1-(2-Naphthyl)benzimidazolium based tripod for fluorescence enhancement based recognition of surfactants in water†
Sandeep
Kumar
,
Shafali
Arora
,
Prabhpreet
Singh
and
Subodh
Kumar
*
Department of Chemistry, Guru Nanak Dev University, Amritsar-143005, Punjab, India. E-mail: subodh_gndu@yahoo.co.in
First published on 23rd August 2012
Abstract
1-(2-Naphthyl)benzimidazolium based fluorogenic tripod TRI-BINAP-1 exhibits highly selective and sensitive recognition of surfactants SDBS and SDS in water amongst a series of inorganic and organic anions. The titration studies, Job's plot, 1H NMR studies and HRMS conspicuously reveal the co-operative participation of the three arms of the tripod for encapsulation of SDS or SDBS in the tripod cavity. The long chain carboxylates exhibit change in fluorescence at much higher concentrations and Job's plot shows the non-cooperative binding of the three arms of the tripod to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (tripod
3 (tripod![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) carboxylate) stoichiometric complex. The lowest detection limit for SDBS and SDS by fluorescence is 0.25 μM. The reduced flipping of the naphthyl rings on complexation of TRI-BINAP-1 with SDBS or SDS decreases thermal deactivation processes and leads to enhanced fluorescence.
carboxylate) stoichiometric complex. The lowest detection limit for SDBS and SDS by fluorescence is 0.25 μM. The reduced flipping of the naphthyl rings on complexation of TRI-BINAP-1 with SDBS or SDS decreases thermal deactivation processes and leads to enhanced fluorescence.
Sodium dodecylbenzenesulfonate (SDBS) and sodium dodecylsulfate (SDS) are one of the most widely used anionic surfactants in domestic as well as industrial formulations.1 As a result of their anthropogenic origin and extensive use, their presence in natural waters can cause serious environmental problems2 by inhibiting biological activity and due to foam formation and transfer of other pollutants i.e. petroleum products, oil, pesticides etc. to the water. So, there is a need for the simple and effective analytical methods that enable the analysis of these surfactants in water at trace levels for environment control.3
A variety of procedures such as direct spectrophotometric determination,4 high-performance liquid chromatography (HPLC),5 gas chromatography-mass spectrometry (GC–MS),6 and capillary electrophoresis7 have been developed to determine SDBS, SDS and their homologues. The ion-selective electrodes8 have provided an alternate direct method but lack the reproducibility and signal stability.
More recently, imidazolium salts anchored hybrid materials9viz. MCM-41 or silica nano-particles based on their complimentary interaction with negatively charged carboxylate, sulfonate or sulfate anion and consequent release of the pre-adsorbed dye into the solution to signal the presence of the surfactant have been developed but exhibit many limitations. However, single molecule based fluorescent probes for selective recognition of surfactants have eluded the chemists though such probes are available for other anions10 are found to be sensitive and cost effective.
The positively charged imidazolium and its benzo derived molecular architects11 due to their electrostatic and hydrogen bonding interactions with negatively charged species have been widely used for the recognition of anions. Usually, in these researches, sp3 hybridized carbon atoms are attached at both nitrogen atoms of the (benz)imidazolium12 moiety. More recently, the presence of the combination of sp3 alkyl and sp2 aryl substituents at the nitrogen atoms of the (benz)imidazolium core13 has been beneficially used for understanding the mesomorphic properties of ionic crystals14 and the aggregation behavior of surfactants15etc.
During our continuing interest in developing anion chemosensors, the significance of this sp3, sp2 carbon atom combination on nitrogen atoms of the (benz)imidazolium moiety in the development of new anion chemosensors16 has been reported, especially with their high tolerance to the competitive protic solvents. However, in all these cases the 1-arylbenzimidazolium core is linked to chromogenic or fluorogenic moiety to achieve the sensing behaviour.
Now, we have synthesized 1-(2-naphthyl)benzimidazolium based tripod TRI-BINAP-1 which on excitation at 270 nm exhibits emission at 450 nm with a Stokes shift of 180 nm and does not require the presence of any additional fluorescent moieties to study the sensing behaviour. TRI-BINAP-1 exhibits highly selective fluorescence enhancement with SDBS and SDS in 95% aqueous medium (5% DMSO required for solubility of the tripod). Titration based stoichiometry determination, Job's plot, 1H NMR titration studies and HRMS of its complex with SDBS and SDS confirm the encapsulation of SDBS and SDS in the tripodal cavity of TRI-BINAP-1 to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex. To the best of our knowledge, this is the first example of molecular recognition based chemosensor for SDBS and SDS.
1 stoichiometric complex. To the best of our knowledge, this is the first example of molecular recognition based chemosensor for SDBS and SDS.
1-(2-Naphthyl)benzimidazole (2) was prepared by CuI and benzotriazole catalyzed N-arylation of benzimidazole with 2-bromonaphthalene by the procedure reported earlier for the synthesis of other N-arylbenzimidazoles.17 The solution of 1-(2-naphthyl)benzimidazole 2 and tribromide 3 in DMF on heating at 90 °C under nitrogen, and subsequent exchange of Br− with hexafluorophosphate gave TRI-BINAP-1 in 79% overall yield (Scheme 1). The monopod 3-butyl-1-(2-naphthyl)benz-imidazolium PF6− (4) was prepared by heating 2 with 1-bromobutane at 100 °C followed by exchange of Br− with PF6−. 1H NMR, 13C NMR, HRMS and CHN analysis confirmed the structures of TRI-BINAP-1 and 4.
