Facile preparation of hydroxide ion or proton conductive ionic liquids by mixing tetra-n-butylphosphonium hydroxide and benzimidazole†
Yuki
Tsuji
and
Hiroyuki
Ohno
*
Department of Biotechnology, Tokyo University of Agriculture and Technology, Koganei, Tokyo, 184-8588, Japan. E-mail: ohnoh@cc.tuat.ac.jp; Fax: +81-42-388-7024; Tel: +81-42-388-7024
First published on 18th September 2012
Abstract
Tetra-n-butylphosphonium benzimidazolate ([P4444][BzIm]) was prepared by mixing equimolar amounts of tetra-n-butylphosphonium hydroxide ([P4444][OH]) and neutral benzimidazole (BzIm). This salt had a melting point (Tm) of 55 °C and good thermal stability up to 300 °C. The equimolar mixture had ionic conductivity of 1.3 × 10−4 S cm−1 at 60 °C. Unequimolar mixing of these components gave rise either to hydroxide ions or proton conductive materials, depending on the mixing ratio. Addition of excess [P4444][OH] to benzimidazole (molar ratio of benzimidazole:[P4444][OH] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) led to good ionic conductivity (1.3 × 10−3 S cm−1 at 60 °C); this is attributed not only to [P4444] cations and [BzIm] anions but also hydroxide anions. Mixtures containing excess benzimidazole were expected to be proton conductors. As an example, the mixture with molar ratio benzimidazole
2) led to good ionic conductivity (1.3 × 10−3 S cm−1 at 60 °C); this is attributed not only to [P4444] cations and [BzIm] anions but also hydroxide anions. Mixtures containing excess benzimidazole were expected to be proton conductors. As an example, the mixture with molar ratio benzimidazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [P4444][OH] of 3
[P4444][OH] of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 had ionic conductivity of 4.1 × 10−4 S cm−1 at 60 °C and a decomposition temperature (Td) above 300 °C. This conductivity was higher than the value expected from the Tg and other physicochemical properties of [P4444][BzIm], suggesting the contribution of proton conduction. Proton conduction was further confirmed by chronoamperometric measurement using electrochemical cells equipped with platinum electrodes.
2 had ionic conductivity of 4.1 × 10−4 S cm−1 at 60 °C and a decomposition temperature (Td) above 300 °C. This conductivity was higher than the value expected from the Tg and other physicochemical properties of [P4444][BzIm], suggesting the contribution of proton conduction. Proton conduction was further confirmed by chronoamperometric measurement using electrochemical cells equipped with platinum electrodes.
Introduction
Ionic liquids (ILs) should be capable of exhibiting good thermal stability and high ionic conductivity. Many studies have been carried out to improve electrochemical properties by exploiting the design of the anions and cations involved.1–5 The negligible vapour pressure and high ionic conductivity of ILs make them attractive materials for use in electrochemical devices including energy conversion systems. The relatively large viscosity of these ILs is a drawback that restricts their application as highly ion conductive materials. The many steps involved in preparing ILs is a further drawback. We have reported a new and simple method for preparing ILs by neutralising tertiary amines with acids.6–9 The resulting ILs have interesting capabilities, including protic and distillable properties. Azoles such as imidazole and benzimidazole are excellent starting materials for preparing cations suitable for use in ILs, because their cationic charge is highly delocalised after protonation.10–12 Several papers have been published on ILs composed of azolate anions; that is, deprotonated azoles.13–19 ILs composed of azolate anions, such as 1-ethyl-3-methylimidazolium triazolate, are not thermally stable and are commonly explosive. It would be valuable to improve the stability of these azole-based ILs to make them suitable for electrochemical use.13Proton conductive ILs can be prepared by exploiting their design. Since ordinary ILs contain no mobile protons, a proton source such as acid must be added to ILs to provide proton conductive properties.20–28 Imidazole has both proton donor and proton acceptor sites in the same molecule. This property is the same as that of water molecules, which have excellent proton conductivity through successive hydrogen bonds. This feature makes imidazole derivatives popular as proton conductive base materials.29,30 Rapid proton conduction is reportedly facilitated by preparing a mixture of proton donors with a slight excess acceptors, rather than an equimolar mixture.21 As cations have been used as proton donors in Brønsted acid–base mixtures, proton acceptors should be added in slight excess to create vacant units for protons. Unfortunately these proton conductive materials gradually deteriorated in their physicochemical properties with increasing proton acceptor fraction. In the design of proton conductive materials it is necessary to reconcile these conflicting effects.
