination at 600 °C for 30 min, the particle diameters are sharply reduced down to 8–10 nm, as indicated in Fig. 1 (c). We supposed that CB particles, the supporting frames and nanoscaled “separators”, would disappear during the thermal treatment in air. As a result, this would lead to the generation of ultra-fine NiO/SnO2 NPs. Fig. 2 (a) shows a typical TEM image of ultra-fine NiO/SnO2 NPs. Noteworthy is that the final products with a diameter range of 8–10 nm are well crystallized. A high-resolution image (Fig. 2 (b) and (c)) reveals the presence of single-crystalline NiO/SnO2 NPs; as can be seen, the interplanar distance of 0.34 nm is highly consistent with the (110) plane of tetragonal rutile SnO2, and the interplanar distance of 0.24 nm is consistent with the (111) plane of NiO. The EDS spectrum shown in Fig. 2 (c) also provides us with the detailed concentration ratio of Sn (55.88%) and Ni (3.28%), respectively.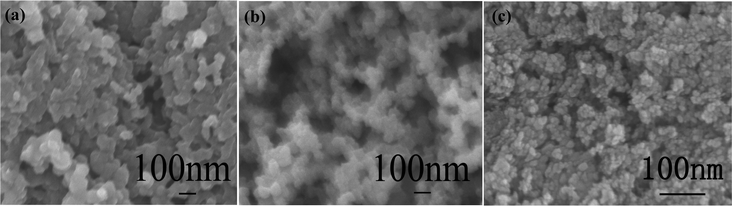 | ||
| Fig. 1 (a) SEM images of the purified CB, (b) the NiO/SnO2 particles calcined at 350 °C for 2 h and (c) the ultra-fine NiO/SnO2 NPs calcined at 600 °C for 20 min. | ||
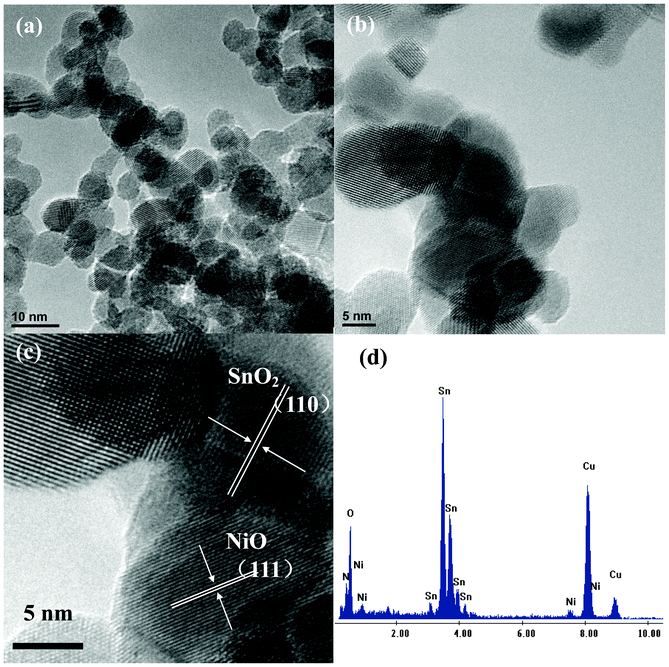 | ||
| Fig. 2 (a) TEM image of the ultra-fine NiO/SnO2 NPs, (b) (c) HRTEM images of the ultra-fine NiO/SnO2 NPs, and (d) EDS spectra showing Sn, Ni, and O concentrations in the sample. | ||
Fig. 3 (a) successively shows XRD patterns of CB powders, the NiO/SnO2/CB precursor and ultra-fine NiO/SnO2 NPs. It is obvious that all the diffraction peaks are quite broad, confirming the small particle size of the products by referring to the Scherrer equation. The diffraction peak centered at 2θ of 23° can be indexed to CB, and is attributed to the partial graphitization of CB particles. After 600 °C thermal treatment in air atmosphere, the peak intensity of SnO2 (JCPDS 77-0448) and NiO (JCPDS 75-0197) obviously becomes larger while the diffraction peak of CB is totally missing. This could result from the combustion of CB frames and the improved crystalline qualities of the oxides. Fig. 3 (b) shows the TGA curve of the NiO/SnO2/CB precursor. The result shows that the weight ratio of oxides (NiO and SnO2) is ∼43.1%. Also, it is found that there is no obvious weight loss when heating at a temperature around 600 °C, which confirms the exhaustion of CB when the NiO/SnO2/CB samples are calcinated at 600 °C in an air atmosphere. To get in-depth information on the ultra-fine NiO/SnO2 NPs, we have performed the N2 adsorption–desorption measurement. Fig. 4 displays the results of the N2 adsorption–desorption isotherm and BJH pore-size distribution measurements of the final NiO/SnO2 NPs. As tested, the ultra-fine samples have a high surface area of 42.506 m2 g−1. The total pore volume and the average pore diameter of this product are 0.22 cm3 g−1 and 7 nm, respectively, agreeing well with our former structural analysis.