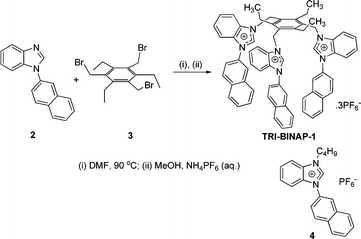 | ||
| Scheme 1 Synthesis of TRI-BINAP-1. | ||
The solution of TRI-BINAP-1 in 95% aqueous DMSO gave an absorption band at 270 nm (Fig. 1) and on excitation at 290 nm gave the emission band centered at 450 nm with Stokes shift of 160 nm. The addition of only 2 equiv. (10 μM) of SDBS and SDS to the solution of TRI-BINAP-1 (5 μM) in 95% aqueous DMSO resulted in >300% increase in the absorbance at 330 nm (Fig. 1) along with the increase in fluorescence intensity associated with a blue shift of the fluorescence band from 450 nm to 415 nm (Fig. 3). The addition of excess of various inorganic anions viz. F−, Cl−, Br−, I−, H2PO4−, NO3−, HSO4−, ClO4−, CN−, and organic anions viz. ethanoate, butyrate (C4), hexanoate (C6), octanoate (C8), decanoate (C10), benzoate etc. (1 mM) to the solution of TRI-BINAP-1 (5 μM) did not cause any change in its UV-Vis and fluorescence spectrum (Fig. 2). The addition of sodium salts of long chain carboxylates viz. laurate (C12, SL), myristate (C14, SM), palmitate (C16) up to 80 μM did not cause any change in the UV-Vis or fluorescence spectrum of TRI-BINAP-1 but at higher concentrations (>200 μM) lead to ∼200% increase in the fluorescence intensity at 415 nm. The addition of other alkyl sulfonate viz. methyl sulfonate (MeSO3–), butyl sulfonate (BuSO3–), octyl sulfonate (OctSO3–), decyl sulfonate (DecSO3–), octyl sulfate (OctOSO3–), decyl sulfate (DecSO4–) etc. even up to 1 mM did not cause any change in the fluorescence intensity of the solution of TRI-BINAP-1. The monopod 4 on excitation at 270 nm gave the emission band at 450 nm quite similar to the one observed in the case of TRI-BINAP-1 but this emission band remained unaffected even on addition of 1 mM solutions of all the anions discussed above and highlights the significance of the molecular architect of TRI-BINAP-1 in the recognition of SDBS and SDS.
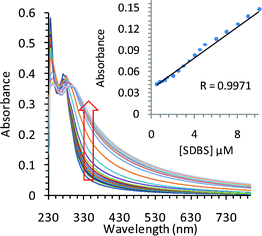 | ||
| Fig. 1 Effect of gradual addition of SDBS on the UV-Vis spectrum of TRI-BINAP-1 (5 μM) in 95% aqueous DMSO. | ||
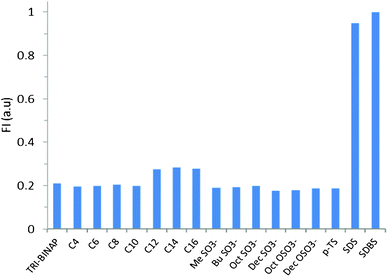 | ||
| Fig. 2 Fluorescence intensity of the solution of TRI-BINAP-1 in 95% aqueous DMSO at 415 nm before and after addition of sodium salts of various carboxylates (C4–C16), sulfonates and sulfates. | ||
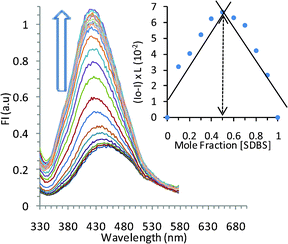 | ||
Fig. 3 Effect of gradual addition of SDBS on the emission spectrum of TRI-BINAP-1 (5 μM) in 95% aqueous DMSO. Inset shows Job's plot pointing to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of TRI-BINAP-1 and SDBS complex. 1 stoichiometry of TRI-BINAP-1 and SDBS complex. | ||
The absorption spectrum of TRI-BINAP-1 (5 μM) in 95% aqueous DMSO underwent an increase in absorption intensity at 330 nm upon gradual addition of the aqueous solution of SDBS and SDS and a plateau was achieved at ∼15 μM of SDBS and SDS. However, in case of sodium laurate (SL), the plateau was achieved on its addition of >200 μM to the solution of TRI-BINAP-1. The spectral fitting of the titration data by non-linear regression analysis program (SPECFIT-32) shows binding constant values of log βL(SDBS) = 4.83 ± 0.13 (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDBS, 1
SDBS, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); log βL(SDS) = 5.28 ± 0.11 (L
1); log βL(SDS) = 5.28 ± 0.11 (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDS, 1
SDS, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); and log βL(SL)3 = 12.13 ± 0.07 (L
1); and log βL(SL)3 = 12.13 ± 0.07 (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SL, 1
SL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), respectively for SDBS, SDS and SL. Therefore, SDBS and SDS form a 1
3), respectively for SDBS, SDS and SL. Therefore, SDBS and SDS form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex with TRI-BINAP-1 but sodium laurate forms a 1
1 stoichiometric complex with TRI-BINAP-1 but sodium laurate forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (TRI-BINAP-1
3 (TRI-BINAP-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SL) complex.
SL) complex.
Similarly, gradual addition of aliquots of SDBS to the solution of TRI-BINAP-1 (5 μM) in 95% aqueous DMSO showed gradual increase in the fluorescence intensity and achieved plateau after addition of 2 equiv. of SDBS. The spectral fitting of the fluorescence data using non-linear regression analysis (SPECFIT-32) shows a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with log β = 5.75 ± 0.09. The analysis of the Job's plot of the fluorescence titration profile in 95% aqueous DMSO showed the inflexion point near to 0.5 and further confirmed the existence of 1
1 with log β = 5.75 ± 0.09. The analysis of the Job's plot of the fluorescence titration profile in 95% aqueous DMSO showed the inflexion point near to 0.5 and further confirmed the existence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric (TRI-BINAP-1
1 stoichiometric (TRI-BINAP-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDBS) complex (Fig. 3). Similarly, SDS forms a 1
SDBS) complex (Fig. 3). Similarly, SDS forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with TRI-BINAP-1 with logβ = 5.41 ± 0.08 (see ESI†). The fluorescence of TRI-BINAP-1 increases linearly with the increase in concentration of SDBS and SDS between 0–10 μM and the lowest detection limit for both SDBS and SDS is 2.5 × 10−7 M (Fig. 4). The addition of sodium laurate, sodium myristate and sodium palmitate even up to 80 μM did not affect the fluorescence of TRI-BINAP-1 but at higher concentrations induced an increase in fluorescence with the plateau at ∼200 μM (see ESI†). The spectral fitting of these data shows the formation of a 1
1 complex with TRI-BINAP-1 with logβ = 5.41 ± 0.08 (see ESI†). The fluorescence of TRI-BINAP-1 increases linearly with the increase in concentration of SDBS and SDS between 0–10 μM and the lowest detection limit for both SDBS and SDS is 2.5 × 10−7 M (Fig. 4). The addition of sodium laurate, sodium myristate and sodium palmitate even up to 80 μM did not affect the fluorescence of TRI-BINAP-1 but at higher concentrations induced an increase in fluorescence with the plateau at ∼200 μM (see ESI†). The spectral fitting of these data shows the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (TRI-BINAP-1
3 (TRI-BINAP-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SL) complex with log βL(SL)3 of 11.52 ± 0.06. This stoichiometry is also confirmed by the inflexion point near to 0.7 in the Job's plot (see ESI†).