It is also important to improve the thermal stability of hydroxide ion conductive materials.31–35 Thermal stability could also be improved with the use of ILs. However, reports of ILs having hydroxide ion conductivity are scarce. This is believed to be due to the small size of the hydroxide ion, which gives a stronger electrostatic interaction. When hydroxide ion conductive properties are added to ILs, a wide range of applications of the ILs should become possible.
In the present study we report novel ILs prepared by mixing tetra-n-butylphosphonium hydroxide ([P4444][OH]) and benzimidazole, which is one of most thermally stable azoles. Three distinct properties of the mixture have been realised simply by changing the mixing ratio of [P4444][OH] and benzimidazole. The proton or hydroxide ion conductive properties should easily be switched by changing their mixing ratio.
Experimental
Materials
Reagent grade benzimidazole (Tokyo Chemical Industry Co., Ltd.) and 40 wt% aqueous solution of tetra-n-butylphosphonium hydroxide ([P4444][OH]) (gift from Hokko Chemical Co., Ltd.) were used without further purification. Equimolar amounts of [P4444][OH] and benzimidazole were mixed slowly in methanol under nitrogen gas atmosphere. The solution was stirred in an ice bath to prepare tetra-n-butylphosphonium benzimidazolate ([P4444][BzIm]) (Scheme 1). We would like to note here that abbreviation with brackets ([ ]) indicates the ions (for example, [BzIm] means deprotonated anion). On the contrary, ‘benzimidazole’ means neutral state. After stirring for a few hours, most of the methanol and water generated were removed using a rotary evaporator. It was then dried under reduced pressure at 45 °C for one day. Similar processes were applied to prepare benzimidazole/[P4444][OH] mixtures with differing amounts (x%) of benzimidazole (x = 16.7–80.0%). (The mixing ratio of these components was expressed as x% in molar ratio.) The water content of these samples was analysed by a Karl–Fischer moisture titrator (MKS–210, Kyoto Electronics Co., Ltd.). All samples confirmed to contain less than 0.3 wt% water were used for further analysis.![Neutralisation of [P4444][OH] with benzimidazole to prepare [P4444][BzIm].](/image/article/2012/RA/c2ra21482b/c2ra21482b-s1.gif) | ||
| Scheme 1 Neutralisation of [P4444][OH] with benzimidazole to prepare [P4444][BzIm]. | ||
Measurements
The structure of [P4444][BzIm] (x = 50.0) and benzimidazole/[P4444][OH] mixtures with differing amounts of benzimidazole (x%, x = 16.7–80%) were confirmed by 1H–NMR measurements (400 MHz, d6–DMSO, δ (ppm) relative to TMS). Benzimidazole: δ = 7.2 (t, 2H), 7.59 (t, 2H), 8.21 (s, 1H). Mixture x = 16.7: δ = 0.90 (m, 3H), 1.38 (m, 4H), 1.59, 2.16 (m, 2H), 6.67 (t, 2H), 7.30 (t, 2H), 7.61(s, 1H). Mixture x = 25.0: δ = 0.90 (m, 3H), 1.38 (m, 4H), 1.58, 2.15 (m, 2H), 6.66 (t, 2H), 7.27 (t, 2H), 7.60(s, 1H). Mixture x = 33.3: δ = 0.90 (m, 3H), 1.39 (m, 4H), 1.57, 2.16 (m, 2H), 6.67 (t, 2H), 7.28 (t, 2H), 7.61(s, 1H). Mixture x = 37.5: δ = 0.90 (m, 3H), 1.40 (m, 4H), 1.57, 2.14 (m, 2H), 6.66 (t, 2H), 7.28 (t, 2H), 7.60(s, 1H). Mixture x = 44.4: δ = 0.90 (m, 3H), 1.41 (m, 4H), 1.59, 2.15 (m, 2H), 6.68 (t, 2H), 7.23 (t, 2H), 7.60(s, 1H). Mixture x = 50.0: δ = 0.91 (t, 3H), 1.42 (m, 4H), 2.17 (t, 2H), 6.67 (t, 2H), 7.28 (t, 2H), 7.61(s, 1H). Mixture x = 54.5: δ = 0.91 (t, 3H), 1.41 (m, 4H), 2.15 (t, 2H), 6.76 (t, 2H), 7.33 (t, 2H), 7.73(s, 1H). Mixture x = 60.0: δ = 0.90 (t, 3H), 1.42 (m, 4H), 2.17 (t, 2H), 6.82 (t, 2H), 7.39 (t, 2H), 7.82(s, 1H). Mixture x = 66.7: δ = 0.91 (t, 3H), 1.41 (m, 4H), 2.13 (t, 2H), 6.92 (t, 2H), 7.44 (t, 2H), 7.94(s, 1H). Mixture x = 75.0: δ = 0.90 (t, 3H), 1.38 (m, 4H), 2.13 (t, 2H), 7.03 (t, 2H), 7.52 (t, 2H), 8.10(s, 1H). Mixture x = 80.0: δ = 0.91 (t, 3H), 1.42 (m, 4H), 2.15 (t, 2H), 7.06 (t, 2H), 7.52 (t, 2H), 8.09(s, 1H).Thermal properties of samples were evaluated using a differential scanning calorimeter (DSC, a SEIKO Instruments DSC-120) and by thermogravimetric analysis (TG/DTA) using a TG/DTA–220. DSC measurement was carried out from −120 °C to +200 °C at a sweeping rate of 5 °C min−1. TG/DTA was carried out from room temperature to 500 °C under a nitrogen gas atmosphere at a heating rate of 10 °C min−1. The bulk viscosity was determined using a Brookfield DV − I+ Viscometer. Viscosity was measured after the sample reached the predetermined temperature, from 20 °C to 80 °C. The ionic conductivity of the samples was determined by means of complex impedance spectrometry using a Schlumberger Solartron-1260 impedance/gain phase analyser over the frequency range from 10 Hz to 106 Hz. All measurements were made in a glove box (UNICO 650F) filled with dry nitrogen gas. The sample was sandwiched with a stainless steel plate and an ITO-coated glass electrode, with the aid of a polyethylene terephthalate spacer. The spacer was 0.10 mm thick and had an effective opening area of 0.28 cm2. The impedance was dynamically measured from 10 to 120 °C at a heating rate of 2.0 °C min−1.36 The conductivity was calibrated against that of 0.10 mol L−1 KCl aqueous solution.
The contribution of proton conduction to the ionic conductivity in the samples was confirmed by chronoamperometric measurements, using an electrochemical cell equipped with two platinum electrodes under nitrogen or hydrogen gas atmosphere without humidification. The applied voltage was 50 mV or 100 mV at 50 °C.
Results and discussion
[P4444][BzIm] salt was prepared as an equimolar mixture of [P4444][OH] and benzimidazole (x = 50.0), with a melting point (Tm) of 55.3 °C (peak top); see Table 1. According to TG/DTA analysis, the decomposition temperature (Td) of the neutral benzimidazole was 207 °C, whereas that of the equimolar mixture (x = 50.0) was 317 °C. The considerable increase in Td was attributed to formation of the salt.37 Furthermore, there were no decomposition signals of benzimidazole or [P4444][OH] in the TG/DTA chart showing one step thermogravimetric weight loss. These data imply the formation of [P4444][BzIm] salt without other derivatives. The very good thermal stability of this salt is explained by the use of phosphonium cations instead of imidazolium ones. The trend in the thermal properties was in a good agreement with previous reports.38,39| Bzlm (%) | T d/°C (10 °C min−1) | T m/°C | T g/°C | η/cP at 60 °C | σ i /S cm−1 at 60 °C | State at 20 °C |
|---|---|---|---|---|---|---|
| a —: not measured, Td: shoulder, Tm: peak top | ||||||
| 16.7 | 160/289 | 47.3 | — | 11.4 | 1.13 × 10−3 | solid |
| 25.0 | 158/285 | 21.1 | — | 17.7 | 1.30 × 10−3 | solid |
| 33.3 | 165/301 | — | −73.6 | 36.6 | 1.30 × 10−3 | liquid |
| 37.5 | 173/311 | — | −65.3 | 44.8 | 8.86 × 10−4 | liquid |
| 44.4 | 183/305 | 58.1 | −57.0 | 99.7 | 5.06 × 10−4 | solid |
| 50.0 | 317 | 55.3 | −48.8 | 127.7 | 1.27 × 10−4 | solid |
| 54.5 | 315 | 43.3 | −51.7 | 155 | 4.46 × 10−4 | solid |
| 60.0 | 311 | — | −55.0 | 189 | 4.09 × 10−4 | liquid |
| 66.7 | 209/306 | — | −47.9 | 195 | 2.44 × 10−4 | liquid |
| 75.0 | 214/319 | — | −44.6 | 244 | 1.81 × 10−4 | liquid |
| 100.0 | 207 | 170 | — | — | — | solid |
The [P4444][BzIm] salt gave 1H-NMR signals at 0.3–0.6 ppm higher magnetic field than neutral benzimidazole.37 The shift of these peaks to higher magnetic field was attributed to the neutralisation. These data all confirm that [P4444][BzIm] salt was formed by mixing equimolar amounts of [P4444][OH] and benzimidazole (x = 50.0).