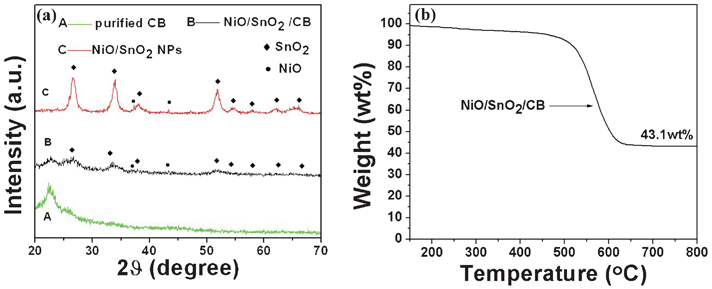 | ||
| Fig. 3 (a) XRD patterns of the ultra-fine NiO/SnO2 NPs, the NiO/SnO2/CB nanocomposites, and the purified CB. (b) TGA curves of the NiO/SnO2/CB nanocomposites. | ||
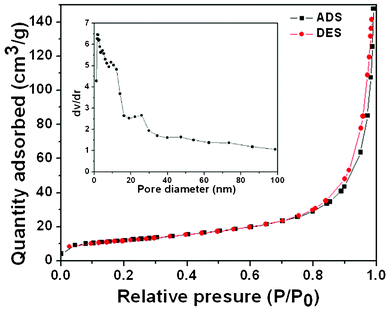 | ||
| Fig. 4 N2 adsorption–desorption isotherm and BJH pore size distribution of the ultra-fine NiO/SnO2 NPs. | ||
XPS measurements have been further conducted to study the surface composition and chemical states of the elements existing in the final products. The survey spectrum (Fig. 5 (a)) illustrates that only Ni, Sn, and O elements exist in the NiO/SnO2 NPs. Fig. 5 (b) shows the high-resolution spectrum of Ni2p, where the peaks at 855.6 and 862.3 eV are well indexed to the Ni2p3/2 peaks and the peak located at 871.8 eV is attributed to Ni2p1/2. The Ni2p3/2 peaks are assigned to Ni(II) ions in the NiO/SnO2/CB NP samples. The peak at 855.6 eV was attributed to NiO5 or Ni2+ in pyramidal symmetry according to the experimental results and theoretical calculation of Soriano et al.11 The spin orbit components (3d3/2 and 3d5/2) of the Sn3d peak are both observed at approximately 495.8 eV and 487.5 eV, as shown in Fig. 5 (c), corresponding to Sn4+ in a tetragonal rutile structure. All of these results give the insight that the samples are composed of NiO and SnO2.
