Multicomponent assembly of luminescent hybrid materials of ZnO-lanthanide polymer complex functionalized SBA-15 mesoporous host by chemical bonds†
Yan-Fei
Shao
,
Bing
Yan
* and
Zhao-Yan
Jiang
Department of Chemistry, Tongji University, State Key Lab of Water Pollution and Resource Reuse (Tongji University), Shanghai, 200092, China. E-mail: byan@tongji.edu.cn; Fax: +86-21-65982287; Tel: +86-21-65984663
First published on 2nd August 2012
Abstract
In this paper, methacrylic-group-modified ZnO nanoparticles (designated ZnO-MAA) are prepared through the sol–gel reaction between zinc methacrylate and LiOH, which is then functionalized with 2-hydroxyethyl methacrylate (HEMA) to form the ZnO-MAA-PHEMA hybrid system after addition polymerization reaction. Then ZnO-MAA-PHEMA unit is modified with 3-(triethoxysilyl)-propyl isocyanate (TEPIC) through the addition reaction and subsequently to achieve the functionalized SBA-15 mesoporous hybrids (ZnO-MAA-PHEMA-SBA-15) by co-condensation of tetraethoxysilane (TEOS) and modified polymer ligand (HEMASi) in the presence of Pluronic P123 surfactant as template. Finally, lanthanide (Eu3+, Tb3+, Nd3+) complex systems with β-diketonates (2-thenoyltrifluoroacetonate (TTA), 1,3-diphenyl-1,3-propanedionate (DBM), 4,4,4-trifluoro-1-phenyl-1,3-butanedionate (BTA) and nicotinate (NTA)) are introduced to obtain multicomponent organic–inorganic hybrid materials through the coordination bonds between the lanthanide ion and the carbonyl group of ZnO-MAA-PHEMA-SBA-15. The detailed physical characterization and especially the photoluminescence of these hybrid materials are studied in detail. The results provide a strategy to assemble luminescent lanthanide hybrids with multicomponent units such as semiconductors, polymers and mesoporous silica and lanthanide complexes.
Introduction
Lanthanide inorganic–organic hybrid materials have attracted great interest in the last twenty years, as they possess improved chemical stability, thermal stability and mechanical resistance.1 To date, chemically bonded lanthanide hybrid materials have been assembled with covalent bonds, coordination bonds, in which the photoactive lanthanide species could be either embedded in an inorganic host matrices (silica, titania, alumina, etc.) 2–4 or fabricated in an organic polymer host.5 These researches were extended to functionalized ordered mesoporous silica and other inorganic oxide networks6–8 or the inorganic–organic polymeric substrate.9,10 In fact, the hybrid materials involved extensive fields, not limited by the lanthanide species. For example, the nanocomposites including the host–guest assembly were noteworthy and studied widely. The nanocomposite realized the composition of all kinds of typical building block such as nanoscale inorganic units (metal, alloy, oxide and non-oxide), inorganic or organic polymer. Nowadays, the relevant modification path or technology was developed to compose these different kinds of materials.11,12 So the tendency gradually shifted to multicomponent composition and functional integration, which undoubtedly displayed the versatility of chemical synthesis.13,14Mesoporous materials with ordered arrays of uniform nanochannels were engaged in the host for luminescent lanthanide complexes.6–8 To date, all kinds of typical mesoporous host have been functionalized to construct photoactive lanthanide hybrid materials, such as MCM-41, SBA-15, SBA-16 and the periodic mesoporous organosilicas (PMOs), etc.6,8
To further assemble organic/organic hybrids and nanocomposites, an inorganic crystalline framework was introduced to the hybrid system to modify their physical and chemical properties.15 Functional semiconductor-based nanocomposites were the most important system and all kinds of them were fabricated with the luminescent lanthanide hybrids.14 At the root of these researches, it was expected to be able to realize the function integration of these multi-components. A color-tunable emitter comprising Eu complex-capped ZnSe quantum dot (QD) organic-inorganic hybrid nanocrystals (NCs) was simply synthesized by a hot-injection method together with the addition of an Eu precursor, which could potentially serve as a light source in a white display by using the emission of both the QDs and lanthanide complexes.13a Zinc oxide nanoparticles (NPs) are environmentally friendly, cheap and chemically stable materials. It was interesting that ZnO NPs were prepared by a sol–gel route through the hydrolysis of a zinc salt by LiOH in alcohol and were modified using protective organic groups grafted to the surface of ZnO.15 Furthermore, lanthanide ions were sensitized by ZnO quantum dots (QDs) and molecular lanthanide complexes grafted to ZnO matrix are of profound significance and have important implications.16 Ulrich Schubert particularly reviewed inorganic–organic hybrid polymers based on surface-modified metal oxide clusters, including ZnO.17 We constructed hybrids of the above ZnO core-shell nanoparticles and the lanthanide complexes with covalent bonds through polymer linkages.18
In this paper, we tried to assemble multicomponent photofunctional systems with ZnO nanocomposites and lanthanide hybrids through polymer functionalized mesoporous silica. Considering the successful construction of ZnO nanoparticles and lanthanide hybrids of organically modified siloxanes through polymer linkage,18 it was predicted that the construction of ZnO nanoparticles and lanthanide hybrids of ordered mesoporous silica should be practical through polymer linkages. So ZnO was synthesized and further modified with special polymers (poly methacrylic acid (PMAA) and poly 2-hydroxyethyl methacrylate (PHEMA)), and then was grafted to lanthanide complex-functionalized mesoporous SBA-15 silica. This strategy could realize a multicomponent composition of ZnO polymer core-shell nanoparticles, mesoporous silica and lanthanide complexes through coordination bonds and polymer linkage functionalization of SBA-15. The detailed physical characterizations of these materials were carried out and the photophysical properties of them were discussed deeply.
