Pickering emulsions stabilized by in situ grown biologically active alkyl gallate microneedles
Elena
Tervoort
a,
André R.
Studart
b,
Claude
Denier
a and
Ludwig J.
Gauckler
*a
aInorganic Nonmetallic Materials, Department of Materials, ETH Zurich, 8093 Zurich, Switzerland. E-mail: ludwig.gauckler@mat.ethz.ch
bComplex Materials, Department of Materials, ETH Zurich, 8093 Zurich, Switzerland
First published on 17th July 2012
Abstract
We report on the stabilization of water-in-oil Pickering emulsions through the in situ growth of surface and biologically active alkyl gallate microneedles. Octyl gallate needles are shown to form a rigid interlocked network upon adsorption at the oil–water interface, generating emulsions that are stable for several months. Emulsions containing food-grade oils with potentially high antioxidant and antimicrobial activities were successfully prepared using solely octyl gallate microneedles as the surface active species. The safety and efficacy of alkyl gallates as antioxidant and antimicrobial agents combined with their ability to form strong interwoven needle-like structures at the oil–water interface makes this a promising approach for the preparation of functional, ultrastable Pickering emulsions for food, cosmetic and pharmaceutical applications.
Introduction
Emulsions stabilized by colloidal particles, known as Pickering emulsions, exhibit remarkable resistance against coalescence and coarsening due to the irreversible adsorption of particles at the liquid–liquid interface.1–3 Particles with high aspect ratios are particularly efficient in the stabilization of emulsions and foams,4,5 because they increase effective droplet coverage, induce capillary forces6 and if flexible are able to intertwine at interfaces.5 As a result, rod-shaped, anisotropic particles typically out-perform equivalent spherical solid particles as emulsion and foam stabilizers.7–9Alkyl gallates are biologically active molecules obtained from the esterification between gallic acid and fatty alcohols. Esters of gallic acid have been shown to have many significant biological activities as a result of their antioxidant, antimicrobial and antiviral effects.10,11 Propyl, octyl and lauryl gallates, in particular, are EU-approved and are often used as antioxidants in food, cosmetics and pharmaceuticals (E-numbers E310, E311 and E312, respectively).12 Emulsions are common vehicles used to deliver such antioxidant additives. The position of the antioxidant in such emulsions plays an important role, since it has been shown that antioxidant activity is enhanced at the oil–water interface where oxidation reactions are expected to occur.13,14 In typical formulations, emulsifiers competitively adsorb with gallates at the oil–water interface, which strongly influences the solubilization and partitioning behavior of the antioxidant. Therefore, the use of alkyl gallates as both antioxidants and emulsifiers in such emulsions would be a major advantage over current formulations.
Here, we show that initially isotropic crystals of alkyl gallates containing 4 to 8 carbons in the hydrocarbon chain (Fig. 1a) can undergo extensive in situ growth in oil–water mixtures to generate surface active microneedles that effectively stabilize water-in-oil Pickering emulsions.
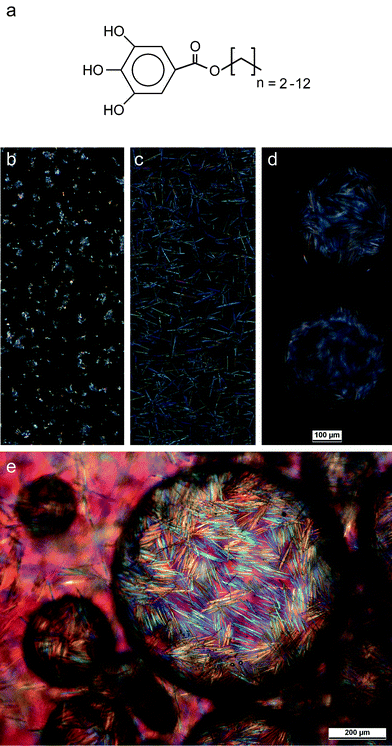 | ||
| Fig. 1 (a) The alkyl gallate molecules investigated in this study. (b and c) Optical images of toluene suspensions containing: (b) alkyl gallate particles (0.08 mol L−1 octyl gallate) in the absence of water and (c) containing alkyl gallate needles in the presence of 0.05 vol% water (with respect to toluene). (d and e) Optical images of the water-in-toluene emulsion obtained by adding 50 vol% water to the gallate-containing toluene suspension. The initial concentration of octyl gallate in toluene was 0.08 mol L−1. The water droplets are effectively stabilized by interfacially-adsorbed octyl gallate microneedles at the water–oil interfaces. Images (b–d) were taken at the same magnification. | ||
Materials and methods
Methyl (≥98%), ethyl (98%), propyl (≥98%), and lauryl (≥99%) gallates were purchased from Fluka. Gallic acid n-butyl ester (>97%) and n-octyl gallate (98%) were obtained from TGI and Alfa Aesar, respectively. Toluene (>99.7%, Fluka), decalin (98%, Sigma Aldrich), hexane (96%, Schalau), octane-fraction (Fluka), soybean oil (Sigma) and limonene 145 oil (99%, Fluka) were used without further purification as the dispersion media. Double deionized water was used in all the experiments.To prepare Pickering emulsions, alkyl gallate powder was added into the oil and the resulting oil suspension was ball-milled for 22 h using alumina balls. Emulsification was performed by vigorously stirring water and the suspension of gallate in oil for 3 min using a household mixer at 1040 rpm (Multimix 350 W, Braun, Spain). The proportion of the immiscible liquids in the emulsions was always 50/50 by volume.
