Improved recovery of ionic liquids from contaminated aqueous streams using aluminium-based salts†
Catarina M. S. S.
Neves
,
Mara G.
Freire
and
João A. P.
Coutinho
*
Departamento de Química, CICECO (www.ciceco.ua.pt), Universidade de Aveiro, 3810-193 Aveiro, Portugal. E-mail: jcoutinho@ua.pt; Fax: +351 234 370 084; Tel: +351 234 370 200
First published on 12th September 2012
Abstract
The number of applications involving ionic liquids has dramatically increased in the past few years, and their production and use in a large scale will inevitably lead to their dispersion into water streams (either by wastewater disposal or accidental leakage). Studies on the removal and recovery of ionic liquids from wastewater streams are therefore of crucial importance, yet particularly scarce. In this work, the use of aluminium salts is proposed to concentrate and remove ionic liquids from aqueous solutions. Two aluminium-based salts (Al2(SO4)3 and AlK(SO4)2·12H2O) were used to treat various aqueous solutions of ionic liquids containing imidazolium-, pyridinium- and phosphonium-based fluids. The gathered results show the enhanced ability of these salts to remove and recover ionic liquids from aqueous media. The minimum recovery efficiency achieved was 96%, whereas for a large array of systems recoveries of circa 100% of ionic liquid were attained. The residual concentrations of ionic liquids in water range from 0.01 to 6 wt%. The results reported disclose a novel promising technique for the recovery and treatment of aqueous effluents contaminated with ionic liquids by using salts commonly employed in water treatment processes, allowing thus its easy scale-up and adaptation to new processes involving ionic liquids.
Introduction
The beneficial use of ionic liquids in a diverse range of applications has become increasingly evident.1–3 Since ionic liquids are constituted by ionic species, they present outstanding properties not common in molecular solvents, such as a negligible vapour pressure, non-flammability, high thermal and chemical stabilities, among others. Moreover, the possibility of directing their properties, by the adequate manipulation of the cation and/or the anion, allows the design of these solvents aiming at presenting a selected set of properties for a particular application.4,5 This tailoring ability makes ionic liquids excellent alternative solvents, that coupled with their negligible vapour pressures, contributed to their classification as potential “green solvents” of industrial interest.6 The intrinsic non-volatile nature of ionic liquids provides the enhanced opportunity to reduce, or even completely eliminate, hazardous and toxic emissions to the atmosphere. However, although ionic liquids cannot contribute to air pollution, most fluids (even those considered hydrophobic) present a non-negligible solubility in water.7–12 Therefore, this water miscibility, even if limited, will unavoidably lead to aquatic environmental impacts. It is well known that ionic liquids that have aromatic rings (like imidazolium or pyridinium-based fluids) are more toxic to aquatic environments than ionic liquids composed of saturated rings.13–18 On the other hand, ionic liquids constituted by cations that have functionalized groups in their alkyl chain are less toxic.13–18 Thus, despite the variable toxicity level imposed by the chemical structures of ionic liquids, their use in a large scale, either at the academic or industrial level, should also involve a treatment approach aiming at “cleaning” the discarded aqueous effluents, as well as to recover and recycle the ionic liquid used. In this context, the research and development of novel methods to remove and recover ionic liquids from wastewater streams with the final goal of creating “greener” and more sustainable processes is of vital significance.An approach already described in literature for the treatment of aqueous solutions contaminated with ionic liquids relays on their physicochemical degradation. This type of treatment addresses the oxidative,19–21 thermal22 and photocatalytic23–28 degradations. These processes are only applicable at diluted solutions and do not allow the recovery of the ionic liquid. Nevertheless, it should be stressed that the products of the reactions–hydrocarbons, water and CO2–are usually less toxic than the ionic liquids.22,24 Another approach that has been studied for the removal of ionic liquids consists of their adsorption in activated carbon, commercially available or modified.29–31 Anthony et al.29 were the first to propose the use of activated carbon for the adsorption of 1-butyl-3-methylimidazolium hexafluorophosphate. More recently, Palomar and co-workers30,31 reported a detailed study on the adsorption of different ionic liquids into activated carbon. Besides the adsorption step, the recovery of the ionic liquid was further attempted by the addition of acetone.31 Again, this approach is only possible in diluted solutions and degradation reactions between acetone and some ionic liquids are prone to occur.
Taking into consideration previous works32–39 which reported aqueous biphasic systems composed of ionic liquids and salting-out species, we explored herein such a possibility as an alternative pathway for recovering ionic liquids from aqueous effluents. Ionic-liquid-based aqueous biphasic systems (ABS) are formed when two mutually incompatible, though both miscible with water, ionic liquid/salt aqueous solutions are mixed. Above a critical concentration of those components, spontaneous phase separation takes place. The pioneering work of Rogers and co-workers35 demonstrated that the addition of a “kosmotropic” salt (like K3PO4) to an aqueous solution of a given ionic liquid can induce the phase separation: a top ionic-liquid-rich phase and a bottom salt-rich phase. After this proof of principle, several ionic-liquid-based ABS were further tested32,36,38,39 with the aim of recovering ionic liquids from aqueous effluents. Deng et al.32 presented the recovery of 1-allyl-3-methylimidazolium chloride with three inorganic salts: K3PO4, K2HPO4 and K2CO3. They found that, for the same concentration of salt, the recovery efficiency of the ionic liquid follows the order: K3PO4 > K2HPO4 > K2CO3,32 which is in good agreement with the Hofmeister series.40 The authors32 also concluded that the increase of the concentration of the salt increases the recovery efficiency of the ionic liquid. They reached a maximum recovery efficiency of 96.80% using 46.48 wt% of K2HPO4.32 In the same line of research, Li et al.36 studied the effect of some sodium-based salts to recover the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate from aqueous media. The salts used were Na3PO4, Na2CO3, Na2SO4, NaH2PO4 and NaCl, and the highest extraction efficiency (98.77%) was achieved with 16.94 wt% of Na2CO3.36 In a different perspective, and with the goal of reducing the use of charged species to recuperate ionic liquids from aqueous solutions, Wu et al.38,39 reported the recovery of different ionic liquids from aqueous solutions making use of carbohydrates. A recovery efficiency of 65% of 1-allyl-3-methylimidazolium bromide and 63% of 1-allyl-3-methylimidazolium chloride was achieved by the addition of sucrose.39 On the other hand, with a distinct ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate, and changing the sugar, the recovery efficiencies observed were higher, but still not satisfactory: 74% with sucrose, 72% with xylose, 64% with fructose and 61% with glucose.38 Despite the possibility of employing carbohydrates to remove ionic liquids from aqueous media, one must be aware that it increases the amount of organic matter in the aqueous streams if large scale applications are envisaged. In addition, the tests with tetrafluoroborate-based ionic liquids must be carried out with special care since these fluids suffer hydrolysis in contact with water.41
Taking into account these previous results,32,36,38,39 and our previous experience on the effect of salts upon the solubility of ionic liquids in aqueous media8 as well as on the preparation of ABS for extraction purposes,33,34,37 we tested here the ability of two strong salting-out inducing species–Al2(SO4)3 and AlK(SO4)2–to create ABS and recover ionic liquids from aqueous solutions. Besides the strong salting-out aptitude of these salts (according to the Hofmeister series40) that may easily induce the phase separation of ionic liquids from aqueous media, they are actually used in water treatment processes.42 According to the USEPA (United States Environmental Protection Agency), alum, chlorine, lime and coagulant aids can be added directly to the public water storage and distribution systems to treat water, and make it suitable for public consumption.42 Therefore, here, ABS based on aluminium-based salts with imidazolium-, pyridinium- and phosphonium-based ionic liquids were studied, and are here reported, with the goal of developing improved systems for the removal and recovery of ionic liquids from aqueous streams. All the ternary systems studied were additionally characterized by measuring some of their tie-lines and tie-line lengths. The physical properties of the coexisting phases, namely density, viscosity, conductivity and pH values were also determined to fully characterize and gather a broader picture on the applicability of these systems. The adequacy of this approach to a series of removal/recovery cycles is also reported.