Experimental section
Materials
Lanthanide nitrates were obtained by dissolving Eu2O3, Tb4O7, and Nd2O3 in concentrated nitric acid, respectively. 2-Thenoyltrifluoroacetonate (TTA, Aladdin), 1,3-diphenyl-1,3-propanedione (DBM, Aladdin), 4,4,4-trifluoro-1-phenyl-1,3-butanedione (BTA, Aladdin), nicotinic acid (NTA, Aladdin), Pluronic P123 (EO20PO70EO20, Aldrich), tetraethoxysilane (TEOS, Aldrich) and 3-(triethoxysilyl)-propyl isocyanate (TEPIC, Lancaster) are used without further treatment. 2-Hydroxyethyl methacrylate (HEMA) was purchased from Aladdin-reagent company. Methacrylic acid (MAA) and lithium hydroxide monohydrate (LiOH·H2O) were purchased from Shanghai YaoHua chemical plant. The solvents are tetrahydrofuran (THF) and N,N-dimethylformamide (DMF). All the reagents were analytically pure and used as received.Preparation of ZnO-PMAA-PHEMA nanoparticles
Firstly, [H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CO2]2Zn solution was synthesized from the reaction of Zn(OH)2 and H2C
C(CH3)CO2]2Zn solution was synthesized from the reaction of Zn(OH)2 and H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)COOH (MAA) aqueous solution at room temperature. Then the solution was evaporated in a rotating vaporizer and further dried in a vacuum oven at 60 °C. The resulting product was dissolved in absolute ethanol. Methacrylic-group-modified ZnO nanoparticles (designated ZnO-MAA) were obtained through a sol–gel reaction by refluxing [H2C
C(CH3)COOH (MAA) aqueous solution at room temperature. Then the solution was evaporated in a rotating vaporizer and further dried in a vacuum oven at 60 °C. The resulting product was dissolved in absolute ethanol. Methacrylic-group-modified ZnO nanoparticles (designated ZnO-MAA) were obtained through a sol–gel reaction by refluxing [H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CO2]2Zn ethanol solution for about 3 h, then adding LiOH ethanol solution at the molar ratio [LiOH]/[Zn] = 3.5 to form a ZnO-MAA colloid.15,18 The colloid concentration was determined by titration using ethylenedinitrilotetraacetic acid (EDTA) and eriochrome black T (EBT) at pH = 10. Such as-prepared colloids were concentrated by removing the solvent ethanol using a rotary vacuum evaporator at 40 °C until some ZnO-MAA solid emerged, then purified by centrifugation and further dried in a vacuum at 60 °C. The freshly dried solid was obtained. Then ZnO-MAA solid (1 mmol) is dissolved in ethanol solution containing the initiator benzoyl peroxide (BPO, 0.005 g), and HEMA (1 mL) was added into the solution. The addition polymerization reaction was carried out under a purging argon atmosphere. The mixture was agitated magnetically and thermal treatment was performed at 60 °C for about 6 h. The products were centrifuged and purified to obtain ZnO-MAA-PHEMA nanoparticles.
C(CH3)CO2]2Zn ethanol solution for about 3 h, then adding LiOH ethanol solution at the molar ratio [LiOH]/[Zn] = 3.5 to form a ZnO-MAA colloid.15,18 The colloid concentration was determined by titration using ethylenedinitrilotetraacetic acid (EDTA) and eriochrome black T (EBT) at pH = 10. Such as-prepared colloids were concentrated by removing the solvent ethanol using a rotary vacuum evaporator at 40 °C until some ZnO-MAA solid emerged, then purified by centrifugation and further dried in a vacuum at 60 °C. The freshly dried solid was obtained. Then ZnO-MAA solid (1 mmol) is dissolved in ethanol solution containing the initiator benzoyl peroxide (BPO, 0.005 g), and HEMA (1 mL) was added into the solution. The addition polymerization reaction was carried out under a purging argon atmosphere. The mixture was agitated magnetically and thermal treatment was performed at 60 °C for about 6 h. The products were centrifuged and purified to obtain ZnO-MAA-PHEMA nanoparticles.