SL) complex with log βL(SL)3 of 11.52 ± 0.06. This stoichiometry is also confirmed by the inflexion point near to 0.7 in the Job's plot (see ESI†).
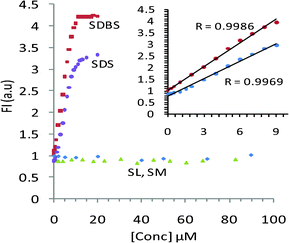 | ||
| Fig. 4 Concentration vs. emission intensity plots of SDBS, SDS, SL and SM on titration against TRI-BINAP-1. | ||
These data clearly point that in case of SDBS and SDS, the three arms of the tripod TRI-BINAP-1 act co-operatively to interact with the three oxygen atoms of the sulfonate/sulfate moiety to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with SDBS and SDS but in the case of long alkyl chain carboxylate ions due to unsymmetrical nature of the cavity with respect to Y-shaped carboxylate ion, the three arms act separately and individually to form 1
1 complex with SDBS and SDS but in the case of long alkyl chain carboxylate ions due to unsymmetrical nature of the cavity with respect to Y-shaped carboxylate ion, the three arms act separately and individually to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex each with one carboxylate anion.
1 complex each with one carboxylate anion.
Mechanism for sensing SDS and SDBS : 1H NMR, HRMS and theoretical studies
In order to rationalize the mechanism of interaction of TRI-BINAP-1 with these surfactants, a series of experiments were performed. The 1H NMR spectrum of TRI-BINAP-1 in the presence of SDS and SDBS separately confirms the molecular cavity based recognition of these surfactant anions. The titrations were performed both by the addition of TRI-BINAP-1 to the solution of SDBS or SDS and by the addition of SDBS and SDS to the solution of TRI-BINAP-1. The results were same in both the cases. The addition of 1 equiv. of SDBS to a 5 mM solution of TRI-BINAP-1 in CD3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) resulted in downfield shift of the BimC2–H proton by 0.28 ppm and points to the electrostatic interaction of sulfonate/sulfate anion with highly polarized C2–H protons. However, on performing these titrations in CD3CN–D2O (1
1) resulted in downfield shift of the BimC2–H proton by 0.28 ppm and points to the electrostatic interaction of sulfonate/sulfate anion with highly polarized C2–H protons. However, on performing these titrations in CD3CN–D2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in place of CD3CN–H2O (1
1) in place of CD3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the C2–H proton underwent deuterium exchange and disappeared without affecting the rest of the spectrum but provided more neat and clean 1H NMR titration data. The aromatic protons of SDBS in CD3CN–D2O (1
1), the C2–H proton underwent deuterium exchange and disappeared without affecting the rest of the spectrum but provided more neat and clean 1H NMR titration data. The aromatic protons of SDBS in CD3CN–D2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution appeared at δ 7.33 and 7.73 which were shifted up-field by 0.61 and 0.55 ppm resepctively18 to δ 6.72 and 7.18, on addition of 1 equiv. of TRI-BINAP-1 (Fig. 5a). The aliphatic protons corresponding to SDBS were also shifted up-field (Fig. 5b). However, the aromatic protons of the TRI-BINAP-1 exhibited change in their chemical-shifts only to a small extent. Similarly, on addition of 1 equiv. of TRI-BINAP-1 to the solution of SDS, the −O3S–OCH2CH2 protons of SDS were shifted up-field by 0.37 ppm from δ 3.98 to 3.61 and δ 1.67 to 1.30 (Fig. 6b). The aromatic protons corresponding to TRI-BINAP-1 exhibited change in their chemical shifts to a relatively smaller extent (Fig. 6a). The addition of higher equiv. of TRI-BINAP-1 to the solution of SDS or SDBS did not further cause any change in the chemical-shift of proton signals. Clearly, SDBS and SDS form a 1
1) solution appeared at δ 7.33 and 7.73 which were shifted up-field by 0.61 and 0.55 ppm resepctively18 to δ 6.72 and 7.18, on addition of 1 equiv. of TRI-BINAP-1 (Fig. 5a). The aliphatic protons corresponding to SDBS were also shifted up-field (Fig. 5b). However, the aromatic protons of the TRI-BINAP-1 exhibited change in their chemical-shifts only to a small extent. Similarly, on addition of 1 equiv. of TRI-BINAP-1 to the solution of SDS, the −O3S–OCH2CH2 protons of SDS were shifted up-field by 0.37 ppm from δ 3.98 to 3.61 and δ 1.67 to 1.30 (Fig. 6b). The aromatic protons corresponding to TRI-BINAP-1 exhibited change in their chemical shifts to a relatively smaller extent (Fig. 6a). The addition of higher equiv. of TRI-BINAP-1 to the solution of SDS or SDBS did not further cause any change in the chemical-shift of proton signals. Clearly, SDBS and SDS form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 compelx with TRI-BINAP-1 and the aromatic and some of aliphatic protons of SDBS and −O3SOCH2CH2 protons of SDS are under the influence of ring currents of the naphthyl rings of TRI-BINAP-1. The addition of sodium laurate did not cause any change in the 1H NMR spectrum of TRI-BINAP-1 (see ESI†).