Results and discussion
The ternary phase diagrams of the two salts (Al2(SO4)3 and AlK(SO4)2) and a large array of ionic liquids were firstly determined to obtain a wide characterization of these novel ABS. Contrarily to previous works,32,36,38,39 a large array of ionic liquids were studied here. The ionic liquids investigated, and that could be recovered from aqueous solutions by the addition of the Al2(SO4)3, were [C2mim][CF3SO3], [C4mim][CF3SO3], [C4mim][Tos], [C4mim][N(CN)2], [C8py][N(CN)2], [C7H7mim][C2H5SO4], [Pi(4441)][Tos], [P4444]Br, [P4444]Cl and [P1444][CH3SO4]. On the other hand, with AlK(SO4)2·12H2O, from the series of ionic liquids investigated, only [C4mim][CF3SO3] and [C8py][N(CN)2] could be recovered. Moreover, the ionic liquids [C4mim]Br, [C4mim][C2H5SO4] and [C8mim]Cl were also tested and found to be not sufficiently “hydrophobic” to undergo liquid–liquid demixing in the presence of the acidic aqueous solution of Al2(SO4)3. Since we are dealing with acidic aqueous solutions, only a limited range of ionic liquids are capable of creating ABS, as shown in a previous work where we provided a critical assessment regarding the formation of ionic-liquid-based ABS under acidic media.43 Besides the salting-out ability of the inorganic salt, the pH of the aqueous solution seems to play an important role on the formation of ionic-liquid-based ABS.The structures of the ionic liquids that were successfully removed are depicted in Fig. 1.
![Chemical structures of the ionic liquids used to form ABS: (i) [C2mim][CF3SO3]; (ii) [C4mim][CF3SO3]; (iii) [C4mim][N(CN)2]; (iv) [C4mim][Tos]; (v) [C8py][N(CN)2]; (vi) [C7H7mim][C2H5SO4]; (vii) [Pi(444)1][Tos]; (viii) [P4444]Br; (ix) [P4444]Cl; (x) [P4441][CH3SO4].](/image/article/2012/RA/c2ra21535g/c2ra21535g-f1.gif) | ||
| Fig. 1 Chemical structures of the ionic liquids used to form ABS: (i) [C2mim][CF3SO3]; (ii) [C4mim][CF3SO3]; (iii) [C4mim][N(CN)2]; (iv) [C4mim][Tos]; (v) [C8py][N(CN)2]; (vi) [C7H7mim][C2H5SO4]; (vii) [Pi(444)1][Tos]; (viii) [P4444]Br; (ix) [P4444]Cl; (x) [P4441][CH3SO4]. | ||
The experimental solubility phase diagrams obtained, at 298 K and at atmospheric pressure, for the ternary systems composed of ionic liquid + salt + water are presented in Fig. 2.
![Ternary phase diagrams for systems composed of ionic liquid + aluminium-based salt + water at 298 K and atmospheric pressure (weight fraction units): ■, [C2mim][CF3SO3]; ×, [C4mim][CF3SO3]; +, [C4mim][Tos]; ♦, [C4mim][N(CN)2]; ▲, [C8py][N(CN)2]; △, [C7H7mim][C2H5SO4]; , [Pi(444)1][Tos]; ◇, [P4444]Br; , [P4444]Cl; □, [P4441][CH3SO4]; ○, critical point.](/image/article/2012/RA/c2ra21535g/c2ra21535g-f2.gif) | ||
Fig. 2 Ternary phase diagrams for systems composed of ionic liquid + aluminium-based salt + water at 298 K and atmospheric pressure (weight fraction units): ■, [C2mim][CF3SO3]; ×, [C4mim][CF3SO3]; +, [C4mim][Tos]; ♦, [C4mim][N(CN)2]; ▲, [C8py][N(CN)2]; △, [C7H7mim][C2H5SO4];  , [Pi(444)1][Tos]; ◇, [P4444]Br; , [Pi(444)1][Tos]; ◇, [P4444]Br;  , [P4444]Cl; □, [P4441][CH3SO4]; ○, critical point. , [P4444]Cl; □, [P4441][CH3SO4]; ○, critical point. | ||
The phase diagrams are separated by salts, and reported in weight fraction percentage units (the phase diagrams in molality units are provided in ESI†). Aiming at providing a general trend and to allow the reader to know the mass fraction content at a given point, Table 1 presents the parameters obtained by regression of the experimental binodal curves (in weight fraction percentage units) by the application of eqn (1)44 described according to,
| [IL] = Aexp[(B × [Salt]0.5) − (C × [Salt]3)] | (1) |
| Ionic liquid | A ± σ | B ± σ | 105 (C ± σ) | R 2 |
|---|---|---|---|---|
| Al2(SO4)3 | ||||
| [C2mim][CF3SO3] | 84 ± 1 | −0.267 ± 0.004 | 2.4 ± 0.1 | 0.9994 |
| [C4mim][CF3SO3] | 102 ± 3 | −0.469 ± 0.013 | 6.6 ± 0.1 | 0.9888 |
| [C4mim][Tos] | 118 ± 2 | −0.355 ± 0.007 | 4.0 ± 0.2 | 0.9994 |
| [C4mim][N(CN)2] | 73 ± 1 | −0.259 ± 0.003 | 4.2 ± 0.1 | 0.9992 |
| [C8py][N(CN)2] | 111 ± 1 | −0.598 ± 0.004 | 3.8 ± 0.4 | 0.9983 |
| [C7H7mim][C2H5SO4] | 91 ± 1 | −0.265 ± 0.002 | 2.3 ± 0.1 | 0.9998 |