Synthesis of the cross-linking precursors containing Si–O chemical bonds (ZnO-MAA-PHEMA-Si)
The as-prepared ZnO-MAA-PHEMA nanoparticles were first dissolved in THF solvent (20 mL), and NaH (2 mmol, 0.048 g) was then added into the solution with stirring at a temperature of 65 °C. Two hours later, 2.0 mmol (0.495 g) of TEPIC was added dropwise into the refluxing solution. The mixture was heated at 65 °C in a covered flask for approximately 12 h in a nitrogen atmosphere. After isolation and purification, a viscous liquid was obtained.Synthesis of functionalized SBA-15 mesoporous materials (ZnO-MAA-PHEMA-SBA-15)
Functionalized SBA-15 mesoporous materials were synthesized with the following molar composition 0.0172 P123: 0.96 TEOS: 0.04 ZnO-MAA-PHEMA-Si: 6HCl: 208.33 H2O. P123 (1.0 g) was dissolved in deionized water (7.5 g) and 2 M HCl solution (30 g), and stirred and heated to 38 °C. Then a mixture of TEOS and ZnO-MAA-PHEMA-Si was added into the above solution drop by drop with stirring for 24 h and transferred into a Teflon bottle sealed in an autoclave for the hydrothermal reaction at 100 °C for 48 h. The prepared product was filtrated and washed with abundant deionized water. Then it was air-dried overnight. Copolymer surfactant P123 was removed by Soxhlet extraction with ethanol under reflux for 2 days. After being filtered and dried, we obtained functionalized SBA-15 mesoporous materials.Synthesis of SBA-15 mesoporous materials and assistant ligands covalently bonded with Ln3+ complexes (Ln(ZnO-MAA-PHEMA-SBA-15)(L)3, Ln = Eu, Nd, Tb; L = β-diketonates, TTA, NTA, BTA, DBM)
The precursor ZnO-MAA-PHEMA-SBA-15 and assistant ligands (TTA, BTA and DBM for Eu, NTA for Tb and DBM for Nd) were dissolved in DMF solvent, then Ln(NO3)3·xH2O ethanol solution was added with the molar ratio Ln3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO-MAA-PHEMA-SBA-15
ZnO-MAA-PHEMA-SBA-15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TTA/BTA/DBM/NTA = 1
TTA/BTA/DBM/NTA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The mixture was stirred for 12 h at room temperature. The resulting product was centrifuged and washed with EtOH. After being dried at 60 °C under vacuum, the end product was obtained as shown in Fig. 1.
3. The mixture was stirred for 12 h at room temperature. The resulting product was centrifuged and washed with EtOH. After being dried at 60 °C under vacuum, the end product was obtained as shown in Fig. 1.
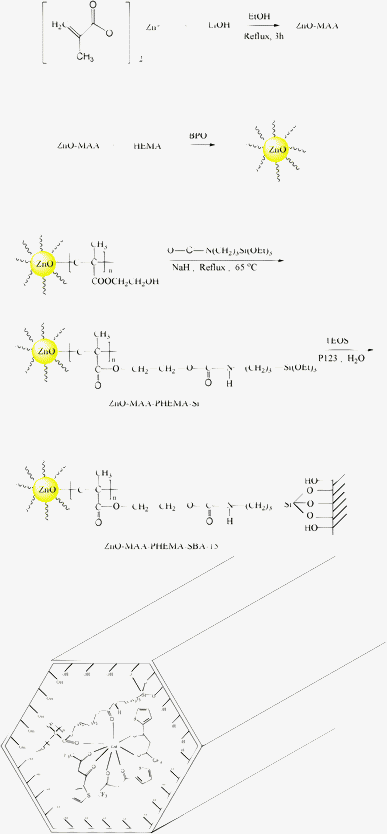 | ||
| Fig. 1 Scheme of synthesis process of ZnO-MAA, ZnO-MAA-PHEMA, ZnO-MAA-PHEMA-Si, ZnO-MAA-PHEMA-SBA-15 and the final hybrids Ln(ZnO-MAA-PHEMA-SBA-15)(L)3. | ||
The contents of RE3+ ions (Eu3+, Nd3+, Tb3+) and Zn2+ in the hybrids were determined with ICP-OES. For Eu(ZnO-MAA-PHEMA-SBA-15)(TTA)3: Eu 4.35%, Zn 3.70%; for Eu(ZnO-MAA-PHEMA-SBA-15)(BTA)3: Eu 4.27%, Zn 3.68%; for Eu(ZnO-MAA-PHEMA-SBA-15)(DBM)3: Eu 4.16%, Zn 3.61%; for Tb(ZnO-MAA-PHEMA-SBA-15)(NTA)3: Tb 4.25%, Zn 3.53%; for Nd(ZnO-MAA-PHEMA-SBA-15)(DBM)3: Nd 4.20%, Zn 3.65%. According to the content of RE3+ and Zn2+, it was predicted that the molar ratio of RE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn is close to 1
Zn is close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
Physical measurements
Fourier transform infrared (FTIR) spectra were measured within the 4000–400 cm−1 region on a Nexus 912 AO446 spectrophotometer with the KBr pellet technique. The content of metal elements (RE, Zn) were determined on the Perkin Optima 2100DV Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES). X-ray powder diffraction patterns (XRD) were recorded on a Rigaku D/max-rB diffractometer equipped with a Cu anode in a 2θ range from 10–70°. The ultraviolet-visible diffuse reflectance spectra were acquired by a BWS003 spectrophotometer. Transmission electron microscope (TEM) experiments were conducted on a JEOL2011 microscope operated at 200 kV or on a JEM-4000EX microscope operated at 400 kV. Thermogravimetric analysis (TG) was performed on a Netzsch STA 409 under nitrogen atmosphere in the Al2O3 crucibles at a heating rate of 15 °C min−1 from 30 to 1000 °C. The fluorescence excitation and emission spectra were obtained on a RF-5301 spectrophotometer. Luminescence lifetime measurements were carried out on an Edinburgh FLS920 phosphorimeter.Results and discussion