1 compelx with TRI-BINAP-1 and the aromatic and some of aliphatic protons of SDBS and −O3SOCH2CH2 protons of SDS are under the influence of ring currents of the naphthyl rings of TRI-BINAP-1. The addition of sodium laurate did not cause any change in the 1H NMR spectrum of TRI-BINAP-1 (see ESI†).
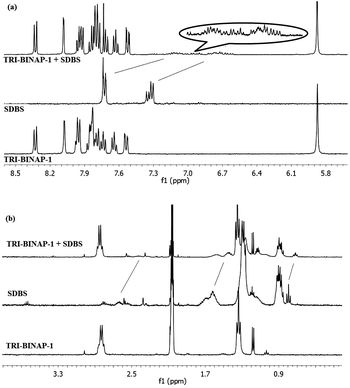 | ||
Fig. 5
1H NMR spectrum of TRI-BINAP-1 (5 mM) before and after addition of SDBS in CD3CN–D2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (a) aromatic region (b) aliphatic region. 1) (a) aromatic region (b) aliphatic region. | ||
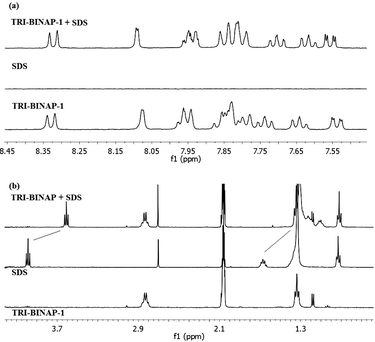 | ||
Fig. 6
1H NMR spectrum of TRI-BINAP-1 (5 mM) before and after addition of SDS in CD3CN-D2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (a) aromatic region (b) aliphatic region. 1), (a) aromatic region (b) aliphatic region. | ||
The insight into the encapsulation of the SDBS and SDS anions in the cavity of TRI-BINAP-1 has been further revealed by the geometrical optimization of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexes of TRI-BINAP-1 with SDBS and SDS. Both the structures of these complexes were optimized at DFT B3LYP 6-31G(d,p) basis set using Gaussian 09W package. The optimized structures clearly show that sulfonate and sulfate anions are stabilised through interactions with BIMC2–H and aromatic C–H moieties. The phenyl ring of SDBS and the CH2 group directly attached to the phenyl ring are facing the ring currents of naphthyl rings and support the observed up-field shift of SDBS's aryl and CH2 protons in the above discussed 1H NMR titration data. Similarly, in case of the optimized structure of a 1
1 stoichiometric complexes of TRI-BINAP-1 with SDBS and SDS. Both the structures of these complexes were optimized at DFT B3LYP 6-31G(d,p) basis set using Gaussian 09W package. The optimized structures clearly show that sulfonate and sulfate anions are stabilised through interactions with BIMC2–H and aromatic C–H moieties. The phenyl ring of SDBS and the CH2 group directly attached to the phenyl ring are facing the ring currents of naphthyl rings and support the observed up-field shift of SDBS's aryl and CH2 protons in the above discussed 1H NMR titration data. Similarly, in case of the optimized structure of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex of TRI-BINAP-1 with SDS, 4–5 CH2 units attached to sulfonate groups face the π currents (Fig. 7) of the three naphthyl rings and again confirm the up-field shift of these protons in the 1HNMR spectrum of a 1
1 stoichiometric complex of TRI-BINAP-1 with SDS, 4–5 CH2 units attached to sulfonate groups face the π currents (Fig. 7) of the three naphthyl rings and again confirm the up-field shift of these protons in the 1HNMR spectrum of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of TRI-BINAP-1 and SDS.
1 solution of TRI-BINAP-1 and SDS.
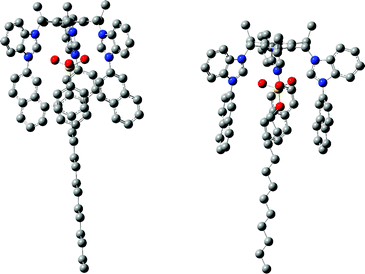 | ||
Fig. 7 The optimized structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of TRI-BINAP-1 with SDBS and SDS. For clarity the hydrogen atoms have been omitted. 1 complexes of TRI-BINAP-1 with SDBS and SDS. For clarity the hydrogen atoms have been omitted. | ||
The HRMS of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solutions of TRI-BINAP-1 with SDBS and SDS gave the parent ion peaks respectively at 1403.6240 [trication + PF6− + dodecylbenzenesulfonate (DBS)] and 1343.5683 [trication +PF6− + dodecyl sulfate (DS)] (see ESI†) and further corroborated that TRI-BINAP-1 forms 1
1 solutions of TRI-BINAP-1 with SDBS and SDS gave the parent ion peaks respectively at 1403.6240 [trication + PF6− + dodecylbenzenesulfonate (DBS)] and 1343.5683 [trication +PF6− + dodecyl sulfate (DS)] (see ESI†) and further corroborated that TRI-BINAP-1 forms 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with both dodecylbenzenesufonate and dodecylsulfate.
1 complexes with both dodecylbenzenesufonate and dodecylsulfate.
Further, to provide insight into the fluorescence mechanism involved in this sensing process, the geometrical optimization of 2 and 4 were made at DFT B3LYP 6-31G(d,p) level and the electron density distribution in HOMO and LUMO was analysed (Fig. 8). It is well documented that in compound 2, on excitation to first excited state, the internal charge transfer (ICT) takes place from benzimidazole core to the aryl ring.19 The present study of monopod 4 shows that on excitation during transfer of an electron from HOMO to LUMO, the ICT takes place from the naphthyl ring to the electron-deficient benzimidazolium cation core. This results in conversion of the benzimidazolium cation core to a neutral radical and naphthyl ring to a radical cationic species. In the excited state, the naphthyl ring is able to accommodate the positive charge more effectively than the benzimidazolium cation and thus leads to reorganization of the excited state to a more stabilized excited state (Scheme 2). This is quite similar to the well documented internal charge transfer in heterocycles including imidazolium moieties.20 The emission from this new lower energy excited state occurs at longer wavelength and the amount of red-shift of the emission depends on the charge stabilization ability of the naphthyl ring.
