Protein as the source for synthesizing fluorescent carbon dots by a one-pot hydrothermal route†
Zhe
Zhang
,
Jinhui
Hao
,
Jing
Zhang
,
Bailin
Zhang
and
Jilin
Tang
*
State Key Laboratory of Electroanaytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: jltang@ciac.jl.cn; Fax: +86 431-85262734; Tel: +86 431-85262734
First published on 17th July 2012
Abstract
Fluorescent carbon dots (C-dots) are prepared directly via a simple hydrothermal method using bovine serum albumin (BSA) as a carbon source in the presence of surface passivation reagents. The obtained C-dots have low cytotoxicity and good biocompatibility, demonstrating that their features are good for application in cell imaging.
Fluorescent semiconductor quantum dots (QDs) have attracted much attention over the past two decades for a variety of purposes and applications, especially in optical imaging agents, electronics, biotechnology, sensors and catalysis due to their high quantum yield and size-tunable fluorescence color.1–7 Unfortunately, QDs are commonly synthesized using toxic heavy metals (e.g., cadmium, lead),8 which limit their widespread use and in vivo biological applications because of potential environmental hazards and long term toxicity concerns. Therefore, considerable efforts have been made to search for environmentally-friendly and low toxicity fluorescent nanomaterials.
Recently, fluorescent carbon dots (C-dots) have emerged as the most alternative fluorescent probes to replace traditional QDs. Compared with QDs, C-dots are highly attractive for bioimaging,9,10 photocatalysis,11 and light emitting devices12 because of their chemical stability, biocompatibility, low toxicity and reasonable photoluminescence.13,14 The C-dots are generally small oxygenous carbon nanoparticles of near spherical geometry with sizes below 10 nm, and they inherently fluoresce in visible upon light excitation. Since they were discovered by Xu et al. while purifying single-walled carbon nanotubes derived from arc-discharge soot, C-dots have been produced via various methods. So far, C-dots can be prepared by two main approaches: top-down and bottom-up routes.15 Top-down methods consist of laser ablation or electrochemical oxidation of graphite,16,17 electrochemical treatment of multiwalled carbon nanotubes,18,19 and chemical oxidation of commercially activated carbon etc.20 Bottom-up methods consist of microwave pyrolysis of saccharides,21,22 combustion soot of candles,23 supported synthetic methods,24,25 chemical or thermal oxidation of suitable precursors,26,27 and hydrothermal treatment of saccharides etc.28,29 However, most of these above-mentioned methods suffer from drawbacks such as the requirement for expensive systems or a large amount of strong acid, which limits their wide application. Furthermore, to the best of our knowledge, biomacromolecules such as protein as precursors for producing C-dots have remained unexplored until now.
In this work, we demonstrate for the first time a simple, “green” synthetic route to prepare C-dots with blue emission by employing a common commercially available protein, bovine serum albumin (BSA) as the precursor by one-pot hydrothermal method. In a typical experiment, BSA (1 g) was added to 20 mL of Milli-Q water with stirring. Half an hour later, 3 mL of 4,7,10-trioxa-1,13-tridecanediamine (TTDDA) was introduced, and the mixture was transferred to a 50 mL Teflon-lined stainless steel autoclave for hydrothermal treatment at 180 °C for carbonization of BSA followed by in situ surface passivation. The excess TTDDA and resulting small molecules were removed by dialyzing against water through a dialysis membrane for 24 h. The high tempreature and high pressure treatment in the autoclave first caused dehydration and pyrolysis of BSA, and then made it brake into small luminescent C-dots. Fig. 1a shows the TEM image of C-dots obtained from BSA, which indicates that the nanoparticles are well dispersed from each other with spherical morphology. The absence of discernible lattice structures of C-dots on the higher magnification TEM image (Fig. S2b†) indicates that the resultant C-dots are amorphous. The corresponding particle size distribution histograms shown in Fig. 1b indicate that these particles have a narrow size distribution in the range of 2–6 nm as estimated from the TEM images. From the elemental analysis, the weight ratio of C, H, N and O elements in the C-dots is 56.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.93
6.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.60
10.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.10.
26.10.