As shown in Fig. 1, the key of the multicomponent hybrid system was the chemical bonds among all kinds of building units within it. The basic composition of the lanthanide mesoporous hybrids could be predicted according to lanthanide and zinc coordination chemistry principles. The first was coordination bonds, which exist between the Zn ions and MMA and the lanthanide ion and PHEMA-SBA-15 unit and beta-diketones ligands. The second was the covalent bond among the MMA, PHEMA and SBA-15 mesoporous host. The tris β-diketonate ligands provided six chelated carbonyl groups to occupy the six coordination spots of Ln3+. Besides, the molar ratio of Ln3+ and PHEMA-SBA-15 unit was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, suggesting that the carbonyl group of PHEMA-SBA-15 could occupy one coordination spot. So the seventh coordination number was occupied and the residual spot might be filled with water molecules. Methacrylic-group-modified ZnO nanoparticles were coordinated to the PHEMA unit through the ester group and PHEMA was further covalently linked with SBA-15 through a Si–O bond.15,18 ZnO colloids produced via the sol–gel process had methacrylic groups, which originated from the polymer precursor adsorbed on the surface of ZnO. 15,18 Then the ZnO polymer nanocomposites were assembled with MAA modified ZnO nanoparticles and monomer EMA (HEMA, HFBMA) through the copolymerization between the MAA group and EMA (HEMA, HFBMA) 15,18. In fact, the polyesters were indirectly connected with ZnO by covalent bonds with the MAA unit as a bridge.15,18 The covalent bonds forming the bridge between ZnO and MAA were proved by the IR spectra.16a,18, 20b The coordination interaction appeared between ZnO (Zn2+) and MAA unit (its carboxylate group) and the charge of COO− is balanced by Li+. Besides, the three nitrates still remained to balance the charge of Ln3+. The polymerization reaction could not be guaranteed to be exact and so it was hard to determine the exact composition within the final complicated hybrid system. The contents of RE and Zn elements were determined to be close to 1
1, suggesting that the carbonyl group of PHEMA-SBA-15 could occupy one coordination spot. So the seventh coordination number was occupied and the residual spot might be filled with water molecules. Methacrylic-group-modified ZnO nanoparticles were coordinated to the PHEMA unit through the ester group and PHEMA was further covalently linked with SBA-15 through a Si–O bond.15,18 ZnO colloids produced via the sol–gel process had methacrylic groups, which originated from the polymer precursor adsorbed on the surface of ZnO. 15,18 Then the ZnO polymer nanocomposites were assembled with MAA modified ZnO nanoparticles and monomer EMA (HEMA, HFBMA) through the copolymerization between the MAA group and EMA (HEMA, HFBMA) 15,18. In fact, the polyesters were indirectly connected with ZnO by covalent bonds with the MAA unit as a bridge.15,18 The covalent bonds forming the bridge between ZnO and MAA were proved by the IR spectra.16a,18, 20b The coordination interaction appeared between ZnO (Zn2+) and MAA unit (its carboxylate group) and the charge of COO− is balanced by Li+. Besides, the three nitrates still remained to balance the charge of Ln3+. The polymerization reaction could not be guaranteed to be exact and so it was hard to determine the exact composition within the final complicated hybrid system. The contents of RE and Zn elements were determined to be close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, corresponding to the reaction ratio of them. On the basis of the above analyses and the discussion in ref. 18, the scheme in Fig. 1 for the hybrid system could be predicted and proved.
2, corresponding to the reaction ratio of them. On the basis of the above analyses and the discussion in ref. 18, the scheme in Fig. 1 for the hybrid system could be predicted and proved.
The transmission electron microscopy (TEM) image for ZnO-MAA was shown in Fig. S1†, which was prepared by using the MAA as the grafting organic ingredient and a molar ratio of [LiOH]/[Zn] = 3.5 through a sol–gel reaction. This exhibited that ZnO nanoparticles were monodisperse and uniform. Furthermore, the nanoparticles were stable under the intense electron beams used for TEM measurement, which indicated a firm bonding between the ZnO and the organic group MAA.