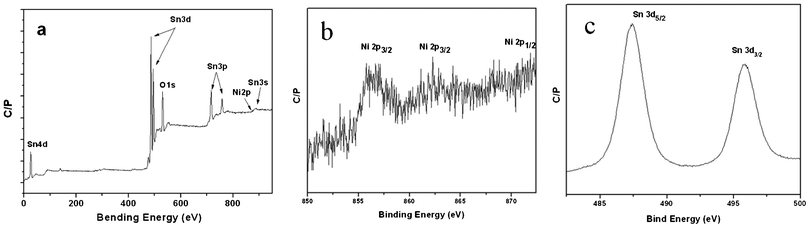 | ||
| Fig. 5 XPS patterns of full spectrum (a), Ni2p (b), and Sn3d (c) spectra of the ultra-fine NiO/SnO2 NPs. | ||
The ethanol-sensing properties of ultra-fine NiO/SnO2 NPs have been investigated. The NiO/SnO2 NPs are coated onto a ceramic tube which is composed of a pair of Au electrodes and a Ni–Cr heating coil in the tube to control the operating temperature to fabricate the gas sensor. The gas sensing properties were detected by a Navigation4000-NMDOG instrument which is produced by the Beijing Wulian Company. Fig. 6 (a) and (b) show the dynamic response/recovery and sensor response curves of the NiO/SnO2 sensor to ethanol. Purposely, the polynomial fitting (polynomial order: 2) has been used for the response–concentration curve of Fig. 6 (b). The sensing measurements ranging from 5 to 100 ppm are conducted around 300 °C, which has been evidenced as the optimal operating temperature by the pioneering work10 and our testing result (please see Fig. 6c). As displayed, the ultra-fine NiO/SnO2 NP sensor can exhibit a high response to ethanol (Smax = 14.9) and very fast response/recovery ability even in a low concentration range (<50 ppm) of ethanol. To account for the functions of CB and NiO in this research, the gas sensing properties of ultra-fine NiO/SnO2 NPs, ultra-fine SnO2 NPs, normal size SnO2 NPs and normal size NiO/SnO2 NPs in 5 ppm ethanol have been comparatively studied (Fig. 6 (d)). On the one hand, it is noted that both the ultra-fine NPs made by using CB as the frame and dispersing agent are able to exhibit a high response. The high response can be attributed to the nanosized effect of SnO2, and CB undoubtedly plays a vital “frame” role to fabricate the ultra-fine nanostructures, totally avoiding the appearance of nanoparticle aggregation. On the other hand, the response/recovery capability of ultra-fine NiO/SnO2 NPs and normal size NiO/SnO2 NPs sensors is much superior to that of normal size NPs. For instant, the tres values of both NiO/SnO2 NPs sensors (upon sensing 5 ppm ethanol) were 3 s and 4 s, respectively, far better than the case of ultra-fine SnO2 NPs (90 s) and normal size SnO2 NPs sensors (100 s) under the same conditions; the trecov values were recorded as 2 s and 3 s, respectively, whereas the trecov values of ultra-fine SnO2 NPs and normal-sized SnO2 NPs sensors were 60 s and 70 s.