Mixtures of [P4444][BzIm] salt containing either [P4444][OH] or benzimidazole were then prepared by changing the mixing ratio (x in %, x = 16.7–80.0 in molar ratio). As seen in Scheme 2, there are three different mixtures, i.e., neutral salt (x = 50.0), [P4444][OH] excess mixtures (x < 50.0), and benzimidazole excess mixtures (50.0 < x). The mixtures with x < 50.0 should contain [P4444] cations, [BzIm] anions (deprotonated benzimidazole) and [OH] anions, so that they were expected to be hydroxide ion conductive materials. Mixtures with x > 50.0 should contain [P4444] cations, [BzIm] anions, and neutral benzimidazole. These mixtures were therefore expected to be proton conductive materials, where benzimidazole would act as both proton donor and acceptor. The [BzIm] anion is expected to act as a proton hopping site. Proton exchange between [BzIm] and benzimidazole may enhance proton conductivity with the aid of rotation of [BzIm] or benzimidazole (or both) at a molecular level. These three distinct conductive properties are all realised by varying the mixing ratio of the starting materials.
![Major component of the mixture with different composition of [P4444][OH] and BzIm.](/image/article/2012/RA/c2ra21482b/c2ra21482b-s2.gif) | ||
| Scheme 2 Major component of the mixture with different composition of [P4444][OH] and BzIm. | ||
Hydroxide ion conductive materials
Mixtures with x < 50.0 are expected to act as hydroxide ion conductive materials. In the 1H-NMR measurements, mixtures with 16.7 ≤ x < 50.0 gave two sets of [P4444] cation peaks, attributed to a methylene group next to the phosphorus atom. Their integrated peak intensity showed agreement with the mixing ratio, and their chemical shifts were almost the same as those for [P4444][BzIm] (x = 50.0) and [P4444][OH]. Details are shown in the ESI.† From these results, it is suggested they were attributed to [P4444][BzIm] and [P4444][OH]. Mixtures with x = 16.7, 25.0, and 44.4 were solids, with Tm = 47.3, 21.1, and 58.1 °C respectively. Mixtures with 33.3 ≤ x ≤ 37.5 were liquid showing no Tm (Table 1). The effect of benzimidazole content (x) on Tg is not simple (see Fig. 1). The lowest Tg (−73.6 °C) was for the mixture with x = 33.3. The value of Tg increased with increasing benzimidazole fraction up to 50.0%, as seen in Fig. 1.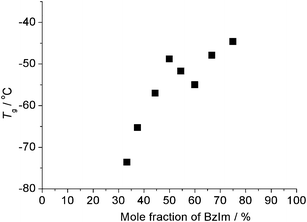 | ||
| Fig. 1 Glass transition temperature (Tg) for a series of mixtures. | ||
Fig. 2 shows the thermal gravimetric curves for mixtures with 16.7 ≤ x ≤ 50.0. The neutral salt with x = 50.0 exhibited one step Td at 317 °C, whereas mixtures with x < 50.0 underwent a two step degradation, at around 160 and 310 °C. The lower temperature was always near the Td of [P4444][OH] (178 °C; determined by analysing an aqueous solution of [P4444][OH]) and the higher one was near Td for [P4444][BzIm] (317 °C). These results strongly suggest that the degradation of mixtures with x < 50.0 seen in Fig. 2 is related to the Td values of [P4444][OH] and [P4444][BzIm]. The two Td values suggest that [P4444][OH] and [P4444][BzIm] species formed clusters without forming any large phase separation in the mixture.