Fig. 2 (A) firstly showed the comparison of the precursor systems (nanoparticles ZnO-MAA and ZnO-MAA-PHEMA-Si). In view of the nanoparticles ZnO-MAA, it was found that the two large bands were very close to those observed typically for an acetate group complexed with a metal such as zinc and corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and C–O stretching, respectively. It was interesting that the C
O stretching and C–O stretching, respectively. It was interesting that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band at 1550 cm−1 and the C–O stretching band at 1456 cm−1 for the acetate groups on the ZnO surface each split into two absorption peaks when the ZnO nanoparticles were dispersed in the methacrylic groups. The doublet bands of the C
O stretching band at 1550 cm−1 and the C–O stretching band at 1456 cm−1 for the acetate groups on the ZnO surface each split into two absorption peaks when the ZnO nanoparticles were dispersed in the methacrylic groups. The doublet bands of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O stretching vibrations with maxima at 1550, 1487 and 1450 and 1421 cm−1, respectively were evidence for methacrylic groups successfully complexed with zinc oxide. In general, three bonding structures are well-known for acetate groups coordinated to a metal ion like Zn2+, which are unidentate, bidentate (chelate), and bridging types, respectively.19Fig. 2 (B) showed the FT-IR spectra of five kinds of multicomponent lanthanide hybrid mesoporous materials Ln(ZnO-MAA-PHEMA-SBA-15)(L)3 (Ln = Eu, Tb, Nd; L = BTA, TTA, DBM, NTA), which presented similar features. The bands located at 3415 cm−1 and 1100 cm−1 originate from the hydroxyl functional group. The bands at 1660 and 1575 cm−1 were attributed to the absorption of C
O and C–O stretching vibrations with maxima at 1550, 1487 and 1450 and 1421 cm−1, respectively were evidence for methacrylic groups successfully complexed with zinc oxide. In general, three bonding structures are well-known for acetate groups coordinated to a metal ion like Zn2+, which are unidentate, bidentate (chelate), and bridging types, respectively.19Fig. 2 (B) showed the FT-IR spectra of five kinds of multicomponent lanthanide hybrid mesoporous materials Ln(ZnO-MAA-PHEMA-SBA-15)(L)3 (Ln = Eu, Tb, Nd; L = BTA, TTA, DBM, NTA), which presented similar features. The bands located at 3415 cm−1 and 1100 cm−1 originate from the hydroxyl functional group. The bands at 1660 and 1575 cm−1 were attributed to the absorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O, indicating that C
O and C–O, indicating that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from HEMA has been coordinated to Ln3+ ions. The low frequency at 460 cm−1 was ascribed to be the coordination of oxygen atoms to Ln3+.20 The region from 1079 to 1165 cm−1 corresponded to the absorption bands of Si–O bond.
O from HEMA has been coordinated to Ln3+ ions. The low frequency at 460 cm−1 was ascribed to be the coordination of oxygen atoms to Ln3+.20 The region from 1079 to 1165 cm−1 corresponded to the absorption bands of Si–O bond.
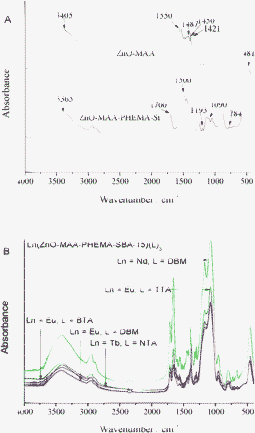 | ||
| Fig. 2 FTIR spectra of precursors ZnO-MAA, Zn-MAA-PHEMA-Si (A) and multicomponent the final hybrids Ln(ZnO-MAA-PHEMA-SBA-15)(L)3 (B). | ||
The selected SAXRD patterns for Tb(ZnO-MAA-PHEMA-SBA-15)(NT)3 and Nd(ZnO-MAA-PHEMA-SBA-15)(TTA)3 were shown in Fig. 3. Both of them showed similar features, which contain three Bragg peaks in the 2θ range of 0.6–2°. The prominent peak should be indexed as the (100) diffraction peak and the other two as the (110) and (200) diffraction peaks, corresponding to the characteristics of a two-dimensional hexagonal (p6mm) structure of the SBA-15 material. The corresponding unit cell parameter a0 (the internal pore diameter plus one pore wall thickness) were calculated according to the formula a0 = 2d100/√3, where d100 was obtained from 2θ angle of the first reflection peak in the XRD pattern by Bragg's equation: 2d100sinθ = λ (λ = 1.5418 Å for the Cu Kaline) (see Table 1). It could be seen that for both the ordered hexagonal mesoporous structure of SBA-15 remained intact after the introduction of the lanthanide ion. It needs to be referred that the two lanthanide mesoporous hybrids show a slight decrease in cell parameters as compared with the parent SBA-15 materials, suggesting that the presence of guest moieties on the mesoporous framework of SBA-15 produces a change of the ordering structure of the mesopores in modified SBA-15.