| [Pi(444)1][Tos] | 154 ± 4 | −0.534 ± 0.010 | 8.6 ± 0.4 | 0.9977 |
| [P4444]Br | 151 ± 6 | −0.666 ± 0.016 | 4.2 ± 0.8 | 0.9912 |
| [P4444]Cl | 145 ± 6 | −0.564 ± 0.014 | 2.3 ± 0.3 | 0.9958 |
| [P4441][CH3SO4] | 132 ± 7 | −0.551 ± 0.023 | 1.5 ± 1.1 | 0.9874 |
| AlK(SO4)2 | ||||
| [C4mim][CF3SO3] | 89 ± 1 | −0.473 ± 0.002 | 1.9 ± 0.1 | 0.9998 |
| [C8py][N(CN)2] | 92 ± 1 | −0.579 ± 0.009 | 1.6 ± 0.2 | 0.9972 |
The larger the biphasic region, the higher the ability of the salt to recover and remove the ionic liquid from solution. From the gathered data, the tendency of the ionic liquids to be recovered by the addition of Al2(SO4)3 follows the order: [C8py][N(CN)2] > [P4444]Br > [C4mim][CF3SO3] > [Pi(444)1][Tos] ≈ [P4441][CH3SO4] ≈ [P4444]Cl > [C4mim][N(CN)2] > [C4mim][Tos] > [C2mim][CF3SO3] > [C7H7mim][C2H5SO4]. Concerning the AlK(SO4)2 salt, [C8py][N(CN)2] is more easily removed from solution than [C4mim][CF3SO3]. Although this latter salt could only salt-out two of the studied ionic liquids it has the advantage that the concentration necessary to promote ABS, and consequently to remove ionic liquids from water streams, is lower than that required by using Al2(SO4)3. In general, the trends found for the ionic liquids ability in creating ABS are in good agreement with our previous works using distinct inorganic salts.37,43,45,46 For these different salts, concentrations of ≈ 22 wt% of Na2SO443 or ≈ 40 wt% of K3PO437,45,46 were needed to salt-out the different ionic liquids.
The tie-lines (TLs) and tie-line lengths (TLLs) for each system were also determined at 298 K. The consistency of the TLs was further checked using the Othmer–Tobias47 and Bancroft48 correlations–the correlation of each system is provided in ESI†. The total mixture compositions, the weight fraction percentage compositions of the coexisting phases (TLs), and the respective TLLs, are reported in Table 2.
| Ionic liquid | weight fraction percentage/wt (%) | TLL | %R | |||||
|---|---|---|---|---|---|---|---|---|
| [IL]IL | [Salt]IL | [IL]M | [Salt]M | [IL]Salt | [Salt]Salt | |||
| Al2(SO4)3 | ||||||||
| [C2mim][CF3SO3] | 56.84 | 2.21 | 44.96 | 9.96 | 6.10 | 35.34 | 60.60 | 97.1 |
| 63.19 | 1.19 | 45.00 | 13.01 | 3.44 | 40.02 | 71.27 | 97.9 | |
| 68.32 | 0.64 | 45.08 | 15.85 | 1.89 | 44.14 | 79.41 | 98.7 | |
| 60.20 | 1.62 | 39.82 | 15.04 | 4.20 | 38.48 | 67.04 | 96.4 | |
| [C4mim][CF3SO3] | 73.51 | 0.47 | 44.97 | 9.96 | 4.49 | 23.43 | 72.74 | 96.1 |
| 76.21 | 0.37 | 44.96 | 13.02 | 1.10 | 30.77 | 81.02 | 99.0 | |
| 82.14 | 0.20 | 44.92 | 15.90 | 0.41 | 34.67 | 88.70 | 99.7 | |
| [C4mim][Tos] | 57.09 | 4.10 | 45.06 | 13.00 | 0.20 | 46.16 | 70.75 | 99.9 |
| 61.49 | 3.31 | 44.98 | 15.88 | 0.06 | 50.08 | 77.21 | 100.0 | |
| 54.02 | 4.74 | 40.10 | 15.00 | 0.33 | 44.31 | 66.69 | 99.8 | |
| [C4mim][N(CN)2] | 58.66 | 0.68 | 45.09 | 9.94 | 0.95 | 40.04 | 69.85 | 99.6 |
| 63.53 | 0.27 | 44.90 | 13.03 | 0.41 | 43.51 | 76.51 | 99.8 | |
| 69.30 | 0.03 | 44.98 | 15.88 | 0.28 | 45.01 | 82.38 | 99.8 | |
| 61.45 | 0.42 | 40.12 | 14.96 | 0.62 | 41.87 | 73.61 | 99.5 | |
| [C8py][N(CN)2] | 59.03 | 1.12 | 24.99 | 14.98 | 3.69 | 23.66 | 59.75 | 91.1 |
| 66.54 | 0.73 | 45.04 | 9.95 | 1.94 | 28.41 | 70.29 | 98.8 | |
| 70.82 | 0.57 | 45.08 | 12.99 | 0.72 | 34.41 | 77.84 | 99.6 | |
| 75.38 | 0.42 | 44.98 | 15.88 | 0.31 | 38.60 | 84.22 | 99.8 | |
| [C7H7mim][C2H5SO4] | 53.66 | 4.01 | 45.02 | 9.98 | 5.46 | 37.35 | 58.61 | 98.1 |
| 59.88 | 2.54 | 44.70 | 13.10 | 2.92 | 42.15 | 69.38 | 98.4 | |
| 65.37 | 1.59 | 44.95 | 15.99 | 1.44 | 46.67 | 78.22 | 99.0 | |
| 56.77 | 3.21 | 39.86 | 15.03 | 3.82 | 40.20 | 64.58 | 97.1 | |
| [Pi(444)1][Tos] | 56.15 | 3.53 | 45.16 | 9.93 | 0.10 | 36.17 | 64.86 | 100.0 |
| 61.88 | 2.89 | 44.87 | 13.04 | 0.02 | 39.81 | 72.04 | 100.0 | |
| 67.99 | 2.32 | 44.80 | 15.93 | 0.01 | 42.22 | 78.82 | 100.0 | |
| [P4444]Br | 63.40 | 1.69 | 44.94 | 9.97 | 1.37 | 29.51 | 67.98 | 99.2 |
| 70.60 | 1.30 | 45.05 | 13.00 | 0.68 | 33.32 | 76.91 | 99.5 | |
| 76.70 | 1.03 | 44.82 | 15.93 | 0.33 | 36.72 | 84.30 | 99.7 | |
| [P4444]Cl | 55.46 | 2.90 | 37.49 | 14.03 | 1.59 | 36.28 | 63.37 | 98.6 |
| 55.44 | 2.90 | 44.85 | 10.00 | 1.01 | 39.42 | 65.55 | 99.6 | |