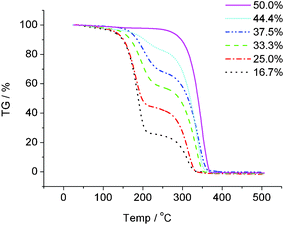 | ||
| Fig. 2 Thermogravimetric curves for a series of mixtures (x = 16.7–50.0). The scan rate was 10 °C min−1. | ||
Viscosity is a good parameter for evaluating ion transport properties. Fig. 3 shows the effect of the benzimidazole mole fraction on the viscosity of the mixture. For the neutral salt, x = 50.0, the viscosity was 128 cP at 60 °C, and decreased with decreasing benzimidazole fraction. For the mixture with x = 16.7 the viscosity was only 11.4 cP. These results strongly suggest that mixtures with smaller benzimidazole fractions allow faster diffusion of hydroxide ions. Fig. 4 shows the effect of the benzimidazole mole fraction on the ionic conductivity of the mixture. The ionic conductivity increased with decreasing x. Higher than 10−3 S cm−1 ionic conductivity of the mixtures at 60 °C was obviously due to their lower viscosity. Additionally, ionic conductivity of the mixtures with 33.3 ≤ x ≤ 50.0 reflects their Tg. Under the same temperature, materials show higher ionic conductivity when they have lower Tg. The Arrhenius plots of the ionic conductivity for all mixtures were depicted to be upper convex curves (Fig. S1† in the ESI). These relations clearly show that the migration of component ions in these samples obeys the vehicle mechanism. The ionic conductivity at x = 50.0 was relatively low (1.27 × 10−4 S cm−1 at 60 °C). This dip in the ionic conductivity at x = 50.0 was attributed presumably to two factors: referred from high Tg and low ion concentration by the major fraction of the neutral salt [P4444][BzIm]. These data all strongly suggest that mixtures with x < 50.0 have the potential to allow hydroxide ion conduction.
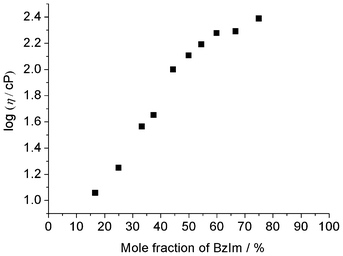 | ||
| Fig. 3 Viscosity for a series of samples with differing mole fractions of BzIm at 60 °C. | ||
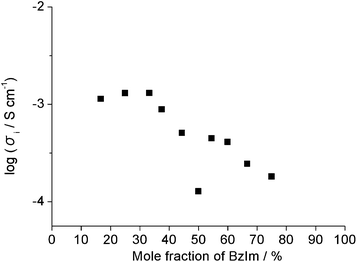 | ||
| Fig. 4 Ionic conductivity for a series of samples with different mole fractions of BzIm at 60 °C. | ||
Proton conductive materials
Thermally stable materials capable of proton conduction are being advocated. The mixtures with x > 50.0 are expected to be proton conductive materials, because they contain both [BzIm] and benzimidazole, as proton acceptor and donor, respectively. There was no difference between [BzIm] and benzimidazole in 1H-NMR signals of mixtures in the range 50.0 < x ≤ 75.0. The signals attributed to [BzIm] and benzimidazole shifted to a lower magnetic field with increasing x value.37 These results suggest the possibility of proton exchange between [BzIm] anion and benzimidazole in this mixture. Since the proton exchange was too fast to detect with 1H-NMR measurements, two species could not be detected; only one signal was found. A shift to the lower magnetic field side was found with increasing x value.Mixtures with x = 54.5 were obtained as a white solid, and mixtures with 60.0 ≤ x ≤ 75.0 were colourless liquids at room temperature. In particular, mixtures with 60.0 ≤ x < 75.0 were obtained as a liquid showing no Tm, but the Tg appeared and increased with increasing x value (Table 1). There was only one Td at around 315 °C for mixtures with x = 54.5 and 60.0. These mixtures had the same thermal stability as the neutral salt and showed no degradation profile of neither neutral benzimidazole nor [P4444][OH].37 These data strongly suggest that there is no free benzimidazole in the [P4444][BzIm]/benzimidazole mixture even containing a slight benzimidazole excess (50.0 < x ≤ 60.0). These data suggest possible complexation, or at least some interactions, between [P4444][BzIm] and neutral benzimidazole. On the other hand, there was a two step Td at approximately 210 °C and 315 °C in the thermogravimetric curves for the mixtures with x > 60.0. These weight losses were attributed to benzimidazole and [P4444][BzIm], respectively. These results indicate that there is some free benzimidazole in the mixture when x > 60, but the mixtures with 50.0 < x ≤ 60.0 are in a kind of equilibrium between [BzIm] and benzimidazole, maintaining the interaction through the proton exchange in view of the single Td value around 315 °C. As shown in Fig. 3, the viscosity gradually increases with increasing benzimidazole fraction (x). This might be due to the effect of π–π interaction among benzimidazoles.40