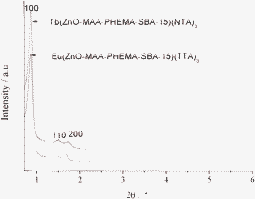 | ||
| Fig. 3 The selected SAXRD patterns of multicomponent terbium and europium mesoporous hybrid materials. | ||
| Sample | d 100 (nm)a | a 0 (nm) | S BET (m2 g−1) | V (cm3 g−1) | D (nm) | t (nm) |
|---|---|---|---|---|---|---|
| a d 100 is the d(100) spacing, a0 the cell parameter (a0 = 2d100/√3), SBET the BET surface area, V the total pore volume, DBJH the average pore diameter, and t the wall thickness, calculated by a0 − D. | ||||||
| SBA-15 | 9.56 | 11.03 | 541 | 0.84 | 6.21 | 4.82 |
| EuL(TTA)3 | 10.29 | 11.88 | 286 | 0.51 | 6.16 | 4.72 |
| TbL(NTA)3 | 9.78 | 11.29 | 304 | 0.46 | 6.05 | 5.24 |
The corresponding nitrogen adsorption–desorption isotherms were presented in Fig. 4 (A for europium hybrids and B for terbium or neodymium ones). Both of A and B present type IV isotherms with distinct H1-type hysteresis loops at high relative pressures, characteristic of high-quality large pore mesoporous materials with uniform mesopores. From the two branches of the adsorption–desorption isotherms, a sharp adsorption step appeared in the P/P0 region from 0.6 to 0.8 and a hysteresis loop appeared at the relative pressure P/P0 > 0.7, which revealed that the materials possessed a well-defined array of regular mesopores. By means of BET and BJH methods, the specific area (SBET) and the pore size (DBJH) were calculated, whose pore volume (V) data were also shown in Table 1. From the SBET, DBJH and V of them, it was observed that these data were less than those typically reported for pure SBA-15 mesoporous silica material.21 This was probably due to the presence of an organic ligand on the pore surface and the co-surfactant effect of the precursor, which interacted with the surfactant and reduced the diameter of the micelles.22
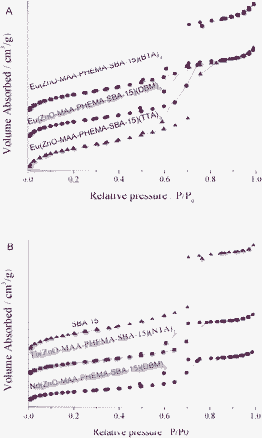 | ||
| Fig. 4 The selected nitrogen adsorption–desorption isotherms of multicomponent lanthanide hybrid materials: (A) europium systems, (B) SBA-15, terbium and neodymium system. | ||
The selected HRTEM images of ternary mesoporous hybrids Eu(ZnO-MAA-PHEMA-SBA-15)(DBM)3 were shown in Fig. 5. The ordered pore structure was substantially conserved after the complexation process. It confirmed the suggested p6mm symmetry and a well-ordered hexagonal structure, which was in agreement with the SAXRD and N2 adsorption–desorption isotherms. The distances between the center of the mesopores for it were similar and estimated to be about 10 nm, which was in good agreement with the values determined from the corresponding XRD analysis (see Table 1).
![Selected TEM image of Eu(ZnO-MAA-PHEMA-SBA-15)(DBM)3 along the [100] (left) and [110] (right) zone axes.](/image/article/2012/RA/c2ra21605a/c2ra21605a-f5.gif) | ||
| Fig. 5 Selected TEM image of Eu(ZnO-MAA-PHEMA-SBA-15)(DBM)3 along the [100] (left) and [110] (right) zone axes. | ||
The thermogravimetric (TG) and differential thermogravimetry (DTG) were performed at a heating rate of 15 °C min−1 to investigate the thermal stabilities of obtained materials (see Fig. 6). From the TG curve we could see that the sample showed three main degradation steps. The first step of weight loss was about 11.6% for Nd(ZnO-MAA-PHEMA-SBA-15)(DBM)3 from 40 to 290 °C, which was attributed to the desorption of physically adsorbed water and residual solvent. The second step of weight loss took place between 290 and 359 °C with a thermal weight loss of 8.4%, which originated from the thermal decomposition of the organic ingredient DBM. The third mass loss was the thermal decomposition of the mesoporous material framework and organic ingredient PHEMA with a weight loss of 23.1% beyond 359 °C. The material retained a mass of about 55.4% up to 1000 °C, which represented the weight of the ZnO and lanthanide oxides.
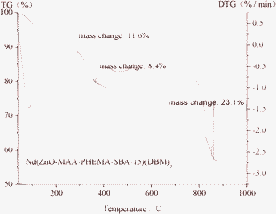 | ||
| Fig. 6 The selected thermogravimetric (TG) and differential thermogravimetry (DTG) curves of hybrid material Nd(ZnO-MAA-PHEMA-SBA-15)(DBM)3. | ||
The UV–Vis diffuse reflection absorption spectra of the hybrid materials were given in Fig. S2† (A for Eu3+ and B for Tb3+ and Nd3+). It was observed that all the spectra exhibit a similar broad absorption band in the UV–Vis range (200–600 nm), which was ascribed to the absorption of the ligands ZnO-MAA-PHEMA-SBA-15 and assistant ligands (three β-diketonates or nicotinate). The strong absorption bands with maximum wavelength ranged in 360–380 nm were mainly attributed to the ZnO-MAA-PHEMA-SBA-15 host and the charge transfer state of Ln3+–O. The short wavelength region at around 250–270 nm corresponded to the β-diketonates or nicotinate. Certainly, there was the overlap between these absorption bands and also overlap with the fluorescence excitation spectra in Fig. 7–9. These wide absorption bands were favorable for the luminescence of the lanthanide ions within the hybrids systems.