| 60.13 | 2.43 | 44.90 | 12.98 | 0.50 | 43.71 | 72.53 | 99.8 | |
| [P4441][CH3SO4] | 58.34 | 2.21 | 44.95 | 9.97 | 2.81 | 34.38 | 64.18 | 98.7 |
| 63.89 | 1.75 | 44.76 | 13.07 | 1.81 | 38.49 | 72.13 | 98.9 | |
| 70.16 | 1.33 | 45.03 | 15.87 | 1.33 | 41.14 | 79.52 | 99.1 | |
| 61.75 | 1.91 | 40.11 | 14.97 | 1.91 | 38.00 | 69.88 | 98.4 | |
| AlK(SO4)2 | ||||||||
| [C4mim][CF3SO3] | 61.21 | 0.61 | 51.00 | 2.18 | 4.77 | 9.27 | 57.11 | 97.4 |
| [C8py][N(CN)2] | 68.62 | 0.26 | 50.99 | 2.18 | 6.00 | 15.86 | 53.07 | 95.8 |
In addition, the critical point of each system (the mixture composition for which the composition of the two aqueous phases are equal) is presented in Table 3. Due to the low solubility of the salt AlK(SO4)2 in water it was only possible to have one TL for each system. Therefore the calculation of the critical points was not carried out with this type-based systems. As an example, Fig. 3 depicts the results obtained for the TLs and critical point for the system composed of [P4444]Br + Al2(SO4)3 + H2O.
![Binodal curve, TLs and critical point for the system composed of [P4444]Br + Al2(SO4)3 + H2O at 298 K: ◇, experimental binodal data; ●, TL data; ▲, TLs relation; ■, critical point.](/image/article/2012/RA/c2ra21535g/c2ra21535g-f3.gif) | ||
| Fig. 3 Binodal curve, TLs and critical point for the system composed of [P4444]Br + Al2(SO4)3 + H2O at 298 K: ◇, experimental binodal data; ●, TL data; ▲, TLs relation; ■, critical point. | ||
| Ionic liquid | Critical Point/wt (%) | |
|---|---|---|
| [IL] | [Salt] | |
| [C2mim][CF3SO3] | 14.32 | 28.72 |
| [C4mim][CF3SO3] | 1.49 | 57.15 |
| [C4mim][Tos] | 17.88 | 20.79 |
| [C4mim][N(CN)2] | 19.72 | 16.68 |
| [C8py][N(CN)2] | 3.13 | 38.55 |
| [C7H7mim][C2H5SO4] | 16.91 | 27.50 |
| [Pi(444)1][Tos] | 14.86 | 14.78 |
| [P4444]Br | 7.72 | 23.25 |
| [P4444]Cl | 35.03 | 6.29 |
| [P4441][CH3SO4] | 12.05 | 19.04 |
Aiming at exploring the viability of the proposed systems for wastewater treatment and to recover ionic liquids from aqueous effluents, Table 2 presents the values of the recovery efficiencies of the various ionic liquids determined according to,
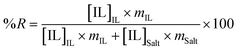 | (2) |
For an easier analysis of the recovery efficiencies of all ionic liquids, all the gathered results are depicted in Fig. 4 and 5.
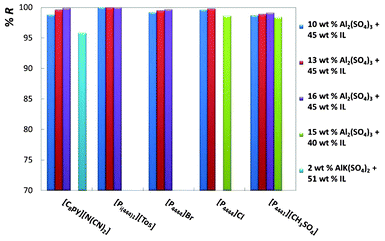 | ||
| Fig. 4 Percentage recovery efficiencies (%R) of phosphonium- and pyridinium-based ionic liquids at diverse mixture compositions. | ||
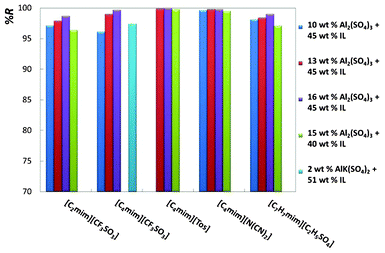 | ||
| Fig. 5 Percentage recovery efficiencies (%R) of imidazolium-based ionic liquids at diverse mixture compositions. | ||
Fig. 4 shows the results for phosphonium- and pyridinium-based ionic liquids, whereas Fig. 5 illustrates the effect of the ionic liquid anion and cation composing imidazolium-based fluids.
Fig. 4 and 5, and Table 2, show that remarkable results on the recovery of ionic liquids from aqueous media can be obtained. The recovery efficiencies of the diverse ionic liquids are always above 96% and for a large number of systems circa 100% of recuperation was achieved. In particular, the fluids [C4mim][Tos], [Pi(444)1][Tos] and [C4mim][N(CN)2] show recovery efficiencies above 99.5%, and for [C4mim][N(CN)2] the amount of inorganic salt at the IL-rich phase is inferior to 0.7 wt%. In addition, the ionic liquid concentration in the salt-rich phase is low, and ranges from 0.01 wt% with [Pi(444)1][Tos] to 6.10 wt% with [C2mim][CF3SO3], both with the salt Al2(SO4)3. For a fixed concentration of ionic liquid, and increasing the concentration of inorganic salt, there is an increase on the recovery efficiency of the ionic liquid, as depicted in Fig. 4 and 5. These results agree with the observations reported before by other authors.32,36 Regarding the AlK(SO4)2, the recoveries of the ionic liquids are also high (97.4% for [C4mim][CF3SO3] and 95.8% for [C8py][N(CN)2]), and remarkably they were achieved using only 2 wt% of salt in the total mixture composition. The main handicap of this salt is its inability to promote ABS with a larger matrix of ionic liquids.