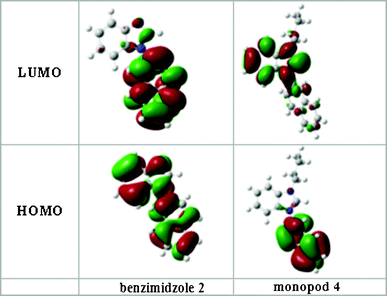 | ||
| Fig. 8 Molecular orbital diagrams of 1-(2-naphthyl)benzimidazole (2) and its benzimidazolium salt 4 showing the localization of charge in the HOMO's and LUMO's. | ||
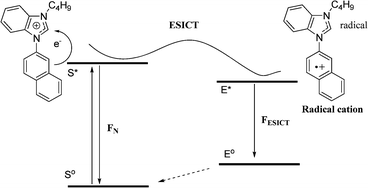 | ||
| Scheme 2 Proposed schematic presentation of ICT phenomenon in N-arylbenzimidazolium derivatives. | ||
The encapsulation of SDS or SDBS into the tripod cavity restricts the flipping of the naphthyl rings and decreases the participation of non-radiative stabilization processes and thus increases the efficiency of fluorescence emission. The interaction of sulfonate/sulfate anion decreases the positive charge on the benzimidazolium core and results in blue-shift of the emission band.
In order to understand the energetics of the association process between TRI-BINAP-1 and surfactants, enthalpy and entropy factors in binding of TRI-BINAP-1 with SDBS/SDS were determined (Fig. 9). The −ΔGo values increased linearly with the increase in temperature between 293 K to 308 K. The results clearly show that the increase in entropy (TΔSo 27.21 Kcal mol−1 for SDS and 17.88 Kcal mol−1 for SDBS) is the major contributing factor for these complexation processes (ΔGo −7.21 and −6.59 Kcal mol−1 for SDS and SDBS). The release of water molecules from the cavity of the TRI-BINAP-1 and the surroundings of the SDBS and SDS molecules during complex formation contributes positively to the molecular recognition of SDBS and SDS.21
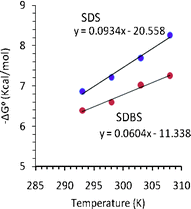 | ||
| Fig. 9 Free energy change vs. temperature plots of TRI-BINAP-1 against SDBS and SDS. | ||
In conclusion, we have prepared a tripod TRI-BINAP-1 which selectively recognizes SDBS and SDS in 95% aqueous medium. The cooperative binding of three imidazolium C–H protons with symmetrically placed three oxygen atoms of the surfactant anion and the hydrophobic interactions of naphthyl rings with aryl or alkyl protons of SDBS and SDS lead to high selectivity.
Experimental
General experimental conditions
All reagents were purchased from commercial suppliers and used without further purification. Solvents used were purified and dried by standard methods prior to use. TLC analyses were performed on silica gel plates and column chromatography was conducted over silica gel (mesh 100–200). 1H NMR spectra and titrations were carried out using JEOL A1 spectrometer operating at 300 MHz at GNDU, Amritsar or using Brucker 400 MHz machine at RSIC, Chandigarh. 13C NMR sepctra were recorded at 75 MHz. All chemical shifts are reported in ppm relative to the TMS as an internal reference. UV-Vis studies were carried out on a Shimadzu UV-1601 PC or Shimadzu UV-2400 machines using slit width of 1.0 nm and matched quartz cells. HRMS spectra were recorded on Brucker MicroToff/QII. The fluorescence experiments were performed on a Shimadzu 1501 fluorescence spectrophotometer. Elemental analysis was performed on a Flash EA-1112 series CHNS–O analyser instrument.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent gave pure compound 2 as a thick liquid (2.28 g). Yield 75%.1H NMR (CDCl3): δ 7.34–7.96 (m, 10H, ArH), 8.04 (d, J = 8.7 Hz, 1H, ArH), 8.21 (s, 1H, BIM C2–H). 13C NMR (CDCl3): δ 110.5, 120.6, 122.1, 122.2, 122.8, 123.7, 126.8, 127.3, 127.9, 130.2, 132.4, 133.6, 133.7, 133.8, 142.5, 144.0.
1) as eluent gave pure compound 2 as a thick liquid (2.28 g). Yield 75%.1H NMR (CDCl3): δ 7.34–7.96 (m, 10H, ArH), 8.04 (d, J = 8.7 Hz, 1H, ArH), 8.21 (s, 1H, BIM C2–H). 13C NMR (CDCl3): δ 110.5, 120.6, 122.1, 122.2, 122.8, 123.7, 126.8, 127.3, 127.9, 130.2, 132.4, 133.6, 133.7, 133.8, 142.5, 144.0.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture. Yield 79%; M. pt. 216 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.08 (t, J = 6.7 Hz, 9H, 3 × CH3), 2.88 (q, J = 7.5 Hz, 6H, 3 × CH2), 5.89 (s, 6H, 3 × CH2), 7.67–7.71 (m, 6H, ArH), 7.77 (t, J = 7.6 Hz, 3H, ArH), 7.83 (t, J = 7.8 Hz, 3H, ArH), 7.91 (d, J = 7.6 Hz, 3H, ArH), 7.94–8.01 (m, 6H, ArH), 8.09 (d, J = 8.2 Hz, 3H, ArH), 8.35 (d, J = 1.9 Hz, 3H, ArH), 8.48 (d, J = 8.4 Hz, 3H, ArH), 9.65 (s, 3H, BIM C2–H); 13C NMR (DMSO-d6, 100 MHz): δ 15.3, 23.6, 45.3, 113.9, 114.3, 123.0, 124.8, 127.4, 127.8, 127.9, 128.0, 128.1, 128.2, 128.4, 130.1, 130.2, 131.6, 131.9, 132.5, 133.0, 141.1, 148.5; HRMS m/z (TOF MS ES+) calculated for C66H57N6F12P2+ [M+], 1223.3928; found 1223.3926. Found C, 57.78; H, 4.31; N, 6.11%; C66H57N6P3F18 requires C, 57.90; H, 4.20; N, 6.14%.