The stability of the emulsions was determined by measuring the droplet size distribution over time using an optical microscope in transmission mode (Polyvar MET, Reichert-Jung, Austria) connected to a digital camera (DC 300, Leica, Switzerland). Using the same instrument, optical micrographs of wet suspensions and emulsions were obtained with and without crossed polarizers. To obtain statistically relevant data, five pictures were taken randomly from different locations of the same sample. The droplet size was measured by the linear intercept method using Lince software (TU Darmstadt, Germany).
The structure of the dried emulsions was investigated by scanning electron microscopy (SEM) (LEO 1530, Germany). Samples for SEM analysis were sputtered with Pt for 35 s at 40 mA.
The crystal structures of the as-supplied octyl gallate powder and of the octyl gallate microneedles were investigated by X-ray diffraction of Cu-Kα radiation using an acceleration voltage of 45 kV and a current of 40 mA (X'Pert Pro MPD diffractometer, PANalytical, Netherlands). Needle-like crystals were obtained by adding water (50 vol%) to toluene containing 0.08 mol L−1 of the as-supplied octyl gallate.
Results and discussion
We found that isotropic octyl gallate crystals initially dispersed in toluene can rapidly grow into microneedles upon the addition of minor amounts of water, as shown in Fig. 1. Due to its low solubility in toluene and high initial concentration (0.08 mol L−1), octyl gallate forms shape-less particle agglomerates in the absence of water (Fig. 1b). Surprisingly, the addition of water in concentrations below the solubility limit in toluene (<0.03 vol%) is sufficient to enable homogeneous and rapid rearrangement of the gallate agglomerates into needle-like crystals. X-Ray diffraction measurements on the initial octyl gallate powder and the octyl gallate needles show that both exhibit the same crystalline structure, indicating that the formation of the needles does not involve any phase transformation. Further water addition results in the complete transformation of all the octyl gallate into needle-like anisotropic particles (0.05 vol%, Fig. 1c) and eventually leads to the formation of very stable water-in-oil Pickering emulsions (50 vol% water, Fig. 1d). Each water droplet in the resulting emulsion is covered by a dense shell of interfacially adsorbed gallate microneedles that extend into the oil phase. Each microneedle adsorbed on the water droplet surface is approximately 100 μm in length and 2 μm in diameter. The needles tightly pack, overlap, and form oriented patchy domains at the oil–water interface (Fig. 1e and 2a). The resulting interlocked structure at the liquid–liquid interface is strong enough to preserve the shape of the original water droplet after drying (Fig. 2b). In contrast, a network of interfacially-adsorbed spherical particles never remains intact