The results gathered show that it is possible, by a proper selection of the salting-out species used, to achieve an enhanced recovery of ionic liquids from aqueous solutions. Previous works reported recovery efficiencies of ionic liquids substantially lower: 61–74% with carbohydrates38,39 and 96.80% with the addition of 46.48 wt% of K2HPO433 or 98.77% with the addition of 16.94 wt% of Na2CO3.36 With aluminium-based salts, commonly used in water treatment processes, the recovery efficiencies of ionic liquids are greatly improved. Moreover, the amount of inorganic salt used in this work is substantially lower than those previously reported with other salts.32,36
Based on the enhanced recovery efficiencies obtained, the use of aluminium-based two-phase systems, and their scale-up and adaptation to new processes involving ionic liquids, is straightforwardly envisaged. A graphical representation of the process configuration is depicted Fig. 6. This process was tested with the ionic liquid [Pi(444)1][Tos] in several recovery cycles, and the results obtained are depicted in Fig. 7. For each cycle, the recovery is always circa to 100% and proves the recyclability of the inorganic salt.
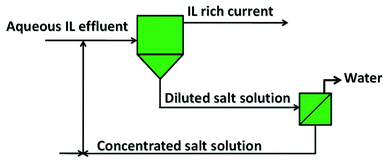 | ||
| Fig. 6 Configuration of the global process for the recovery of ionic liquids from aqueous effluents. | ||
![Recovery of [Pi(444)1][Tos] in several cycles using 16 wt% Al2(SO4)3 + 45 wt% IL, and concentration of salt and ionic liquid in both aqueous phases at the end of each cycle.](/image/article/2012/RA/c2ra21535g/c2ra21535g-f7.gif) | ||
| Fig. 7 Recovery of [Pi(444)1][Tos] in several cycles using 16 wt% Al2(SO4)3 + 45 wt% IL, and concentration of salt and ionic liquid in both aqueous phases at the end of each cycle. | ||
Since we are dealing with two charged species, one of the main concerns is the possibility of ion exchange. Therefore, aiming at evaluating if any ion exchange is occurring during the formation of ABS and further phase separation–to check if the ionic liquid recovered is a non-contaminated fluid–several analytical and spectroscopic techniques were applied to the characterization of the recovered coexisting phases of the systems composed of [Pi(444)1][Tos] and [C4mim][N(CN)2]. The detection of the sulfate anion was carried out with Fourier Transform Infrared spectroscopy. The Al3+ and [Pi(444)1]+ cations, at both aqueous phases, were quantified by Inductively Coupled Plasma Optical Emission Spectrometry. The imidazolium cation and tosylate anion contents were quantified by UV-Vis spectroscopy. From the results obtained we can guarantee that there is no ion exchange in these systems, and that the amount of each ion at each phase is in good agreement with those estimated from the tie-lines.
The thermophysical properties of the coexisting phases are of crucial relevance to evaluate the applicability and potential scale up of the process here proposed. Therefore, the density, viscosity, conductivity and pH values of the coexisting phases were measured for ABS composed of 15 wt% of Al2(SO4)3 + 45 wt% of water + 40 wt% of ionic liquid, and 2 wt% of AlK(SO4)2 + 45 wt% of water + 51 wt% of ionic liquid. The data obtained at 298 K are reported in Table 4. The compositions of each phase correspond to the TLs compositions reported in Table 2.
| Ionic liquid | Phase | pH | κ/(mS cm−1) | ρ/(g cm−3) | η/(mPa s) |
|---|---|---|---|---|---|
| Al2(SO4)3 | |||||
| [C2mim][CF3SO3] | IL | 1.72 | 46.4 | 1.2394 | 3.7823 |
| Salt | 1.63 | 13.7 | 1.2911 | 12.214 | |
| [C4mim][CF3SO3] | IL | 1.84 | 46.4 | 1.2374 | 3.7387 |
| Salt | 1.72 | 19.7 | 1.2899 | 12.128 | |
| [C4mim][Tos] | IL | 2.58 | 13.4 | 1.1355 | 9.3852 |
| Salt | 1.58 | 6.20 | 1.2969 | 15.568 | |
| [C4mim][N(CN)2] | IL | 4.82 | 44.1 | 1.0415 | 4.5958 |
| Salt | 2.53 | 13.5 | 1.3135 | 17.177 | |
| [C8py][N(CN)2] | IL | 5.07 | 24.6 | 1.0105 | 11.372 |
| Salt | 2.61 | 17.9 | 1.2630 | 8.8931 | |
| [C7H7mim][C2H5SO4] | IL | 1.81 | 20.3 | 1.1642 | 6.9866 |
| Salt | 1.51 | 13.2 | 1.3061 | 15.242 | |
| [Pi(444)1][Tos] | IL | 1.45 | 10.5 | 1.0709 | 14.363 |
| Salt | 0.98 | 13.2 | 1.2802 | 10.527 | |
| [P4444]Br | IL | 1.23 | 15.8 | 1.0591 | 19.382 |
| Salt | 1.00 | 21.0 | 1.2696 | 8.1799 | |
| [P4444]Cl | IL | 1.35 | 21.6 | 0.9999 | 12.795 |
| Salt | 1.20 | 7.78 | 1.2928 | 13.020 | |
| [P4441][CH3SO4] | IL | 1.87 | 12.8 | 1.0495 | 10.385 |
| Salt | 1.52 | 7.94 | 1.3045 | 14.579 | |
| AlK(SO4)2 | |||||
| [C4mim][CF3SO3] | IL | 3.83 | 29.2 | 1.1632 | 3.9547 |
| Salt | 3.03 | 32.0 | 1.0596 | 1.3749 | |
| [C8py][N(CN)2] | IL | 5.77 | 25.4 | 1.0088 | 6.921 |
| Salt | 3.74 | 18.8 | 1.0396 | 1.6005 | |
The results obtained indicate that for almost all systems the two phases present low pH values. Although these low pH values are a result of the acidic nature of the inorganic salt by itself (pH = 2.12 for an aqueous solution with 15 wt% of Al2(SO4)3 and pH = 3.15 for an aqueous solution with 5 wt% of AlK(SO4)2), the influence of the ionic liquid is also noted. The greatest impact is shown in ABS containing ionic liquids with the alkaline anion [N(CN)2]− that increases the pH values of the phases (albeit remaining acidic). This trend was previously verified by us with a distinct inorganic salt and a broad number of ionic liquids.43
The conductivity of the coexisting phases in ionic-liquid-based ABS is here reported for the first time. This property is also crucial since it reflects the charge density of the phase and the mobility of the ions in aqueous solutions. The values obtained range between (10.5 and 46.4) mS cm−1 for [Pi(444)1][Tos] and [CF3SO3]-based ionic liquids, respectively, in the ionic-liquid-rich phase and with the salt Al2(SO4)3. Regarding the salt-rich phase the values range between 6.20 mS cm−1 for [C4mim][Tos] and 32.0 mS cm−1 for the system composed of [C4mim][CF3SO3] and AlK(SO4)2.