The ionic conductivity of mixtures with 50.0 ≤ x < 100 has been analysed as the function of x. All the Arrhenius plots were depicted to be not linear lines but upper convex curves.37 This obviously implies that the conduction of mobile ions in these mixtures conforms to the typical vehicle mechanism in the viscous matrix. The ionic conductivity of the mixture with x = 54.5 was 4.46 × 10−4 S cm−1 at 60 °C; this value is greater than the conductivity of other mixtures in the range 50.0 < x < 100 at the same temperature (Fig. 4). For general proton conduction in a mixture of proton donors and acceptors, it is important to prepare the mixture of proton donor with a slight excess acceptor than their equimolar mixture.21 Protonated amines as cations have been used as proton donors in the case of Brønsted acid–base mixtures, and the thermal stability of these mixtures tends to decrease. The salt properties are also impaired with an increasing proton acceptor fraction. For example, when acid was mixed with a slight excess of imidazole to prepare protic ILs, the resulting imidazolium bis(trifluoromethanesulfonyl)imide ([Im][Tf2N]) had Td (10% weight loss) at 379 °C, whereas for a mixture of [Im][Tf2N] and a slight excess neutral imidazole (Im), such as [Im][Tf2N]/Im = 3/4, the value of Td (10% weight loss) was 212 °C. This considerable decrease in Td is a serious drawback in proton conductors.21
We have successfully prepared thermally stable mixtures even in the presence of a little excess proton acceptor (x = 54.5 and 60.0) and having the same Td as salts. We found that mixtures in the range 50.0 < x ≤ 60.0 have good thermal stability. The thermal stability is necessary for future proton conductors, especially for advanced fuel cells.
The ionic conductivity at 60 °C of mixtures with x > 60.0 decreased with increasing x value. The ionic conductivity of mixtures with x ≥ 50.0 reflects Tg, which suggested that this decrease in the ionic conductivity is due mainly to the increase in the Tg. On the other hand, as mentioned above, the drop in the ionic conductivity of the mixture with x = 50.0 was explained by following two reasons, high Tg and lack of sufficient number of protons to contribute ionic conductivity. We accordingly determined the contribution of proton conduction to the total ionic conductivity. Chronoamperometric measurements were made to confirm and compare proton conduction in mixtures with x = 60.0, 66.7, and 75.0, using the cell composed of two platinum electrodes.37 A steady-state current was observed as a result of the reduction of protons when a suitable potential was applied under hydrogen gas atmosphere. Fig. 5 shows that there is a steady state current observed when a constant potential (50 and 100 mV) was applied to the cell containing the mixture with x = 60.0 under a hydrogen gas atmosphere. In contrast, no current was observed when the same potential was applied to the cell under a nitrogen gas atmosphere. The observed current was roughly proportional to the given potential under a hydrogen gas atmosphere. Furthermore, comparison of these mixtures revealed that the current reflected their ionic conductivities, as shown in Table 2. These data support the hypothesis that the protons migrated in these mixtures (x = 60.0, 66.7, and 70.0). In every case, almost no current was found under N2 regardless of the given potential (see Table 2). Based on the Arrhenius plots of ionic conductivity, we propose that the proton conduction of these mixtures is based on the vehicle mechanism. Effective proton conduction can be realised by designing the ions to facilitate rapid proton exchange and successive transport, via rotation41 of benzimidazole, for example.