after drying droplets of this size. Closer examination of the emulsions during and after drying shows that the shell exhibits sharp local bends at the boundaries between the patches of oriented needles, creating a faceted three-dimensional structure after drying (Fig. 2a and b). Moreover, the wall thickness corresponds to at least two layers of particles, or about 4 μm. Such a thick wall generates strong steric repulsion between the adsorbed layers of particles, preventing droplet coalescence and Ostwald ripening and thus ensures effective emulsion stabilization.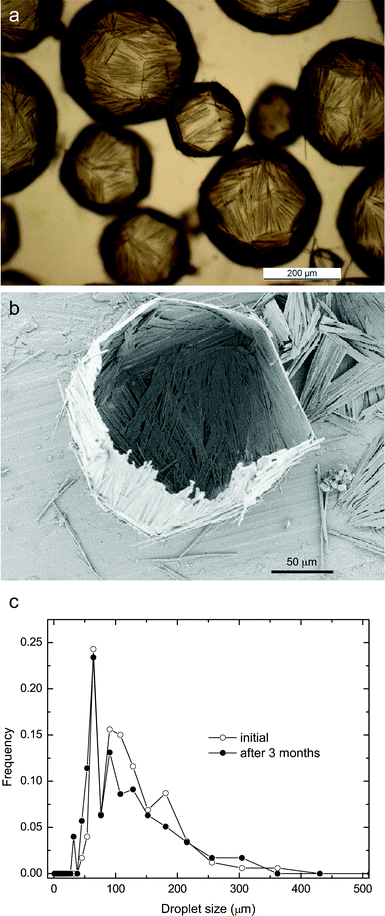 | ||
| Fig. 2 (a) Optical image of water-in-toluene droplets stabilized by interfacially-adsorbed octyl gallate microneedles. The needles grow in situ upon the addition of water to a toluene suspension containing 0.08 mol L−1 octyl gallate. (b) SEM image showing the faceted solid capsules obtained after drying the emulsion displayed in (a). (c) The time dependence of the droplet size distribution of a water-in-decalin emulsion stabilized by 0.08 mol L−1 octyl gallate. | ||
A number of different types of gallates such as methyl gallate, ethyl gallate, butyl gallate, octyl gallate and lauryl gallate were evaluated as emulsifiers in water–toluene mixtures (Fig. 1a). All of them, including the poorly water-soluble lauryl gallate, were able to form needles in the presence of water. However, only butyl and octyl gallate led to needle-like particles with the appropriate wettability to strongly adsorb at the oil–water interface and thus form very stable emulsions.15–17
The extraordinary stability of the emulsions prepared with gallate microneedles of optimum hydrophobicity is illustrated in Fig. 2c. The droplet size distribution of a water-in-decalin emulsion containing 0.08 mol L−1 octyl gallate remains nearly unchanged for more than three months. The stability of such needle-stabilized emulsions depends on the initial concentration of gallate in the oil phase. Each type of gallate exhibits an optimum concentration for emulsion stabilization. Generally, the concentration should be sufficient to produce enough needles to stabilize the whole oil–water interfacial area; however, too many needles prevent emulsification due to a significant increase in the viscosity of the gallate-containing oil phase. The optimum concentration increases for gallates with shorter hydrocarbon tail lengths due to their lower hydrophobicity. For example, the optimum concentration of butyl gallate is 0.15 mol L−1, as compared to 0.08 mol L−1 for octyl gallate.
To demonstrate the applicability of this system to food, cosmetics and pharmaceutical formulations we also prepared emulsions with edible oils. Emulsions comprising of 50% water in either soybean oil or limonene oil were effectively stabilized using 0.2 mol L−1 butyl gallate and 0.16 mol L−1 of octyl gallate, respectively (Fig. 3).