The density and viscosity are relevant properties when envisaging the application of these systems in a large scale at an industrial level. Concerning the Al2(SO4)3, the viscosity of the ionic-liquid-rich phase ranges from 3.74 mPa s−1 for the system containing [C4mim][CF3SO3] to 19.4 mPa s−1 for the system composed of [P4444]Br. On the other hand, in the salt-rich phase, the values range between (8.18 and 17.2) mPa s−1 for the ionic liquids [P4444]Br and [C4mim][N(CN)2], respectively. Albeit it was expected that the ionic-liquid-rich phase would be more viscous, this is not observed for most of the systems evaluated. Indeed, this trend is only verified in the systems composed of [C8py][N(CN)2], [P4444]Br and [Pi(444)1][Tos]. These ionic liquids present a larger biphasic region and, consequently, their concentration at the salt-rich phase is smaller when compared with other ionic liquids. This can also be explained by the amount of water at the coexisting phases, since these systems present lower water contents at the ionic-liquid-rich phase. Comparing the viscosity of [C4mim][CF3SO3]-based ABS formed with other salts49 and sugars,33 the data here reported indicate slightly higher viscosities, albeit well below to those displayed by common polymer-based ABS.50–52 With the salt AlK(SO4)2, the viscosity is higher for the ionic-liquid-rich phase (3.95 mPa s−1 for the system containing [C4mim][CF3SO3] and 6.92 mPa s−1 for the system with [C8py][N(CN)2]) when compared with the salt-rich phase, and is generally lower when compared with those displayed by the salt Al2(SO4)3.
The density data shows that, for almost all systems, the salt-rich phase is denser than the ionic-liquid-rich phase. The exception to that rule is the system with [C4mim][CF3SO3] and AlK(SO4)2, and where an inversion of the phases was observed. These results are in close agreement with other systems previously reported.34,46,50 This is a direct consequence of the low concentration of salt at the ionic-liquid-rich phase and of the high density of the fluorinated ionic liquid.33 The values for the ionic-liquid-rich phase range from 1.00 g cm−3 for the system containing the [P4444]Cl and 1.24 g cm−3 for the system employing the [C2mim][CF3SO3]. For the salt-rich phase the values range between 1.04 g cm−3 for the system with [C8py][N(CN)2] + AlK(SO4)2 and 1.31 g cm−3 for the aqueous system composed of [C4mim][N(CN)2] + Al2(SO4)3. In general, the systems here reported present lower densities than the ones already reported in literature with ionic liquids and other salts,45,49 as well as those involving polymers.50–52
Conclusions
This work discloses a novel and efficient method to remove and recover ionic liquids from contaminated water streams. It makes use of the strong salting-out inducing ability of aluminium-based salts to create ABS that allow the removal of hydrophilic ionic liquids from diluted aqueous solutions by concentrating them into a second liquid phase. Aiming at gathering a broader picture on the amount of salts necessary to treat the contaminated effluents, the ternary phase diagrams, critical points, TLs and TLLs were determined at 298 K. The addition of Al2(SO4)3 and AlK(SO4)2 to aqueous solutions containing ionic liquids leads, for most of the systems, to a recovery efficiency of circa 100%, while reducing the ionic liquid concentration in the aqueous solution from 45 wt% to values around 1 wt%. By applying several cycles in the process here proposed, it was possible to guarantee that the salt can be recycled and the ionic liquid is completely recovered.The results here obtained constitute the first report on a complete recovery of ionic liquids from aqueous solutions making use of inorganic salts already used in water treatment processes, and could represent a major contribution for the development of industrial processes based on ionic liquids.
Experimental
Materials
Several ABS composed of the following ionic liquids: 1-ethyl-3-methylimidazolium trifluoromethanesulfonate (triflate), [C2mim][CF3SO3], purity 99 wt%; 1-butyl-3-methylimidazolium trifluoromethanesulfonate (triflate), [C4mim][CF3SO3], purity 99 wt%; 1-butyl-3-methylimidazolium tosylate, [C4mim][Tos], purity 98 wt%; 1-butyl-3-methylimidazolium dicyanamide, [C4mim][N(CN)2], purity >98 wt%; 1-octylpyridinium dicyanamide, [C8py][N(CN)2], purity >98 wt%; 1-benzyl-3-methylimidazolium ethylsulfate, [C7H7mim][C2H5SO4], purity 98 wt%; tri(isobutyl)methylphosphonium tosylate, [Pi(444)1][Tos], purity 98 wt%; tetrabutylphosphonium bromide, [P4444]Br, purity 95 wt%; tetrabutylphosphonium chloride, [P4444]Cl, purity 97 wt%; tributylmethylphosphonium methylsulfate, [P4441][CH3SO4], purity 96–98 wt% were investigated in this work. All imidazolium- and pyridinium-based ionic liquids were purchased from Iolitec. The phosphonium-based ionic liquids were kindly supplied by Cytec Industries Inc. To reduce the volatile impurities contents to negligible values, individual samples of ionic liquids were further purified under constant agitation at vacuum and moderate temperature (343 K) for a minimum of 24 h. After this purification approach, the purity of each ionic liquid was checked by 1H and 13C NMR spectra, and 19F and 31P NMR spectra whenever applicable, and found to be according to the purities given by the suppliers.The inorganic salts Al2(SO4)3 and AlK(SO4)2·12H2O were acquired from Himedia (purity ≥ 98.0 wt%) and from Carlo Erba (purity ≥ 99.5 wt%), respectively. The water employed was double distilled, passed across a reverse osmosis system and further treated with Milli-Q plus 185 water purification equipment. The buffers used in the calibration of the pH meter equipment were solutions with pH values of 4.00 and 7.00, acquired from Panreac. In order to calibrate the conductivity meter, standard solutions of KCl with concentrations of 0.1 and 0.01 M were diluted from a standard solution of 1 M supplied by Mettler Toledo and used.