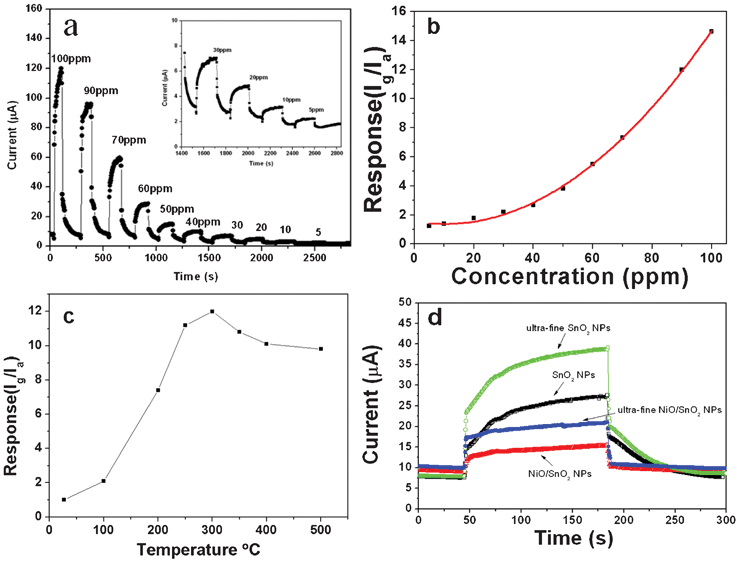 | ||
| Fig. 6 (a) Dynamic response and recovery curve of the sensor based on the prepared ultra-fine NiO/SnO2 NPs exposure to ethanol, inset shows the low concentration curve, and (b) the comparison of their gas concentration dependent sensitivities. (c) Gas sensing properties of ultra-fine NiO/SnO2 NPs, normal size NiO/SnO2 NPs, ultra-fine SnO2 NPs and normal size SnO2 NPs exposed to 5 ppm ethanol. | ||
As demonstrated, the ultra-fine NiO/SnO2 NP sensor shows typical gas-sensing properties of n-type semiconductors. The introduction of NiO could bring about several advantages. Prime is the formation of p–n heterojunctions between the NiO and SnO2 interfaces. As reported, the contact between p- and n-type semiconductors could result in the band bending happening in depletion layers on both sides of the physical interfaces so as to accommodate the equalization of the Fermi levels.12,22 In the case of air atmosphere, a thicker charge depletion layer would be formed near the grain surface of SnO2 due to the p–n junction. Hence, electrons associated with these charged species can be drawn from the conduction band of the bulk material, causing a much higher resistance than that of single-phased SnO2. The detected ethanol gas would increase the number of electrons in SnO2 and meantime decreases the concentration of holes in NiO, giving rise to a lower resistance and therefore upgrading the detecting sensitivity.10 In addition, NiO can act as a catalyst to facilitate the occurrence of the oxidation reaction. In a single-phased n-type semiconductor, like SnO2, the concentration of surface oxygen is several orders of magnitude lower than the full monolayer concentration because the chemisorption of oxygen occurs in order to compensate the oxygen deficiency, which may retard the recovery reaction involving the chemisorption of oxygen.23 Thus, the abundant, negatively charged surface oxygens at the surface of NiO can facilitate the transfer of electrons or of negatively charged adsorbed oxygen (O−) to the surface of SnO2 during the recovery reaction. This can explain the fast response and recovery of NiO-functionalized SnO2 NP sensors.10
Conclusions
We present ultra-fine NiO/SnO2 NPs synthesized by a novel and facile method and their gas sensing properties. In particular, CB was creatively adopted as a frame and separator during the typical synthesis. The ultra-fine NiO/SnO2 NPs were obtained by the mix of NiCl2/SnCl2 salts and CB followed by thermal treatment. After the heating treatment at 600 °C in air, the CB frame was removed but the NiO/SnO2 could still maintain the former ultra-fine particle diameter (less than 10 nm). When tested as gas sensors, the ultra-fine NiO/SnO2 particles exhibited a high response and a fast response/recovery capability. It is found that the high response was contributed to by the nano-sized effects of NiO/SnO2 NPs while the rapid response/recovery characteristics were enhanced remarkably by the addition of NiO. The simplicity of the process outlined in this report offers a new way of preparing novel metal–oxide particles with catalytic adulteration for applications in real-time monitoring gas sensors with fast response and recovery speed. This technique provides a general, easy, and convenient approach for the preparation of SnO2 nanomaterials on the desired substrates.Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 50872039), Natural Science Foundation of Hubei Province (No. 2010CDB04005), National Natural Science Foundation of Jiangsu Province (No: BK2009724), Industry R&D program in Jiangsu Province (No: GY2009003), and the Center for Electron Microscopy of Wuhan University for TEM experiments.References
- N. Yamazoe, Toward innovations of gas sensor technology, Sens. Actuators, B, 2005, 108, 2 CrossRef.