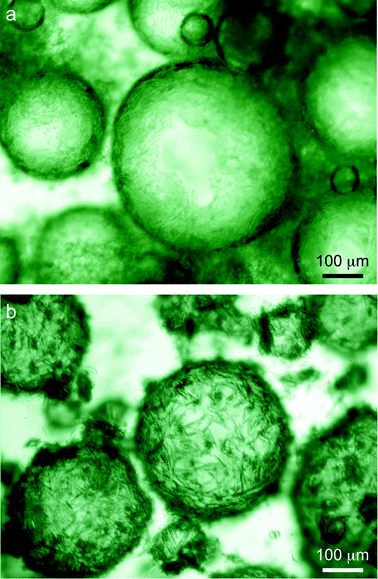 | ||
| Fig. 3 Optical images of (a) a water-in-soybean oil emulsion stabilized by butyl gallate microneedles (0.20 mol L−1) and (b) a water-in-limonene oil emulsion stabilized by octyl gallate microneedles (0.16 mol L−1). | ||
The mechanism of in situ growth of large needle-like particles from initially isotropic alkyl gallate crystals is probably related to the enhanced solubility of the gallate molecules in the continuous oil phase in the presence of dissolved water. Due to its high dipole moment, water molecules interact favorably with the polar hydroxyl groups of the alkyl gallate molecules, presumably decreasing the energy penalty associated with the interactions of such groups with the toluene molecules. Such a high solubility increases the Ostwald ripening between the particles, eventually leading to the formation of larger and more thermodynamically stable needle-like crystals. To better evaluate this hypothesis, we analyzed the formation of anisotropic gallate crystals in 50/50 vol% water-in-oil emulsions prepared with oils exhibiting different water solubility limits. Given their amphiphilic character, the alkyl gallate molecules are expected to be more soluble in oils containing a higher concentration of dissolved water. The results showed that stable particle-stabilized emulsions were obtained for all the evaluated oils. After a mixing time of 3 min, gallate crystals were found to grow up to 90 μm in emulsions containing toluene, as compared to only 10–15 μm in the other tested oils (Fig. 4). Such results compare well with the water solubility limits of the oils, which is as high as 0.033% w/w for toluene and lies equally or below 0.01% w/w for the other oils. Assuming that the measured needle length represents a snapshot of the Ostwald ripening process, the larger crystals observed in toluene may indeed result from the higher solubility of octyl gallate in this oil, which should ultimately lead to a faster growth rate of the needle particle.
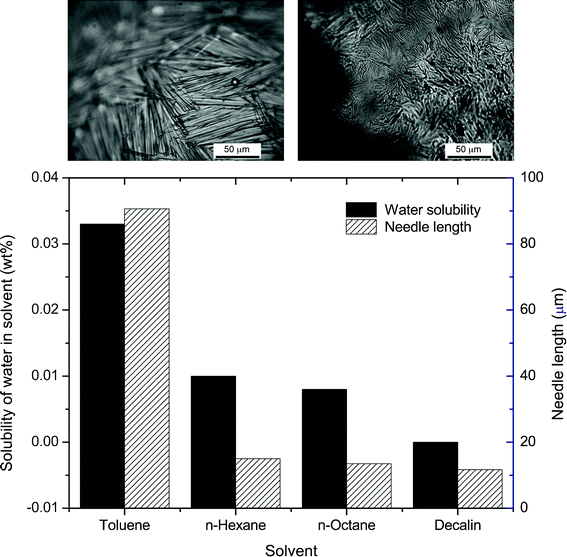 | ||
Fig. 4 The length of octyl gallate microneedles obtained by adding water to different apolar solvents containing an initial gallate concentration of 0.08 mol L−1 (water : solvent volume ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). For comparison, the solubility limits of water in the different solvents are also shown. The optical images show microneedles adsorbed on the surface of water droplets in toluene (left) and decalin (right). 50). For comparison, the solubility limits of water in the different solvents are also shown. The optical images show microneedles adsorbed on the surface of water droplets in toluene (left) and decalin (right). | ||
Conclusions
Water-in-oil Pickering emulsions exhibiting remarkable stability and antioxidant properties can be easily formed through the in situ growth and adsorption of alkyl gallate microneedles on the surface of water droplets. The gallate microneedles are formed upon the addition of water to an oil suspension of alkyl gallate isotropic particles during the emulsification process. Water dissolved in the oil presumably increases the solubility of the alkyl gallate molecules in the continuous phase and thus favors the growth of large, anisotropic crystals through Ostwald ripening. In line with the proposed growth mechanism, the aspect ratio of the gallate anisotropic particles increases in oils exhibiting higher water solubility limits. For the case of butyl and octyl gallates, the resulting microneedles are hydrophobic enough to adsorb at the oil–water interface and thus form a rigid, interlocking structure that protects the droplets from coalescence for time periods of more than 3 months. The in situ growth and stabilization of water-in-oil emulsions with gallate microneedles was also demonstrated using food-grade edible oils like limonene and soybean oil. The use of alkyl gallate microneedles as both emulsifiers and biologically active agents in the absence of any other synthetic molecules opens new possibilities for the formulation of ultrastable and functional Pickering emulsions for pharmaceutical, cosmetic and food applications.Acknowledgements
The authors are indebted to Dr K. Feldman for help with the optical micrographs and Elizaveta Pustovgar for experimental assistance.References
- R. Aveyard, B. P. Binks and J. H. Clint, Emulsions stabilised solely by colloidal particles, Adv. Colloid Interface Sci., 2003, 100–102, 503–546 CrossRef CAS.