![Time dependence of the current under DC polarisation for the mixture of benzimidazole and [P4444][OH] (x = 60.0) under a H2 or N2 atmosphere at 50 °C. (red): given potential is 100 mV under H2, (blue): 50 mV under H2, (green): 100 mV under N2, and ⋯: 50 mV under N2.](/image/article/2012/RA/c2ra21482b/c2ra21482b-f5.gif) | ||
Fig. 5 Time dependence of the current under DC polarisation for the mixture of benzimidazole and [P4444][OH] (x = 60.0) under a H2 or N2 atmosphere at 50 °C. ![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) (red): given potential is 100 mV under H2, (red): given potential is 100 mV under H2, ![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) (blue): 50 mV under H2, (blue): 50 mV under H2, ![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) (green): 100 mV under N2, and ⋯: 50 mV under N2. (green): 100 mV under N2, and ⋯: 50 mV under N2. | ||
Conclusions
A novel IL, namely [P4444][BzIm], was prepared by neutralisation of [P4444][OH] and benzimidazole. For the equimolar mixture of [P4444][OH] and benzimidazole, Tm was found at 55 °C and thermal stability was confirmed above 300 °C. Unequimolar mixtures of [P4444][BzIm] salt and either [P4444][OH] or benzimidazole were readily prepared by changing the amount of benzimidazole. The addition of an excess of one component yielded either hydroxide anions or proton conductors, depending on the mixing ratio. These samples were all prepared by one step mixing, and the carrier ions were easily controlled by the mixing ratio. The mixture with x = 33.3, which is expected to have hydroxide ion conductivity, had the highest ionic conductivity, of 1.3 × 10−3 S cm−1 at 60 °C, reflecting the low value of Tg (−73.6 °C). Mixtures with x = 54.5 or x = 60.0, which are expected to be proton conductors, exhibited only a single Td (317 °C). Based on the chronoamperometric measurements with an electrochemical cell equipped with platinum electrodes under a hydrogen atmosphere, mixtures with x = 60.0, 66.7, and 75.0 were confirmed to pass a steady-state current, suggesting proton conduction in the matrix. These mixtures are expected to act as a thermally stable proton conductive matrix suitable for application over a wide range of temperature.Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 21225007). Chronoamperometric measurements were carried out with the assistance of the Nissan Motor Co.References
- S. Tobishima, Electrochem., 2002, 70, 198–202 CAS.
- M. Doyle, S. K. Choi and G. Proulx, J. Electrochem. Soc., 2000, 147, 34–37 CrossRef CAS.
- A. B. McEwen, H. L. Ngo, K. LeCompte and J. L. Goldman, J. Electrochem. Soc., 1999, 146, 1687–1695 CrossRef CAS.
- H. Matsumoto, T. Matsuda, T. Tsuda, R. Hagiwara, Y. Ito and Y. Miyazaki, Chem. Lett., 2001, 30, 26–27 CrossRef.
- P. Wang, S. M. Zakeeruddin, P. Comte, I. Exnar and M. Grätzel, J. Am. Chem. Soc., 2003, 125, 1166–1167 CrossRef CAS.
- M. Hirao, K. Ito and H. Ohno, Electrochim. Acta, 2000, 45, 1291–1294 CrossRef CAS.
- M. Hirao, H. Sugimoto and H. Ohno, J. Electrochem. Soc., 2000, 147, 4168–4172 CrossRef CAS.
- M. Yoshizawa, W. Ogihara and H. Ohno, Electrochem. Solid-State Lett., 2001, 4, E25–E27 CrossRef CAS.
- H. Ohno and M. Yoshizawa, Solid State Ionics, 2002, 154–155, 303–309 CrossRef CAS.
- J. D. Holbrey, W. M. Reichert, R. P. Swatloski, G. A. Broker, W. R. Pitner, K. R. Seddon and R. D. Rogers, Green Chem., 2002, 4, 407–413 RSC.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC.
- P. Bonhôte, A. P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef.
- W. Ogihara, M. Yoshizawa and H. Ohno, Chem. Lett., 2004, 33, 1022–1023 CrossRef CAS.
- H. Xue, Y. Gao, B. Twamley and J. M. Shreeve, Inorg. Chem., 2005, 44, 5068–5072 CrossRef CAS.
- C. F. Ye, J. C. Xiao, B. Twamley and J. M. Shreeve, Chem. Commun., 2005, 2750–2752 RSC.