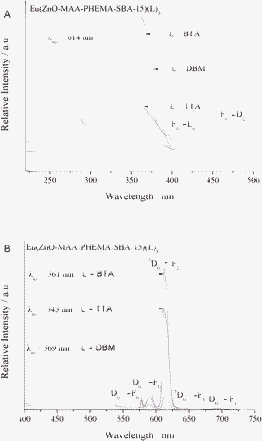 | ||
| Fig. 7 The excitation (A) and emission (B) spectra of hybrid mesoporous materials Eu(ZnO-MAA-PHEMA-SBA-15)(L)3 (L = BTA, TTA, DBM). | ||
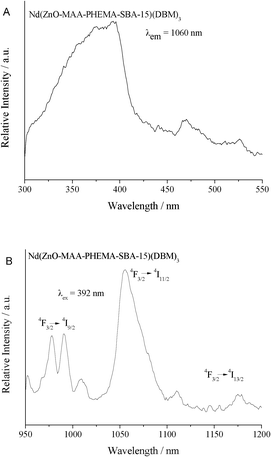 | ||
| Fig. 8 The excitation (A) and emission (B) spectra of hybrid mesoporous materials Nd(ZnO-MAA-PHEMA-SBA-15)(DBM)3. | ||
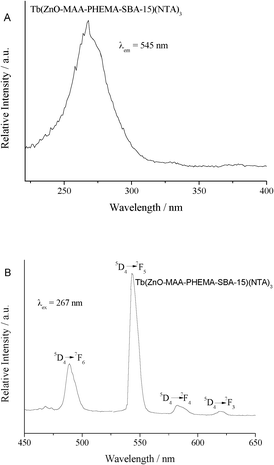 | ||
| Fig. 9 The excitation (A) and emission (B) spectra of hybrid mesoporous materials Tb(ZnO-MAA-PHEMA-SBA-15)(NTA)3. | ||
The excitation and emission spectra of three europium hybrid mesoporous materials Eu(ZnO-MAA-PHEMA-SBA-15)(L)3 (L = BTA, TTA, DBM) were shown in Fig. 7(A) and (B), respectively. The excitation spectra monitored at 614 nm for Eu3+ exhibited a broad excitation band from 220 to 400 nm centered at about 350 nm in the ultraviolet region, which was attributed to absorption of the ZnO composite mesoporous silica host and the Eu–O charge transfer transition state (CTS) transition caused by an interaction between the organic groups and the Eu3+ ions coordinated to oxygen atoms of the three β-diketonates ligands), both of the two kinds of absorption bands overlapped. The two absorption bands both existed, which was revealed by their different excitation intensities. The three kinds of europium hybrids possessed the same ZnO-MAA-PHEMA-SBA-15 host but different β-diketonates; the effective luminescence of Eu3+ was obtained through energy transfer process between the wide band absorption of host and CTS to Eu3+. The peaks at around 397 nm and 464 nm were observed owing to the narrow f–f absorption transition (7F0→5L6 and 7F0→5D2) of the Eu3+ ion.23 Besides, there were some excitation bands at the short wavelength ultraviolet region of around 260 nm, suggesting the absorption of the three β-diketonates. Among the excitation intensity was the strongest for the hybrids of Eu(ZnO-MAA-PHEMA-SBA-15)(L)3, which was reasonable as TTA was the most effective ligand for Eu3+ owing to its optimum energy match to Eu3+.24 The intensity of the f–f absorption transition was much weaker than the absorption of the host and CTS so that it was not checked in the excitation spectra. As a result, five main emission peaks in the emission spectra of the three hybrid materials were observed. They were located at 579, 591, 614, 649 and 684 nm, respectively, corresponding to 5D0→7FJ transitions (J = 0, 1, 2, 3, 4). In addition, there was one weak broad emission at the range of 400 to 550 nm, corresponding to the emission of the ZnO NPs of the ZnO composite of polymer and the mesoporous silica (SBA-15) unit within the hybrid system, as both of them were optically active.15,25 This suggested that the luminescence of the host was quenched by Eu3+ for the energy transfer between them and the multicomponent hybrid system was effective for the luminescence of Eu3+.
In addition, among these emission peaks of the materials chelating Eu3+, a high intensity ratio of I(5D0→7F2)/I(5D0→7F1) (red/orange) was obtained. The intensity ratio of the 5D0→7F2 (electric-dipolar transition) transition to the 5D0→7F1 (magnetic-dipolar transition) transition was widely used as an indicator of the local environment of Eu3+.26 Because the 5D0→7F1 transition did not depend on the chemical environments around the Eu3+, it was used as a reference to compare luminescent intensities of different Eu3+-containing materials. The intensity ratios of the three curves in the three hybrid materials Eu(ZnO-MAA-HEMA-SBA-15)(L)3, L = BTA, DBM and TTA were 6.70, 7.15, and 7.90, respectively, indicating that Eu3+ was located in the similar local coordination environments for the same ZnO-MAA-PHEMA-SBA-15 composite host. The different β-diketonate ligands had little influence on the coordination environment and the red/orange intensity ratio.
