In situ fabrication of Li4Ti5O12@CNT composites and their superior lithium storage properties
Jie
Shu
*,
Lu
Hou
,
Rui
Ma
,
Miao
Shui
,
Lianyi
Shao
,
Dongjie
Wang
,
Yuanlong
Ren
and
Weidong
Zheng
Faculty of Materials Science and Chemical Engineering, Ningbo University, Ningbo 315211, Zhejiang Province, People's Republic of China. E-mail: sergio_shu@hotmail.com; shujie@nbu.edu.cn (Dr Jie Shu); Fax: +86-574-87609987; Tel: +86-574-87600787
First published on 4th September 2012
Abstract
In this paper, Li4Ti5O12@CNT composites are fabricated by a controlled in situ growth of CNTs on the surface of Li4Ti5O12. The formation of coiled CNTs occurs by the electroless loading of Ni–P catalysts on the surface of Li4Ti5O12. Coiled CNTs provide electron bridges interconnecting the Li4Ti5O12 particles to form a nano/micro-structured conductive hyper-network. The electronic conductivity of Li4Ti5O12@CNT composites is better than that of the sample before coating. As a result, Li4Ti5O12@CNT composites show superior lithium storage properties comparable to the pristine Li4Ti5O12. Based on electrochemical analysis, it is obvious that Li4Ti5O12@CNT composites can deliver reversible charge capacities of 149.2, 102.6, 73.3 and 47.5 mA h g−1 at 0.2 C, 10 C, 20 C and 50 C, respectively.
1 Introduction
Lithium titanate oxide (Li4Ti5O12) is a high performance anode material for high power lithium-ion batteries. Based on a reversible conversion reaction between Li4Ti5O12 and Li7Ti5O12, a theoretical capacity of 175 mA h g−1 can be delivered by the pristine sample, which corresponds to the redox platforms at around 1.5 V in the charge–discharge curves. Attributed to the low electronic conductivity (10−9 S cm−1) of Li4Ti5O12, the pristine sample shows poor lithium storage properties. Therefore, various doping, mixing and coating techniques are used to improve the electronic conductivity and electrochemical properties of Li4Ti5O12.1–5For fabricating an effective conductive network, carbon nanotubes (CNTs) or nanofibers (CNFs) have been introduced as electron bridges interconnecting the Li4Ti5O12 particles in recent years. X. Li6 described the preparation of Li4Ti5O12/CNT composites by a simple solid state technique. It is found that the presence of CNTs has great effect on the particle size, surface morphology and electrochemical properties of Li4Ti5O12. To fabricate similar nano/micro-structure, commercial CNTs can be dispersed into the Li4Ti5O12 particles or precursors by a simple sol–gel method, as reported by J. J. Huang7 and H. I. Kim.8 Cycled at 5 C, the as-prepared Li4Ti5O12/CNT composites show a reversible lithium storage capacity of 142 mA h g−1 after 500 cycle. Besides, K. Naoi9 and H. S. Choi10 also reported the preparation of CNFs@Li4Ti5O12 composites with nano-Li4Ti5O12 loaded onto commercial CNFs by a sol–gel method and electrospinning technique, respectively. Used as a negative electrode in hybrid supercapacitor, they show higher energy density and power densities comparable to conventional electric double-layer capacitors. In these previous reports, the CNTs are purchased and added into the pristine Li4Ti5O12 samples to form nano/micro-structure composites. However, it is difficult to form a well-dispersed conductive hyper-network in the Li4Ti5O12/CNT composites with the ex situ introduction of CNTs.
In this paper, we report the in situ fabrication of Li4Ti5O12@CNT composites and research on their lithium storage properties. Good conductive pathways interconnecting Li4Ti5O12 particles are constructed by in situ formed CNTs with high electronic conductivity. These novel nano/micro-structured composites show lower impedance, higher reversible capacity and better rate performance than those of the pristine Li4Ti5O12.