Methods
Tie-lines (TLs) were determined by a gravimetric method originally proposed by Merchuk et al.44 For that purpose, ternary mixtures of known total composition at the biphasic region and composed of ionic liquid + salt + water were gravimetrically prepared within ±10−4 g, vigorously agitated, and left to equilibrate for at least 12 h at 298 (±1) K, aiming at a complete separation of both phases. After a careful separation step, both ionic liquid and salt phases were weighed within ±10−4 g. Each TL was determined by a mass balance approach through the relationship between the ionic-liquid-rich mass phase composition and the overall system composition, and for which the following system of four equations (eqn (3)–(6)) and four unknown values ([IL]IL, [IL]Salt, [Salt]IL and [Salt]Salt) was solved:44
| [IL]IL = Aexp[(B × [Salt]0.5IL) − (C × [Salt]3IL)] | (3) |
| [IL]Salt = Aexp[(B × [Salt]0.5Salt) − (C × [Salt]3Salt)] | (4) |
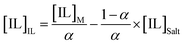 | (5) |
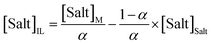 | (6) |
For the calculation of individual tie-line lengths (TLLs), the following equation was employed:
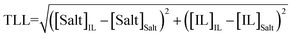 | (7) |
The critical point (the mixture composition at which the composition of the two aqueous phases becomes identical) of each system was also estimated.54,55 This was accomplished by extrapolating the TL slopes of individual systems followed by a further fitting using eqn (8),
| [IL] = f[Salt] + g | (8) |
The thermophysical properties of the coexisting phases, namely pH, conductivity, density and viscosity, were determined for total mixture compositions of 40 wt% of ionic liquid + 15 wt% of Al2(SO4)3 + 45 wt% of water, and 51 wt% of ionic liquid + 2 wt% of AlK(SO4)2 + 45 wt% of water. For such mixture compositions, the respective TLs were determined in order to know the composition of each aqueous phase.
Acknowledgements
This work was financed by national funding from FCT–Fundação para a Ciência e a Tecnologia, through the projects Pest-C/CTM/LA0011/2011 and PTDC/AAC-AMB/119172/2010. C. M. S. S. N. and M. G. F. also acknowledge FCT for the doctoral grant SFRH/BD/70641/2010 and the postdoctoral grant SFRH/BPD/41781/2007, respectively.References
- M. J. Earle, J. M. S. S. Esperanca, M. A. Gilea, J. N. Canongia Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- J. F. Wishart, Energy Environ. Sci., 2009, 2, 956–961 CAS.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef.
- P. J. Carvalho and J. A. P. Coutinho, Energy Environ. Sci., 2011, 4, 4614–4619 CAS.
- A. Bosmann, L. Datsevich, A. Jess, A. Lauter, C. Schmitz and P. Wasserscheid, Chem. Commun., 2001, 2494–2495 RSC.
- M. G. Freire, P. J. Carvalho, R. L. Gardas, I. M. Marrucho, L. M. N. B. F. Santos and J. A. P. Coutinho, J. Phys. Chem. B, 2008, 112, 1604–1610 CrossRef CAS.
- M. G. Freire, P. J. Carvalho, A. M. S. Silva, L. M. N. B. F. Santos, L. P. N. Rebelo, I. M. Marrucho and J. A. P. Coutinho, J. Phys. Chem. B, 2009, 113, 202–211 CrossRef CAS.
- M. G. Freire, C. M. S. S. Neves, P. J. Carvalho, R. L. Gardas, A. M. Fernandes, I. M. Marrucho, L. M. N. B. F. Santos and J. A. P. Coutinho, J. Phys. Chem. B, 2007, 111, 13082–13089 CrossRef CAS.
- M. G. Freire, C. M. S. S. Neves, K. Shimizu, C. E. S. Bernardes, I. M. Marrucho, J. A. P. Coutinho, J. N. C. Lopes and L. P. N. Rebelo, J. Phys. Chem. B, 2010, 114, 15925–15934 CrossRef CAS.
- M. G. Freire, C. M. S. S. Neves, S. P. M. Ventura, M. J. Pratas, I. M. Marrucho, J. Oliveira, J. A. P. Coutinho and A. M. Fernandes, Fluid Phase Equilib., 2010, 294, 234–240 CrossRef CAS.
- C. M. S. S. Neves, M. L. S. Batista, A. F. M. Cláudio, L. M. N. B. F. Santos, I. M. Marrucho, M. G. Freire and J. A. P. Coutinho, J. Chem. Eng. Data, 2010, 55, 5065–5073 CrossRef CAS.
- D. J. Couling, R. J. Bernot, K. M. Docherty, J. K. Dixon and E. J. Maginn, Green Chem., 2006, 8, 82–90 RSC.
- N. Papaiconomou, J. Estager, Y. Traore, P. Bauduin, C. Bas, S. Legeai, S. Viboud and M. Draye, J. Chem. Eng. Data, 2010, 55, 1971–1979 CrossRef CAS.
- C. Pretti, C. Chiappe, D. Pieraccini, M. Gregori, F. Abramo, G. Monni and L. Intorre, Green Chem., 2006, 8, 238–240 RSC.
- S. Stolte, J. Arning, U. Bottin-Weber, A. Muller, W.-R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2007, 9, 760–767 RSC.
- S. Stolte, M. Matzke, J. Arning, A. Boschen, W.-R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2007, 9, 1170–1179 RSC.
- S. P. M. Ventura, C. S. Marques, A. A. Rosatella, C. A. M. Afonso, F. Gonçalves and J. A. P. Coutinho, Ecotoxicol. Environ. Saf., 2012, 76, 162–168 CrossRef CAS.
- E. Siedlecka and P. Stepnowski, Environ. Sci. Pollut. Res., 2009, 16, 453–458 CrossRef CAS.
- E. M. Siedlecka, M. Golebiowski, J. Kumirska and P. Stepnowski, Chem. Anal. (Warsaw), 2008, 53, 943–951 CAS.
- E. M. Siedlecka, W. Mrozik, Z. Kaczynski and P. Stepnowski, J. Hazard. Mater., 2008, 154, 893–900 CrossRef CAS.
- W. H. Awad, J. W. Gilman, M. Nyden, R. H. Harris, T. E. Sutto, J. Callahan, P. C. Trulove, H. C. DeLong and D. M. Fox, Thermochim. Acta, 2004, 409, 3–11 CrossRef CAS.