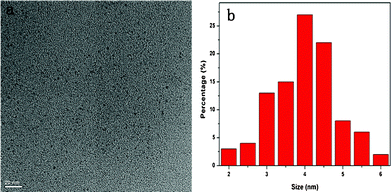 | ||
| Fig. 1 (a) TEM image and (b) corresponding particle size distribution histograms of C-dots. | ||
Fig. 2a shows the UV-vis absorption and photoluminescence (PL) spectra of the C-dots dispersion. The absorption spectrum (dark yellow line) shows a peak centred at 276 nm which is ascribed to the p–p* transition of C-dots. When the C-dots are excited at 360 nm, a strong PL emission peak located at 448 nm (blue line) is observed, indicating that the C-dots are fluorescent. The full width at half maximum (FWHM) excited at 360 nm is about 94 nm, and the quantum yields (QY) is determined to be 11% with quinine sulfate as a reference (for detailed experimental steps, please see ESI†). The inset photographs of the C-dots solution is light yellow, transparent and clear under visible light and exhibits strong blue luminescence under UV light (365 nm). Fig. 2b shows the normalized PL emission spectra of the C-dots dispersion with various excitation wavelengths from 320 nm to 420 nm on the left in 20 nm increments. It is seen that the emission spectrum of the C-dots changes from 430 nm to 470 nm. The red shift of PL emission is due to the co-existence of C-dots with different sizes in each sample, which is common behavior in fluorescent carbon nanoparticles.28 Furthermore, Fourier transform infrared (FTIR) spectra of BSA, TTDDA, and C-dots are used to characterize surface state of C-dots (Fig. S1, ESI†). A broad absorption band at 3200–3500 cm−1 originated from O–H stretching vibrations is observed. Peaks at 1530 and 1660 cm−1 are assigned to the amide II and amide I stretching vibrations, and that at 1455 cm−1 is characteristic of the amide III of C–N stretch. Amide I and amide II bands are two major bands of the protein infrared spectrum. The amide I band is directly related to the backbone conformation and amide II is conformationally sensitive. After high tempreature and high pressure processing in the autoclave, amide II disappears and the intensity of amide I decreases significantly (Fig. S1c†). This indicates that the structure of BSA is destroyed. In presence of TTDDA molecules, the carbonyl moieties on the surface of BSA first react with the amine head groups of TTDDA to form amides. Then, the BSA protected by TTDDA can dehydrate and carbonize into C-dots. This can be confirmed by the disappearance of the N–H stretching band and appearance of a new absorption band of C–O at 1105 cm−1 (Fig. S1c†). The results show that one-pot hydrothermal carbonization of BSA is an effective way of obtaining TTDDA passivated fluorescent C-dots. In the absence of TTDDA, a suspension of carbon aggregates was obtained by following the same procedure with weak luminescence. In contrast, in the presence of TTDDA, a light yellow suspension with strong blue luminescence was obtained. This is due to the fact that TTDDA is simultaneously used as passivation reagents to enhance the luminescence intensity and as stabilizer to prevent C-dots assembling into large carbon particles during hydrothermal carbonization.