2) mixture. Yield 79%; M. pt. 216 °C; 1H NMR (DMSO-d6, 400 MHz): δ 1.08 (t, J = 6.7 Hz, 9H, 3 × CH3), 2.88 (q, J = 7.5 Hz, 6H, 3 × CH2), 5.89 (s, 6H, 3 × CH2), 7.67–7.71 (m, 6H, ArH), 7.77 (t, J = 7.6 Hz, 3H, ArH), 7.83 (t, J = 7.8 Hz, 3H, ArH), 7.91 (d, J = 7.6 Hz, 3H, ArH), 7.94–8.01 (m, 6H, ArH), 8.09 (d, J = 8.2 Hz, 3H, ArH), 8.35 (d, J = 1.9 Hz, 3H, ArH), 8.48 (d, J = 8.4 Hz, 3H, ArH), 9.65 (s, 3H, BIM C2–H); 13C NMR (DMSO-d6, 100 MHz): δ 15.3, 23.6, 45.3, 113.9, 114.3, 123.0, 124.8, 127.4, 127.8, 127.9, 128.0, 128.1, 128.2, 128.4, 130.1, 130.2, 131.6, 131.9, 132.5, 133.0, 141.1, 148.5; HRMS m/z (TOF MS ES+) calculated for C66H57N6F12P2+ [M+], 1223.3928; found 1223.3926. Found C, 57.78; H, 4.31; N, 6.11%; C66H57N6P3F18 requires C, 57.90; H, 4.20; N, 6.14%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture. Yield 86%; M. pt. 98 °C; 1H NMR (CDCl3, 300 MHz): δ 0.92 (t, 3H, J = 7.2 Hz, CH3), 1.39 (hext, 2H, J = 7.2 Hz, CH2), 1.99 (quin, 2H, CH2), 4.52 (t, 2H, J = 7.2 Hz, CH2), 7.59–7.68 (m, 6H, ArH), 7.81 (d, 1H, J = 7.8 Hz, ArH), 7.91 (d, 1H, J = 7.5 Hz, ArH), 7.96 (d, 1H, J = 7.2 Hz, ArH), 8.04 (d, 1H, J = 8.4 Hz, ArH), 8.20 (s, 1H, ArH), 9.26 (s, 1H, Bim C2–H). 13C NMR (CDCl3, 75 MHz): δ 13.3, 19.7, 31.0, 47.9, 113.5, 113.6, 121.3, 124.5, 127.7, 127.9, 128.2, 128.7, 129.8, 130.9, 131.4, 131.7, 133.2, 133.5, 140.3. Found C, 56.55; H, 4.79; N, 6.19%; C21H21N2PF6 requires C, 56.51; H, 4.74; N, 6.28%.
2) mixture. Yield 86%; M. pt. 98 °C; 1H NMR (CDCl3, 300 MHz): δ 0.92 (t, 3H, J = 7.2 Hz, CH3), 1.39 (hext, 2H, J = 7.2 Hz, CH2), 1.99 (quin, 2H, CH2), 4.52 (t, 2H, J = 7.2 Hz, CH2), 7.59–7.68 (m, 6H, ArH), 7.81 (d, 1H, J = 7.8 Hz, ArH), 7.91 (d, 1H, J = 7.5 Hz, ArH), 7.96 (d, 1H, J = 7.2 Hz, ArH), 8.04 (d, 1H, J = 8.4 Hz, ArH), 8.20 (s, 1H, ArH), 9.26 (s, 1H, Bim C2–H). 13C NMR (CDCl3, 75 MHz): δ 13.3, 19.7, 31.0, 47.9, 113.5, 113.6, 121.3, 124.5, 127.7, 127.9, 128.2, 128.7, 129.8, 130.9, 131.4, 131.7, 133.2, 133.5, 140.3. Found C, 56.55; H, 4.79; N, 6.19%; C21H21N2PF6 requires C, 56.51; H, 4.74; N, 6.28%.
Procedure for surfactant sensing
Stock solution of chemosensor TRI-BINAP-1 and control compound 4 were prepared at 10−3 M in DMSO and was then diluted to 5 μm by DMSO–Water mixture (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95). The resulting solution was shaken well before recording the absorption or fluorescence spectra. The solutions of all the anions including surfactants were prepared in de-ionized water. Titration experiments were performed by placing 3 mL of a solution of chemosensor in a quartz cuvette of 1 cm optical path length, and then adding the surfactant or long chain carboxylate stock solution incrementally by means of a micro-pipette. Spectra were recorded 3 min after the addition.
95). The resulting solution was shaken well before recording the absorption or fluorescence spectra. The solutions of all the anions including surfactants were prepared in de-ionized water. Titration experiments were performed by placing 3 mL of a solution of chemosensor in a quartz cuvette of 1 cm optical path length, and then adding the surfactant or long chain carboxylate stock solution incrementally by means of a micro-pipette. Spectra were recorded 3 min after the addition.
All absorption scans and fluorescence spectra were saved as ACS II files and further processed in Excel™ to produce all graphs shown. Stability constants were determined by fitting the absorption and emission spectra recorded during the titrations of the chemosensor with surfactants and long chain carboxylates. The data was fitted with the global analysis program SPECFIT-32. For determining the stoichiometry of the various complexes, Job's plot22 method was used. For this the ratio of intensities Io/I (initial intensity/intensity at specific conc. of SDS/SDBS) vs. mole fraction of SDS/SDBS were plotted. The inflexion point in the Job's plot analysis gives the stoichiometry of the complex.
Computational details
The ground state structural calculations for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexes of chemosensor TRI-BINAP-1 with SDBS and SDS and monopod 4 and naphthylbenzimidazole (2) were computed using Becke's three-parameter hybrid functional and the Lee–Yang–Parr correlation (B3LYP) with 6-31G(d,p) basis set using GAUSSIAN 09W suite of programmes.23 The optimized structures and MO diagrams were generated with the aid of Gauss view 5.0 package.