- H. Xue, B. Twamley and J. M. Shreeve, J. Mater. Chem., 2005, 15, 3459–3465 RSC.
- G.-H. Tao, Y. Guo, Y.-H. Joo, B. Twamley and J. M. Shreeve, J. Mater. Chem., 2008, 18, 5524–5530 RSC.
- A. R. Katritzky, S. Singh, K. Kirichenko, J. D. Holbrey, M. Smiglak, W. M. Reichert and R. D. Rogers, Chem. Commun., 2005, 868–870 RSC.
- M. Smiglak, C. C. Hines, T. B. Wilson, S. Singh, A. S. Vincek, K. Kirichenko, A. R. Katritzky and R. D. Rogers, Chem.–Eur. J., 2010, 16, 1572–1584 CrossRef CAS.
- M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411–15419 CrossRef CAS.
- A. Noda, M. A. B. H. Susan, K. Kudo, S. Mitsushima, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2003, 107, 4024–4033 CrossRef CAS.
- J. Sun, L. R. Jordan, M. Forsyth and D. R. MacFarlane, Electrochim. Acta, 2001, 46, 1703–1708 CrossRef CAS.
- A. Fernicola, S. Panero, B. Scrosati, M. Tamada and H. Ohno, ChemPhysChem, 2007, 8, 1103–1107 CrossRef CAS.
- A. Fernicola, S. Panero and B. Scrosati, J. Power Sources, 2008, 178, 591–595 CrossRef CAS.
- K. M. Johansson, E. I. Izgorodina, M. Forsyth, D. R. MacFarlane and K. R. Seddon, Phys. Chem. Chem. Phys., 2008, 10, 2972–2978 RSC.
- W. Ogihara, H. Kosukegawa and H. Ohno, Chem. Commun., 2006, 3637–3639 RSC.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206–237 CrossRef CAS.
- K. D. Kreuer, S. J. Paddison, E. Spohr and M. Schuster, Chem. Rev., 2004, 104, 4637–4678 CrossRef CAS.
- K. D. Kreuer, A. Fuchs, M. Ise, M. Spaeth and J. Maier, Electrochim. Acta, 1998, 43, 1281–1288 CrossRef CAS.
- K. D. Kreuer, Chem. Mater., 1996, 8, 610–641 CrossRef CAS.
- G. F. McLean, T. Niet, S. Prince-Richard and N. Djilali, Int. J. Hydrogen Energy, 2002, 27, 507–526 CrossRef CAS.
- J. R. Varcoe and R. C. T. Slade, Fuel Cells, 2005, 5(2), 187–200 CrossRef CAS.
- N. J. Robertson, H. A. Kostalik IV, T. J. Clark, P. F. Mutolo, H. D. Abruña and G. W. Coates, J. Am. Chem. Soc., 2010, 132, 3400–3404 CrossRef CAS.
- H. A. Kostalik IV, T. J. Clark, N. J. Robertson, P. F. Mutolo, J. M. Longo, H. D. Abruña and G. W. Coates, Macromolecules, 2010, 43, 7147–7450 CrossRef.
- J. Sun, D. R. MacFarlene and M. Forsyth, Electrochim. Acta, 2003, 48, 1971–1976 CrossRef CAS.
- H. Ohno, Y. Inoue and P. Wang, Solid State Ionics, 1993, 62, 257–264 CrossRef CAS.
- Y. Tsuji, T. Mizumo and H. Ohno, Chem. Commun., 2011, 47, 3132–3134 RSC.
- J. Kagimoto, K. Fukumoto and H. Ohno, Chem. Commun., 2006, 2254–2256 RSC.
- R. E. Del Sesto, C. Corley, A. Robertson and J. S. Wilkes, J. Organomet. Chem., 2005, 690, 2536–2542 CrossRef CAS.
- H. Nakamoto, A. Noda, K. Hayamizu, S. Hayashi, H. Hamaguchi and M. Watanabe, J. Phys. Chem. C, 2007, 111, 1541 CAS.
- T. Mukai, M. Yoshio, T. Kato, M. Y.-Fujita and H. Ohno, Electrochemistry, 2005, 73, 623 CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Arrhenius plots of the ionic conductivity for the mixtures. See DOI: 10.1039/c2ra21482b |
| This journal is © The Royal Society of Chemistry 2012 |