2 Experimental section
Pristine Li4Ti5O12 samples are prepared by a citric acid assisted sol–gel method. Tetrabutyl titanate and lithium oxalate are used as the precursors for Li4Ti5O12 preparation in the experiment. Li4Ti5O12 is formed after a calcination process at 850 °C for 10 h. Li4Ti5O12@CNT composites are prepared by a pre-deposition Ni–P alloys on Li4Ti5O12 by electroless plating as catalysts and then a thermal hydrolysis process of acetylene at 850 °C for 1 h for CNTs growth. It is similar to the preparation technique to fabricate Si/CNFs composites described in a previous report.11The phase characteristics of as-prepared samples are identified by a Riguku X-ray diffraction (XRD) instrument. The surface morphologies and structures of samples are observed by a Hitachi S-3400 scanning electron microscopy (SEM) and a JEOL JEM-2100 transmission electron microscopy (TEM). The electronic conductivities of the pristine Li4Ti5O12 and Li4Ti5O12@CNT composites are measured by a HP4329A high frequency resistance meter at room temperature. Pellets with symmetric silver film coated on both sides as sandwich blocking electrodes are served for electronic conductivity measurements.
The working electrodes for lithium storage analysis are prepared by mixing and casting the as-prepared materials, acetylene black and polytetrafluoroethylene, onto the copper mesh. The working electrode is sealed in a simulated battery and cycled in a LiPF6-based electrolyte with metal lithium as the counter electrode at room temperature. Galvanostatic charge–discharge cycles are recorded on a multichannel Land battery test system in a potential range between 0.0 and 3.0 V. Electrochemical impedance spectroscopy (EIS) patterns are recorded by a CHI660D electrochemical working station in the frequency range between 100 kHZ and 0.005 HZ with an amplitude of 5 mV.
3 Results and discussion
Fig. 1 shows the XRD patterns of pristine Li4Ti5O12, Li4Ti5O12@catalysts and Li4Ti5O12@CNTs. Based on the JCPDS card No. 26-1198, it is known that the primary Li4Ti5O12 sample is a pure spinel-structured compound. After catalyst loading, Ni8P3 can be detected on the surface of Li4Ti5O12 by a weak diffraction peak appearing at 44.7° in the XRD pattern of the Li4Ti5O12@catalysts, which is similar to a previous study.12 By using Ni8P3 as a catalyst, graphitized carbon materials can be formed on the surface of Li4Ti5O12. It is confirmed by the new diffraction peak at 26.2° in the XRD pattern of Li4Ti5O12@CNTs. This Bragg position contributes to the (111) plane of graphite. In addition, some anatase TiO2 can be observed after a thermal hydrolysis process of acetylene at 850 °C, which is attributed to the extra diffraction peaks at 25.4°, 38.0°, 48.1°, 54.0° and 55.1°. It indicates that partial Li4Ti5O12 can decompose into anatase TiO2 and Li2O according to the following equation:| Li4Ti5O12 → 5TiO2 + 2Li2O | (1) |
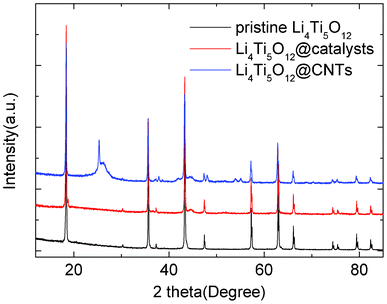 | ||
| Fig. 1 XRD patterns of pristine Li4Ti5O12, Li4Ti5O12@catalysts and Li4Ti5O12@CNTs. | ||
As reported, carbon coated Li4Ti5O12 with high purity can prepared by a high temperature solid state reaction.13–16 It is found that the decomposition of Li4Ti5O12 will not take place using carbon nanotubes as a carbon coating layer.7,8,16–18 Therefore, the appearance of anatase TiO2 is probably associated with the existence of catalysts during the thermal hydrolysis process. It induces and increases the evaporation of Li2O during the high temperature chemical vapor deposition (CVD) reaction.