1 stoichiometric complexes of chemosensor TRI-BINAP-1 with SDBS and SDS and monopod 4 and naphthylbenzimidazole (2) were computed using Becke's three-parameter hybrid functional and the Lee–Yang–Parr correlation (B3LYP) with 6-31G(d,p) basis set using GAUSSIAN 09W suite of programmes.23 The optimized structures and MO diagrams were generated with the aid of Gauss view 5.0 package.
Acknowledgements
We thank UGC, New Delhi for CAS programme and CSIR, New Delhi for fellowship to Sandeep Kumar and DST, New Delhi for financial assistance.References
- (a) B. Chen, S. Wang, Q. Zhang and Y. Huang, Analyst, 2012, 137, 1232–1240 RSC; (b) R. J. Larson, T. M. Rothgeb, R. J. Shimp, T. E. Ward and R. M. Ventullo, J. Am. Oil Chem. Soc., 1993, 70, 645–657 CrossRef CAS; (c) J. A. Perales, M. A Manzano, D. Sales and J. M. Quiroga, Bull. Environ. Contam. Toxicol., 1999, 63, 94–100 CrossRef CAS PubMed; (d) M. Villar, M. Callejon, J. C. Jimeez, E. Alonso and A. Guiraum, Anal. Chim. Acta, 2009, 634, 267–271 CrossRef CAS PubMed.
- (a) K. K. Brandt, M. Hesselsøe, P. Roslev, K. Henriksen and J. Sørensen, Appl. Environ. Microbiol., 2001, 67, 2489–2498 CrossRef CAS PubMed; (b) H. Urakawa, K. Inaba and S. Tsuneda, FEMS Microbiol. Lett., 2008, 282, 166–173 CrossRef CAS PubMed.
- (a) W. H. Chan, A. W. M. Lee and J.-Z. Lu, Anal. Chim. Acta, 1998, 361, 55–61 CrossRef; (b) M. Oshima, S. Motomizu and H. Doi, Analyst, 1992, 117, 1643–1646 RSC; (c) R. Patel and K. S. Patel, Analyst, 1998, 123, 1691–1695 RSC; (d) T. Sakai, H. Harada, X. Liu, N. Ura, K. Takeyoshi and K. Sugimoto, Talanta, 1998, 45, 543–548 CrossRef CAS PubMed; (e) S. Taguchi, K. Takahashi, N. Hata and I. Kasahara, Analyst, 2001, 126, 2078–2081 RSC.
- Y. Y. Hu, Y. Z. He and L. L. Qian, Anal. Chim. Acta, 2005, 536, 251–257 CrossRef CAS.
- (a) M. Villar, M. Callejon, J. C. Jimenez, E. Alonso and A. Guiraum, Anal. Chim. Acta, 2007, 599, 92–97 CrossRef CAS PubMed; (b) P. A. Lara-Martın, M. Petrovic, A. Gomez-Parra, D. Barcelo and E. Gonzalez-Mazo, Environ. Pollut., 2006, 144, 483–491 CrossRef PubMed; (c) P. A. Lara, A. Gomez and E. Gonzalezo, J. Chromatogr., A, 2006, 1114, 205–210 CrossRef PubMed.
- (a) Z. Moldovan, V. Avram, O. Marincas, P. Petrov and T. Ternes, J. Chromatogr., A, 2011, 1218, 343–349 CrossRef CAS PubMed; (b) M. Akyuz, Talanta, 2007, 71, 471–478 CrossRef PubMed; (c) W. H. Ding, S. H. Tzing and J. H. Lo, Chemosphere, 1998, 38, 2597–2606 CrossRef.
- (a) J. Riu and D. Barcelo, Analyst, 2001, 126, 825–828 RSC; (b) K. Heinig, C. Vogt and G. Werner, Analyst, 1998, 123, 349–353 RSC.
- (a) J. Sanchez and M. del Valle, Crit. Rev. Anal. Chem., 2005, 35, 15–29 CrossRef CAS; (b) M. Gerlache, J. M. Kauffmann, G. Quarin, J. C. Vire, G. A. Bryant and J. M. Talbot, Talanta, 1996, 43, 507–519 CrossRef CAS PubMed.
- (a) E. Climent, C. Gimenez, M. Marcos, R. Martınez-Manez, F. Sancenon and J. Soto, Chem. Commun., 2011, 47, 6873–6875 RSC; (b) C. Coll, R. Martınez-Manez, M. D. Marcos, F. Sancenon and J. Soto, Angew. Chem., Int. Ed., 2007, 46, 1675–1678 CrossRef CAS PubMed; (c) C. Coll, R. Casasus, E. Aznar, M. D. Marcos, R. Martınez-Manez, F. Sancenon, J. Soto and P. Amoros, Chem. Commun., 2007, 1957–1959 RSC; (d) C. Coll, R. Martínez-Máñez, M. D. Marcos, F. Sancenón, J. Soto and R. K. Mahajan, Eur. J. Inorg. Chem., 2009, 3770–3777 CrossRef CAS.
- (a) M. Wenzel, J. R. Hiscock and P. A. Gale, Chem. Soc. Rev., 2012, 41, 480–520 RSC; (b) P. A. Gale, Chem. Commun., 2011, 47, 82–86 RSC; (c) Y. Hua and A. H. Flood, Chem. Soc. Rev., 2010, 39, 1262–1271 RSC; (d) A. F. Li, J. H. Wang, F. Wang and Y. B. Jiang, Chem. Soc. Rev., 2010, 39, 3729–3745 RSC; (e) E. Galbrith and T. D. James, Chem. Soc. Rev., 2010, 39, 3831–3842 RSC; (f) P. A. Gale, Chem. Soc. Rev., 2010, 39, 3746–3771 RSC; (g) V. Amendola, L. Fabbrizzi and L. Mosca, Chem. Soc. Rev., 2010, 39, 3889–3915 RSC; (h) Z. Zhang and P. R. Schreiner, Chem. Soc. Rev., 2009, 38, 1187–1198 RSC; (i) P. A. Gale, S. E. Garcia-Garrido and J. Garric, Chem. Soc. Rev., 2008, 37, 151–190 RSC; (j) J. W. Steed, Chem. Soc. Rev., 2009, 38, 506–519 RSC.