- N. Barsan and U. Weimer, Conduction model of metal oxide gas sensors, J. Electroceram., 2001, 7, 143 CrossRef CAS.
- H. C. Chiu and C. S. Y, J. Phys. Chem. C, 2007, 111, 7256 CAS.
- A. Kolmakov, D. O. Klenov, Y. Lilach, S. Stemmer and M. Moskovits, Nano Lett., 2005, 5, 667 CrossRef CAS.
- B. Cheng, J. M. Russell, W. S. Shi, L. Zhang and E. T. Samulski, J. Am. Chem. Soc., 2004, 126, 5972 CrossRef CAS.
- Y. Jia, L. F. He, Z. Guo, X. Chen, F. L. Meng, T. Luo, M. Q. Li and J. H. Liu, J. Phys. Chem. C, 2009, 113, 9581 CAS.
- Y. Jia, X. Chen, Z. Guo, J. Y. Liu, F. L. Meng, T. Luo, M. Q. Li and J. H. Liu, J. Phys. Chem. C, 2009, 113, 20583 CAS.
- H. T. Fang, X. Sun, L. H. Qian, D. W. Wang, F. Li, Y. Chu, F. P. Wang and H. M. Cheng, J. Phys. Chem. C, 2008, 112, 5790 CAS.
- I. S. Hwang, J. K. Choi, S. J. Kim, K. Y. Dong, J. H. Kwon, B. K. Ju and J. H. Lee, Sens. Actuators, B, 2009, 142, 105–110 CrossRef.
- H. R. Kim, K. I. Choi, K. M. Kim, I. D. Kim, G. Z. Cao and J. H. Lee, Chem. Commun., 2010, 46, 5061–5063 RSC.
- L. Soriano, I. Preda, A. Gutiérrez, S. Palacín and M. Abbate, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 233417 CrossRef.
- Z. J. Wang, Z. Y. Li, J. H. Sun, H. N. Zhang, W. Wang, W. Zheng and C. Wang, J. Phys. Chem. C, 2010, 114, 6100 CAS.
- E. Comini, G. Faglia, G. Sberveglieri, Z. Pan and Z. L. Wang, Appl. Phys. Lett., 2002, 81, 1869 CrossRef CAS.
- Y. Wang, X. Jiang and Y. Xia, J. Am. Chem. Soc., 2003, 125, 16176 CrossRef CAS.
- A. Chiorino, G. Ghiotti, F. Prinetto, M. C. Carotta, D. Gnani and G. Marinelli, Sens. Actuators, B, 1999, 58, 338 CrossRef.
- Y. Shimizu, E. D. Bartolomeo, E. Traversa, G. Gusmano, T. Hyodo, K. Wada and M. Egashira, Sens. Actuators, B, 1999, 60, 118 CrossRef.
- Y. Zeng, T. Zhang, H. T. Fan, W. Y. Fu, G. Y. Lu, Y. M. Sui and H. B. Yang, J. Phys. Chem. C, 2009, 113, 19000 CAS.
- P. C. Ma, M. Y. Liu, H. Zhang, S. Q. Wang, R. Wang, K. Wang, Y. K. Wong, B. Z. Tang, S. H. Hong, K. W. Paik and J. K. Kim, ACS Appl. Mater. Interfaces, 2009, 5, 1090 Search PubMed.
- H. Darmstadt and C. Roy, Carbon, 2001, 6, 841 CrossRef.
- S. Goeringer, C. R. Chenthamarakshan, K. Rajeshwar and W. A. Wampler, Carbon, 2001, 4, 515 CrossRef.
- J. R. Li, J. R. Xu, M. Q. Zhang and M. Z. Rong, Carbon, 2003, 12, 2353 CrossRef.
- W. Göpel and K. D. Schierbaum, Sens. Actuators, B, 1995, 1, 26–27 Search PubMed.
- D. Kohl, J. Phys. D: Appl. Phys., 2001, 34, 125 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