Fig. 2 shows the surface morphologies of pristine Li4Ti5O12, Li4Ti5O12@catalysts and Li4Ti5O12@CNTs. It is found that pristine Li4Ti5O12 sample displays irregular polyhedral particles, as shown in Fig. 2a with an average particle size of 500 nm. With electroless plating of catalysts, a large number of Ni8P3 particles with an average particle size of 20–50 nm are attached to the surface of Li4Ti5O12, as shown in Fig. 2b. Attributed to the nano-catalysts, coiled CNTs are grown on the surface of Li4Ti5O12 with the Ni–P alloys on the top, as shown in Fig. 2c–2f. These coiled CNTs are multi-walled hollow tubes with different diameters. As a result, Li4Ti5O12 particles are tightly wrapped by crosslinked CNTs to form Li4Ti5O12@CNT composites, which provide a conductive hyper-network for electron transportation. There are lots of macro-sized channels built by crosslinked CNTs for lithium ion transportation in Li4Ti5O12@CNTs. Therefore, CNTs coated Li4Ti5O12 may show better kinetic properties than Li4Ti5O12@C composites with a continuous carbon coating layer. Based on the XRD and SEM results, the formation process of Li4Ti5O12@CNTs can be described by the schematic view in Fig. 3. It is expected that in situ fabricated CNTs can provide electron bridges interconnecting the Li4Ti5O12 particles to improve the lithium storage properties. As shown in Fig. 3, rapid lithium ion transportation between Li4Ti5O12 and Li7Ti5O12 can be achieved by crosslinked electron pathways and porous nano/micro-structure.
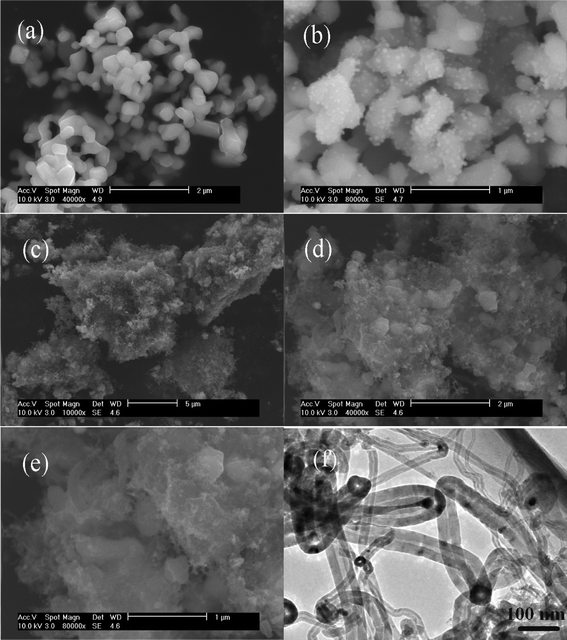 | ||
| Fig. 2 SEM images of pristine Li4Ti5O12 (a), Li4Ti5O12@catalysts (b) and Li4Ti5O12@CNTs (c–f). | ||
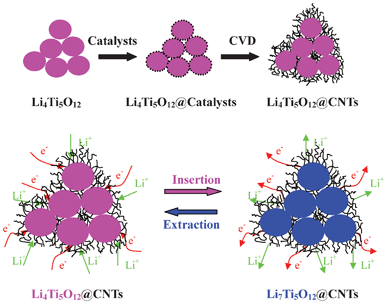 | ||
| Fig. 3 Schematic view of in situ formation process of Li4Ti5O12@CNTs and their lithium storage mechanisms. | ||
EIS results in Fig. 4a show that the introduction of CNTs on the surface of Li4Ti5O12 decreases the direct current resistance and charge transfer impedance for active materials. Furthermore, based on the formula σ = d/AR (where σ is the conductivity, R is the resistance, d is the diameter and A is the specific surface area), the electronic conductivities of Li4Ti5O12 and Li4Ti5O12@CNT composites are 1.6 × 10−8 and 3.4 × 10−5 S cm−1, respectively. As a result, the redox polarization decreases from 90 mV to 30 mV based on the charge–discharge curves at 0.2 C. Furthermore, the kinetic properties of Li4Ti5O12@CNTs are also greatly improved by the CNT coating. Cycled at 10 C, the insertion/extraction polarization reduces from 284 mV to 86 mV as shown in Fig. 4b. Usually, the pristine Li4Ti5O12 sample always shows poor lithium storage properties at different charge–discharge rates due to the low electronic conductivity. As shown in Fig. 4c, the reversible charge capacities of pristine Li4Ti5O12 are 86.2, 34.6, 25.7 and 6.5 mA h g−1 at 0.2 C, 10 C, 20 C and 50 C, respectively. After coated with CNTs, Li4Ti5O12@CNT composites