- (a) Z. Xu, S. K. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 1457–1466 RSC; (b) J. Yoon, S. K. Kim, N. J. Singh and K. S. Kim, Chem. Soc. Rev., 2006, 35, 355–360 RSC; (c) H. Gong, B. M. Rambo, E. Karnas, V. M. Lynch, K. M. Keller and J. L. Sessler, J. Am. Chem. Soc., 2011, 133, 1526–1533 CrossRef CAS PubMed.
- (a) H. N. Kim, J. Lim, H. N. Lee, J. Ryu, M. J. Kim, J. Lee, D. Lee, Y. Kim, S. Kim, K. Lee, H. Lee and J. Yoon;, Org. Lett., 2011, 13, 1314–1317 CrossRef CAS PubMed; (b) Z. Xu, N. J. Singh, S. K. Kim, D. R. Spring, K. S. Kim and J. Yoon, Chem.–Eur. J., 2011, 11, 1163–1170 CrossRef PubMed; (c) D. Wang, X. Zhang, C. He and C. Duan, Org. Biomol. Chem., 2010, 8, 2923–2925 RSC; (d) L. Yang, S. Qin, X. Su, F. Yang, J. You, C. Hu, R. Xie and J. Lan, Org. Biomol. Chem., 2010, 8, 339–348 RSC; (e) C. E. Willans, K. M. Anderson, L. C. Potts and J. W. Steed, Org. Biomol. Chem., 2009, 7, 2756–2760 RSC.
- (a) S. Ahrens, A. Peritz and T. Strassner, Angew. Chem., Int. Ed., 2009, 48, 7908–7910 CrossRef CAS PubMed; (b) D. Meyer and T. Strassner, J. Org. Chem., 2011, 76, 305–308 CrossRef CAS PubMed; (c) R. Giernoth, Angew. Chem., Int. Ed., 2010, 49, 5608–5609 CrossRef CAS PubMed.
- P. H. J. Kouwer and T. M. Swager, J. Am. Chem. Soc., 2007, 129, 14042–14052 CrossRef CAS PubMed.
- (a) L. Shi, N. Li, H. Yan, Y. Gao and L. Zheng, Langmuir, 2011, 27, 1618–1625 CrossRef CAS PubMed; (b) L. Shi, N. Li and L. Zheng, J. Phys. Chem. C, 2011, 115, 18295–18301 CrossRef CAS.
- (a) S. Kumar, V. Luxami and A. Kumar, Org. Lett., 2008, 10, 5549–5552 CrossRef CAS PubMed; (b) S. Kumar and S. Kumar, Tetrahedron Lett., 2009, 50, 4463–4466 CrossRef CAS; (c) S. Kumar, P. Singh and S. Kumar, Tetrahedron Lett., 2012, 53, 2248–2252 CrossRef CAS.
- A. K. Verma, J. Singh, V. K. Sankar, R. Chaudhary and R. Chandra, Tetrahedron Lett., 2007, 48, 4207–4210 CrossRef CAS.
- Recently, the encapsulation of 1-octanesulfonate in pilalr[5]arene has been shown by upfield shift of the 1H NMR signals: Y. Ma, X. Ji, F. Xiang, X. Chi, C. Han, J. He, Z. Abliz, W. Chena and F. Huang, Chem. Commun., 2011, 47, 12340–12342 RSC.
- (a) C. Zhang, L. Liheng and Z. Chen, Chem. Phys. Lett., 2006, 420, 330–335 CrossRef CAS; (b) Z. Chen, C. Zhang and L. Feng, Spectrochim. Acta, Part A, 2005, 62, 592–595 CrossRef PubMed; (c) W. Rettig, Angew. Chem., Int. Ed. Engl., 1986, 25, 971–988 CrossRef; (d) W. Rettig and M. Zander, Chem. Phys. Lett., 1982, 87, 229–234 CrossRef CAS.
- (a) Z. R. Grabowski and K. Rotkiewicz, Chem. Rev., 2003, 103, 3899–4031 CrossRef PubMed; (b) Q. Lu, L. Dong, J. Zhang, J. Li, L. Jiang, Y. Huang, S. Qin, C. Hu and X. Yu, Org. Lett., 2009, 11, 669–672 CrossRef CAS PubMed; (c) S. H. Mashraqui, R. Betkar, M. Chandiramani, D. Quinonero and A. Frontera, Tetrahedron Lett., 2010, 51, 596–599 CrossRef CAS; (d) N. J. Singh, E. J. Jun, K. Chellappan, D. Thangadurai, R. P. Chandran, I. Hwang, J. Yoon and K. S. Kim, Org. Lett., 2007, 9, 485–488 CrossRef CAS PubMed.
- (a) M. Rekharsky, Y. Inoue, S. Tobey, A. Metzger and E. Anslyn, J. Am. Chem. Soc., 2002, 124, 14959–14967 CrossRef CAS PubMed; (b) C. L. D. Gibb and B. C. Gibb, J. Am. Chem. Soc., 2011, 133, 7344–7347 CrossRef CAS PubMed; (c) S. Matsumoto, H. Iwamoto and T. Mizutani, Chem.–Asian. J., 2010, 5, 1163–1170 CrossRef CAS PubMed; (d) N. Deng, P. Zhang, P. Cieplak and L. Lai, J. Phys. Chem. B, 2011, 115, 11902–11910 CrossRef CAS PubMed; (e) X. Salvatella, M. W. Peczuh, M. Gairí, R. K. Jain, J. Sánchez-Quesada, J. Mendoza, A. D. Hamilton and E. Giralt, Chem. Commun., 2000, 1399–1400 RSC.
- P. Job, Ann. Chim., 1928, 9, 113 CAS.
- M. J. Frisch et al. Gaussian 09, revision A.02, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra21247a |
| This journal is © The Royal Society of Chemistry 2012 |