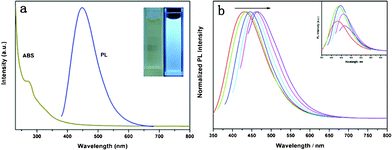 | ||
| Fig. 2 (a) UV-vis absorption (dark yellow line) and PL spectra (blue line) of C-dots dispersion. Inset: images of the C-dots dispersion under visible (left) and UV light (right, 365 nm). (b) Normalized PL spectra at excitation wavelengths from 320 nm to 420 nm on the left in 20 nm increment. Inset: PL emission spectra of C-dots. | ||
Fig. 3a shows the effect of pH on the fluorescence property of C-dots. The fluorescence intensity of the C-dots dispersion decreases upon changing the pH from 2.69 to 12.93, and the fluorescence emission peak gradually red-shifts with increasing pH, which indicates that the fluorescence characteristics of the C-dots strongly depends on the pH value. The pH effect indicates that the fluorescent species in the C-dots should have acidic sites relevant to the blue emssion, because the fluorescue is quenched in basic media.26 However, its mechanism is not understood. Fig. 3b shows the response of normalized PL intensity to continuous irradiation by a UV-lamp at a wavelength of 365 nm for 10 h. The fluorescence from C-dots can keep 85% of the initial value, which indicates that the C-dots possess good photostability. In order to assess the toxicity of our C-dots for a fluorescent marker, we did an 3-[4,5-dimethylthiazol-2-yl]-diphenyltetrazolium bromide (MTT) assay using the colorectal carcinoma cell line HCT116 as a model. Fig. S6† shows the cell viability after incubation with C-dots at different concentrations (12.5–125 μg mL−1 of C-dots) for 24 h. The viability of the cells exceeds 92% in all investigated concentrations after 24 h incubation. These results demonstrate little toxicity of our C-dots as fluorescent imaging agents. Encouraged by low cytotoxicity and good biocompatibility of the C-dots prepared with BSA as a precursor, we used them as a bioimaging agent to label the colorectal carcinoma cell line SW1116. The C-dots solution had been mixed with cell culture media along with cells, incubated for 3 h, and the washed cells were then imaged under bright field and UV excitation. As shown in Fig. 4b, cells become bright blue under UV excitation, but they are colorless in the control sample where no C-dots was used (Fig. S7b†). This suggests that C-dots are successfully internalized by SW1116 cells and localize in the cell membrane and cytoplasm region surrounding the nuclei of the SW1116 cells.
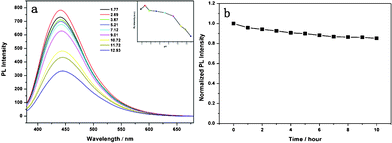 | ||
| Fig. 3 (a) Effect of the solution pH value on C-dot fluorescence (λex = 360 nm). (b) Emission intensity of C-dots during continuous excitation at 365 nm. | ||
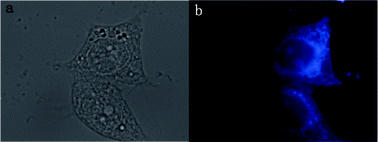 | ||
| Fig. 4 (a) Bright field and (b) fluorescence microscopic images of SW1116 cells cultured in the presence of C-dots for 3 h under UV excitation. | ||
In summary, we have developed a “green” and facile method to prepare fluorescent C-dots with a diameter of 2–6 nm through one-pot hydrothermal treatment of BSA in the presence of TTDDA. In the process, BSA is used as carbon source and TTDDA is used as surface passivation reagent and stabilizer. The fluorescence property of the C-dots is useful for cell imaging applications. Furthermore, low cytotoxicity and good photostability make these fluorescent C-dots have great potential applications for optical imaging agent where cadmium-based QDs show toxic effects.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, No. 2011CB935800).References
- X. H. Gao, Y. Y. Cui, R. M. Levenson, L. W. K. Chung and S. M. Nie, Nat. Biotechnol., 2004, 22, 969–976 CrossRef CAS.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS.
- Y. Y. Li, Y. L. Zhou, H. Y. Wang, S. Perrett, Y. L. Zhao, Z. Y. Tang and G. J. Nie, Angew. Chem., Int. Ed., 2011, 50, 5860–5864 CrossRef CAS.
- Y. H. Xia, Y. L. Zhou and Z. Y. Tang, Nanoscale, 2011, 3, 1374–1382 RSC.
- Y. S. Xia, T. D. Nguyen, M. Yang, B. Lee, A. Santos, P. Podsiadlo, Z. Y. Tang, S. C. Glotzer and N. A. Kotov, Nat. Nanotechnol., 2011, 6, 580–587 CrossRef CAS.
- Y. Qu, W. Li, Y. L. Zhou, X. F. Liu, L. L. Zhang, L. M. Wang, Y. F. Li, A. Iida, Z. Y. Tang, Y. L. Zhao, Z. F. Chai and C. Y. Chen, Nano Lett., 2011, 11, 3174–3183 CrossRef CAS.
- X. F. Liu, Y. Gao, X. M. Wang, S. J. Wu and Z. Y. Tang, J. Nanosci. Nanotechnol., 2011, 11, 1941–1949 CrossRef CAS.