show superior electrochemical performance as shown in Fig. 4b and 4c. Cycled at 0.2 C, Li4Ti5O12@CNT composites deliver a reversible charge capacity of 149.2 mA h g−1 after 10 cycles. Considering the 15.2 weight percent for electrochemically inactive CNTs in Li4Ti5O12@CNT composites, it is obvious that Li4Ti5O12 particles deliver an actual capacity same as the theoretical one. Cycled at high rates, Li4Ti5O12@CNT composites can show excellent high power behaviors, as shown in Fig. 4b. The reversible charge capacities for Li4Ti5O12@CNT composites are 102.6, 73.3 and 47.5 mA h g−1 at 10 C, 20 C and 50 C, respectively. These values are higher than those obtained at the same rates (10 C and 20 C) by H. I. Kim.8 This indicates that the existence of CNTs significantly improves the electrochemical properties of Li4Ti5O12. Therefore, nano/micro-structured Li4Ti5O12@CNT composites fabricated by in situ CNT growth are promising anode materials for high power lithium-ion batteries. It is a general nano/micro-structured M@CNTs system (M = oxide, metal, alloy, etc.) for lithium storage, which is suitable to fabricate other composites as anode or cathode materials for high power lithium-ion batteries. Therefore, this nano/micro-structured system and its preparation technique may have great effect on the rapid development of novel active materials as anode or cathode for high power lithium-ion batteries.
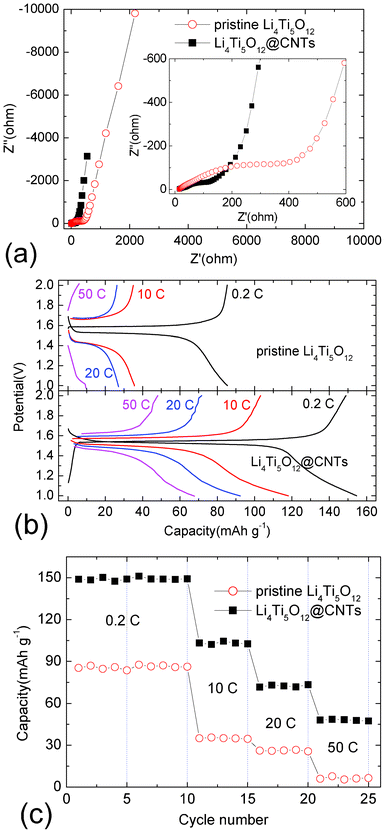 | ||
| Fig. 4 EIS patterns (a), charge–discharge curves (b) and cycling properties (c) of pristine Li4Ti5O12 and Li4Ti5O12@CNTs. | ||
4 Conclusions
In the Li4Ti5O12@CNT composites, pristine Li4Ti5O12 particles are prepared by a citric acid assisted sol–gel method. Based on the loading of nano-catalysts, the coiled CNTs are grown in situ on the surface of Li4Ti5O12 by a thermal hydrolysis of acetylene. The crosslinked CNTs build a hyper-networked conductive pathway in the Li4Ti5O12@CNT composites. As a result, Li4Ti5O12@CNT composites show a lower redox polarization than pristine Li4Ti5O12. Attributed to its low electronic conductivity, the pristine Li4Ti5O12 sample shows poor lithium storage properties. The cycled charge capacities are merely 86.2, 34.6, 25.7 and 6.5 mA h g−1 at 0.2 C, 10 C, 20 C and 50 C, respectively. After coating with electronically conductive CNTs, Li4Ti5O12@CNT composites show superior electrochemical properties. They can deliver reversible lithium storage capacities of 149.2, 102.6, 73.3 and 47.5 mA h g−1 at 0.2 C, 10 C, 20 C and 50 C, respectively.Acknowledgements
This project is sponsored by National Science Foundation of China (No. 51104092), Key Project of Chinese Ministry of Education (No. 210083) and Qianjiang Talent Project of Zhejiang Province (2011R10089). The work is also supported by K. C. Wong Magna Fund in Ningbo University.References
- H. G. Jung, M. W. Jiang, J. Hassoun, Y. K. Sun and B. Scrosati, Nat. Commun., 2011, 2, 516 CrossRef.