- L. Berthon, S. I. Nikitenko, I. Bisel, C. Berthon, M. Faucon, B. Saucerotte, N. Zorz and P. Moisy, Dalton Trans., 2006, 2526–2534 RSC.
- T. Itakura, K. Hirata, M. Aoki, R. Sasai, H. Yoshida and H. Itoh, Environ. Chem. Lett., 2009, 7, 343–345 CrossRef CAS.
- T. Itakura, R. Sasai and H. Itoh, Water Res., 2005, 39, 2543–2548 CrossRef CAS.
- T. Itakura, R. Sasai and H. Itoh, Bull. Chem. Soc. Jpn., 2006, 79, 1303–1307 CrossRef CAS.
- X. Li, J. Zhao, Q. Li, L. Wang and S. C. Tsang, Dalton Trans., 2007, 1875–1880 RSC.
- P. Stepnowski, Aust. J. Chem., 2005, 58, 170–173 CrossRef CAS.
- J. L. Anthony, E. J. Maginn and J. F. Brennecke, J. Phys. Chem. B, 2001, 105, 10942–10949 CrossRef CAS.
- J. Lemus, J. Palomar, M. A. Gilarranz and J. J. Rodriguez, Adsorption, 2011, 17, 561–571 CrossRef CAS.
- J. Palomar, J. Lemus, M. A. Gilarranz and J. J. Rodriguez, Carbon, 2009, 47, 1846–1856 CrossRef CAS.
- Y. Deng, T. Long, D. Zhang, J. Chen and S. Gan, J. Chem. Eng. Data, 2009, 54, 2470–2473 CrossRef CAS.
- M. G. Freire, C. L. S. Louros, L. P. N. Rebelo and J. A. P. Coutinho, Green Chem., 2011, 13, 1536–1545 RSC.
- M. G. Freire, J. F. B. Pereira, M. Francisco, H. Rodríguez, L. P. N. Rebelo, R. D. Rogers and J. A. P. Coutinho, Chem.–Eur. J., 2012, 18, 1831–1839 CrossRef CAS.
- K. E. Gutowski, G. A. Broker, H. D. Willauer, J. G. Huddleston, R. P. Swatloski, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2003, 125, 6632–6633 CrossRef CAS.
- C. Li, J. Han, Y. Wang, Y. Yan, J. Pan, X. Xu and Z. Zhang, J. Chem. Eng. Data, 2010, 55, 1087–1092 CrossRef CAS.
- C. M. S. S. Neves, S. P. M. Ventura, M. G. Freire, I. M. Marrucho and J. A. P. Coutinho, J. Phys. Chem. B, 2009, 113, 5194–5199 CrossRef CAS.
- B. Wu, Y. Zhang and H. Wang, J. Phys. Chem. B, 2008, 112, 6426–6429 CrossRef CAS.
- B. Wu, Y. M. Zhang and H. P. Wang, J. Chem. Eng. Data, 2008, 53, 983–985 CrossRef CAS.
- F. Hofmeister, Naunyn-Schmiedebergs Arch. Pharmacol., 1888, 24, 247–260 Search PubMed.
- M. G. Freire, C. M. S. S. Neves, I. M. Marrucho, J. A. P. Coutinho and A. M. Fernandes, J. Phys. Chem. A, 2010, 114, 3744–3749 CrossRef CAS.
- American Water Works Association (AWWA), in Water quality and treatment: a handbook of community water supplies, McGraw-Hill, 4th edn, 1990 Search PubMed.
- A. F. M. Cláudio, A. M. Ferreira, S. Shahriari, M. G. Freire and J. A. P. Coutinho, J. Phys. Chem. B, 2011, 115, 11145–11153 CrossRef.
- J. C. Merchuk, B. A. Andrews and J. A. Asenjo, J. Chromatogr., Biomed. Appl., 1998, 711, 285–293 CrossRef CAS.
- C. L. S. Louros, A. F. M. Cláudio, C. M. S. S. Neves, M. G. Freire, I. M. Marrucho, J. Pauly and J. A. P. Coutinho, Int. J. Mol. Sci., 2010, 11, 1777–1791 CrossRef CAS.
- S. P. M. Ventura, C. M. S. S. Neves, M. G. Freire, I. M. Marrucho, J. Oliveira and J. A. P. Coutinho, J. Phys. Chem. B, 2009, 113, 9304–9310 CrossRef CAS.
- D. Othmer and P. Tobias, Ind. Eng. Chem., 1942, 34, 693–696 CrossRef CAS.
- P. González-Tello, F. Camacho, G. Blázquez and F. J. Alarcón, J. Chem. Eng. Data, 1996, 41, 1333–1336 CrossRef.
- A. F. M. Cláudio, M. G. Freire, C. S. R. Freire, A. J. D. Silvestre and J. A. P. Coutinho, Sep. Purif. Technol., 2010, 75, 39–47 CrossRef.
- T. A. Graber, H. Galleguillos, J. A. Asenjo and B. A. Andrews, J. Chem. Eng. Data, 2001, 47, 174–178 CrossRef.
- M. Perumalsamy and T. Murugesan, J. Chem. Eng. Data, 2009, 54, 1359–1366 CrossRef CAS.
- S. M. Snyder, K. D. Cole and D. C. Szlag, J. Chem. Eng. Data, 1992, 37, 268–274 CrossRef CAS.
- M. Domínguez-Pérez, L. I. N. Tomé, M. G. Freire, I. M. Marrucho, O. Cabeza and J. A. P. Coutinho, Sep. Purif. Technol., 2010, 72, 85–91 CrossRef.
- C. Yu, J. Han, S. Hu, Y. Yan and Y. Li, J. Chem. Eng. Data, 2011, 56, 3577–3584 CrossRef CAS.
- Y. Zhang, S. Zhang, Y. Chen and J. Zhang, Fluid Phase Equilib., 2007, 257, 173–176 CrossRef CAS.
- P. J. Carvalho, T. Regueira, L. M. N. B. F. Santos, J. Fernandez and J. A. P. Coutinho, J. Chem. Eng. Data, 2009, 55, 645–652 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Weight fraction percentage binodal data for the ternary systems composed of ionic liquid (1), salt (2) and water (3) at 298 K; Othmer–Tobias and Bancroft correlations; density and viscosity values of the coexisting phases in the temperature range between 298.15 K and 328.15 K. See DOI: 10.1039/c2ra21535g |
| This journal is © The Royal Society of Chemistry 2012 |