- G. Kaptay, On the equation of the maximum capillary pressure induced by solid particles to stabilize emulsions and foams and on the emulsion stability diagrams, Colloids Surf., A, 2006, 282–283, 387–401 CrossRef.
- P. Pieranski, Two-Dimensional Interfacial Colloidal Crystals, Phys. Rev. Lett., 1980, 45(7), 569–572 CrossRef CAS.
- N. D. Denkov, I. B. Ivanov, P. A. Kralchevsky and D. T. Wasan, A possible mechanism of stabilization of emulsions by solid particles, J. Colloid Interface Sci., 1992, 150(2), 589–593 CrossRef CAS.
- R. G. Alargova, D. S. Warhadpande, V. N. Paunov and O. D. Velev, Foam superstabilization by polymer microrods, Langmuir, 2004, 20(24), 10371–10374 CrossRef CAS.
- B. Madivala, S. Vandebril, J. Fransaer and J. Vermant, Exploiting particle shape in solid stabilized emulsions, Soft Matter, 2009, 5(8), 1717–1727 RSC.
- R. G. Alargova, V. N. Paunov and O. D. Velev, Formation of polymer microrods in shear flow by emulsification - solvent attrition mechanism, Langmuir, 2006, 22(2), 765–774 CrossRef CAS.
- H. A. Wege, S. Kim, V. N. Paunov, Q. X. Zhong and O. D. Velev, Long-term stabilization of foams and emulsions with in situ formed microparticles from hydrophobic cellulose, Langmuir, 2008, 24(17), 9245–9253 CrossRef CAS.
- A. L. Campbell, B. L. Holt, S. D. Stoyanov and V. N. Paunov, Scalable fabrication of anisotropic micro-rods from food-grade materials using an in shear flow dispersion-solvent attrition technique, J. Mater. Chem., 2008, 18(34), 4074–4078 RSC.
- M. Kawada, Y. Ohno, Y. Ri, T. Ikoma, H. Yuugetu, T. Asai, M. Watanabe, N. Yasuda, S. Akao, G. Takemura, S. Minatoguchi, K. Gotoh, H. Fujiwara and K. Fukuda, Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice, Anti-Cancer Drugs, 2001, 12(10), 847–852 CrossRef CAS.
- H. Yamasaki, M. Uozaki, Y. Katsuyama, H. Utsunomiya, T. Arakawa, M. Higuchi, T. Higuti and A. H. Koyama, Antiviral effect of octyl gallate against influenza and other RNA viruses, International Journal of Molecular Medicine, 2007, 19(4), 685–688 CAS.
- F. S. Agency, Current EU approved additives and their E Numbers, http://www.food.gov.uk/, Accessed June 3rd, 2012.
- L. R. C. Barclay and M. R. Vinqvist, Membrane peroxidation - Inhibiting effects of water-soluble antioxidants on phospholipids of different charge types, Free Radical Biol. Med., 1994, 16(6), 779–788 CrossRef CAS.
- W. A. Pryor, J. A. Cornicelli, L. J. Devall, B. Tait, B. K. Trivedi, D. T. Witiak and M. D. Wu, A rapid screening-test to determine the antioxidant potencies of natural and synthetic antioxidants, J. Org. Chem., 1993, 58(13), 3521–3532 CrossRef CAS.
- U. T. Gonzenbach, A. R. Studart, E. Tervoort and L. J. Gauckler, Ultrastable particle-stabilized foams, Angew. Chem., Int. Ed., 2006, 45(21), 3526–3530 CrossRef CAS.
- D. Megias-Alguacil, E. Tervoort, C. Cattin and L. J. Gauckler, Contact angle and adsorption behavior of carboxylic acids on alpha-Al2O3 surfaces, J. Colloid Interface Sci., 2011, 353(2), 512–518 CrossRef CAS.
- A. R. Studart, R. Libanori, A. Moreno, U. T. Gonzenbach, E. Tervoort and L. J. Gauckler, Unifying model for the electrokinetic and phase behavior of aqueous suspensions containing short and long amphiphiles, Langmuir, 2011, 27(19), 11835–11844 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