- Z. Liang, Y. S. Hu, H. Li, Z. X. Wang and L. Q. Chen, Adv. Mater., 2011, 23, 1385 CrossRef.
- G. N. Zhu, H. J. Liu, J. H. Zhuang, C. X. Wang, Y. G. Wang and Y.Y. Xia, Energy Environ. Sci., 2011, 4, 4016 CAS.
- G. D. Gu, N. Sharma, V. K. Peterson, J. A. Kimpton, D. Z. Jia and Z. P. Guo, Adv. Funct. Mater., 2011, 21, 3990 CrossRef.
- T. F. Yi, J. Shu, X. D. Zhu, Y. R. Zhu, C. B. Yue, A. N. Zhou and R. S. Zhu, Electrochim. Acta, 2009, 54, 7464 CrossRef CAS.
- X. Li, M. Z. Qu and Z. L. Yu, Solid State Ionics, 2010, 181, 635 CrossRef CAS.
- J. J. Huang and Z. Y. Jiang, Electrochim. Acta, 2008, 53, 7756 CrossRef CAS.
- H. I. Kim, J. J. Yang, H. J. Kim, T. Osaka and S. G. Park, J. Kor. Electrochem. Soc., 2010, 13, 235 CrossRef CAS.
- K. Naoi, S. Ishimoto, Y. Isobe and S. Aoyagi, J. Power Sources, 2010, 195, 6250 CrossRef CAS.
- H. S. Choi, T. H. Kim, J. H. Im and C. R. Park, Nanotechnology, 2011, 22, 405402 CrossRef.
- J. Shu, R. Ma, M. Shui, Y. Wang, N. B. Long, D. J. Wang, Y. L. Ren, R. F. Zhang, W. D. Zheng and S. Gao, RSC Adv., 2012, 2, 8323 RSC.
- J. Shu, H. Li, R. Z. Yang, Y. Shi and X. J. Huang, Electrochem. Commun., 2006, 8, 51 CrossRef CAS.
- J. Shu, Electrochim. Acta, 2009, 54, 2869 CrossRef CAS.
- H.-G. Jung, S.-T. Myung, C. S. Yoon, S.-B. Son, K. H. Oh, K. Amine, B. Scrosati and Y.-K. Sun, Energy Environ. Sci., 2011, 4, 1345 CAS.
- G.-N. Zhu, H.-J. Liu, J.-H. Zhuang, C.-X. Wang, Y.-G. Wang and Y.-Y. Xia, Energy Environ. Sci., 2011, 4, 4016 CAS.
- H. F. Ni and L. Z. Fan, J. Power Sources, 2012, 214, 195 CrossRef CAS.
- L. F. Shen, C. Z. Yuan, H. J. Luo, X. G. Zhang, K. Xu and F. Zhang, J. Mater. Chem., 2011, 21, 761 RSC.
- B. Zhang, Y. S. Liu, Z. D. Huang, S. W. Oh, Y. Yu, Y.-W. Mai and J.-K. Kim, J. Mater. Chem., 2012, 22, 12133 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