- C. Kirchner, T. Liedl, S. Kudera, T. Pellegrino, A. M. Javier, H. E. Gaub, S. Stolzle, N. Fertig and W. J. Parak, Nano Lett., 2005, 5, 331–338 CrossRef CAS.
- L. Cao, X. Wang, M. J. Meziani, F. S. Lu, H. F. Wang, P. J. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S. Y. Xie and Y. P. Sun, J. Am. Chem. Soc., 2007, 129, 11318 CrossRef CAS.
- S. T. Yang, L. Cao, P. G. J. Luo, F. S. Lu, X. Wang, H. F. Wang, M. J. Meziani, Y. F. Liu, G. Qi and Y. P. Sun, J. Am. Chem. Soc., 2009, 131, 11308–11309 CrossRef CAS.
- L. Cao, S. Sahu, P. Anilkumar, C. E. Bunker, J. A. Xu, K. A. S. Fernando, P. Wang, E. A. Guliants, K. N. Tackett and Y. P. Sun, J. Am. Chem. Soc., 2011, 133, 4754–4757 CrossRef CAS.
- F. Wang, Y. H. Chen, C. Y. Liu and D. G. Ma, Chem. Commun., 2011, 47, 3502–3504 RSC.
- P. Chakravarty, W. Qian, M. A. El-Sayed and M. R. Prausnitz, Nat. Nanotechnol., 2010, 5, 607–611 CrossRef CAS.
- V. N. Mochalin, O. Shenderova, D. Ho and Y. Gogotsi, Nat. Nanotechnol., 2012, 7, 11–23 CrossRef CAS.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS.
- S. L. Hu, K. Y. Niu, J. Sun, J. Yang, N. Q. Zhao and X. W. Du, J. Mater. Chem., 2009, 19, 484–488 RSC.
- Q. L. Zhao, Z. L. Zhang, B. H. Huang, J. Peng, M. Zhang and D. W. Pang, Chem. Commun., 2008, 5116–5118 RSC.
- X. Y. Xu, R. Ray, Y. L. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS.
- J. G. Zhou, C. Booker, R. Y. Li, X. T. Zhou, T. K. Sham, X. L. Sun and Z. F. Ding, J. Am. Chem. Soc., 2007, 129, 744–745 CrossRef CAS.
- Z. A. Qiao, Y. F. Wang, Y. Gao, H. W. Li, T. Y. Dai, Y. L. Liu and Q. S. Huo, Chem. Commun., 2010, 46, 8812–8814 RSC.
- H. Zhu, X. L. Wang, Y. L. Li, Z. J. Wang, F. Yang and X. R. Yang, Chem. Commun., 2009, 5118–5120 RSC.
- S. Chandra, P. Das, S. Bag, D. Laha and P. Pramanik, Nanoscale, 2011, 3, 1533–1540 RSC.
- H. P. Liu, T. Ye and C. D. Mao, Angew. Chem., Int. Ed., 2007, 46, 6473–6475 CrossRef CAS.
- R. L. Liu, D. Q. Wu, S. H. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598–4601 CrossRef CAS.
- Y. Xiu, Q. A. Gao, G. D. Li, K. X. Wang and J. S. Chen, Inorg. Chem., 2010, 49, 5859–5867 CrossRef CAS.
- D. Y. Pan, J. C. Zhang, Z. Li, C. Wu, X. M. Yan and M. H. Wu, Chem. Commun., 2010, 46, 3681–3683 RSC.
- H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563–5565 CrossRef CAS.
- Y. H. Yang, J. H. Cui, M. T. Zheng, C. F. Hu, S. Z. Tan, Y. Xiao, Q. Yang and Y. L. Liu, Chem. Commun., 2012, 48, 380–382 RSC.
- Z. C. Yang, M. Wang, A. M. Yong, S. Y. Wong, X. H. Zhang, H. Tan, A. Y. Chang, X. Li and J. Wang, Chem. Commun., 2011, 47, 11615–11617 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21217j/ |
| This journal is © The Royal Society of Chemistry 2012 |