In Fig. 8, the excitation spectrum of neodymium hybrids Nd(ZnO-MAA-PHEMA-SBA-15)(DBM)3 monitored at 1060 nm was also composed of a broad band between 300 to 430 nm with a maximum intensity at a wavelength of 392 nm. It was still ascribed the host absorption of the ZnO-MAA-PHEMA-SBA-15 composite and the ligand absorption of DBM was not apparent. This was similar to the excitation of the above Eu hybrid system except for the CTS absorption (only for Eu3+). Moreover, some weak peaks located at 450 to 550 nm probably were due to the characteristic f–f transitions of Nd3+.27 Under the excitation of 392 nm, there were three kinds of dominating emission bands at the range of 950 nm to 1200 nm, which originated from the typical luminescent transitions of 4F3/2→4IJ (J = 9/2, 11/2, 13/2) of Nd3+.28
Fig. 9 showed the excitation (A) and emission (B) spectra of the Tb3+ hybrid material Tb(ZnO-MAA-PHEMA-SBA-15)(NTA)3. The excitation spectra were obtained by monitoring the emission of Tb at 545 nm, which showed a different character from the europium and neodymium hybrid systems. The only an apparent excitation band appeared in the short wavelength ultraviolet region from 220 to 350 nm centered at about 265 nm, which was attributed to the Tb–O CTB (charge transfer band) always appearing at higher energy (lower wavelengths). No obvious f–f transitions or host absorption of the ZnO-MAA-PHEMA-SBA-15 composite were detected. The difference of the excitation spectra was related to the energy match of Ln3+ and ZnO-MAA-PHEMA-SBA-15 composite host. The emissive energy level of Tb3+ is higher and was closer than Eu3+ or Nd3+ to the host excitation band of ZnO composite mesoporous silica host. So the energy transfer between the ZnO composite mesoporous host and Tb3+ was not effective. The emission lines of the hybrid materials were assigned to the transitions from the 5D4→7FJ (J = 6, 5, 4, 3) transitions at about 489, 545, 583 and 622 nm, respectively. The most striking green luminescence (5D4→7F5) was observed during excitation of the precursors at about 350 nm, which indicated that the energy was absorbed by different precursors and transferred to the chelated Tb3+ ions.
The resulting lifetimes of Tb and Eu hybrids were also given in Table 2. The typical decay curve of the Eu3+ and Tb3+ hybrids were all described as a single exponential (ln[S(t)/S0] = −k1t = −t/τ). This estimated that all lanthanide ions occupied the same average coordination environment. The luminescent lifetimes of the three europium hybrid systems only showed little distinction for they belonged to the similar hybrid framework and ligands as around Eu3+. While (ZnO-MAA-PHEMA-SBA-15)(NTA)3 possessed the longer lifetimes than the europium hybrids, this was related to the NTA ligands.
| Ln(ZnO-MAA-PHEMA-SBA-15)(L)3 | I 02/I01 | τ a , b (ms) | A r | A nr | η(%) | n w |
|---|---|---|---|---|---|---|
| a For the 5D0–7F2 transition of Eu3+. b For the 5D4–7F5 transition of Tb3+; error: ±10%. | ||||||
| Ln = Eu, L = BTA | 6.70 | 0.55 | 440 | 1378 | 24.2 | ∼1.5 |
| Ln = Eu, L= DBM | 7.15 | 0.48 | 466 | 1617 | 22.3 | ∼1.5 |
| Ln = Eu, L = TTA | 7.90 | 0.51 | 507 | 1454 | 20.8 | ∼1.5 |
| Ln = Tb, L= NTA | — | 0.88 | — | — | — | — |
By means of the emission spectra and lifetimes of the 5D0 emitting level for the three europium hybrid materials, the emission quantum efficiency (η) (expresses the competition between the radiative processes and nonradiative processes) of the 5D0 level was calculated from the spectra and lifetimes (see Table 2). Assuming that only nonradiative (transition rate Anr) and radiative processes (transition rate Ar) were essentially involved in the depopulation of the 5D0 excited state, η was defined as follows:29
| η = Ar/(Ar + Anr) | (1) |
Nonradiative process affected the experimental luminescence lifetime by the following equation:
| τexp = (Ar + Anr)−1 | (2) |
So quantum efficiency η was calculated from radiative transition rate constant and experimental luminescence lifetime from the equation:
| η = Arτexp | (3) |
A r was obtained by summing over the radiative rates A0J for each 5D0→7FJ transition of Eu3+.
| Ar = ∑A0J = A00 + A01 + A02 + A03 + A04 | (4) |
The branching ratio for the 5D0→7F5,6 transitions was neglected as they both were not detected experimentally, and whose influence was ignored in the depopulation of the 5D0 excited state. Since 5D0→7F1 belongs to the isolated magnetic dipole transition, it is practically independent of the chemical environments around the Eu3+ ion, and thus can be considered as an internal reference for the whole spectrum. The experimental coefficients of spontaneous emission, A0J, was calculated according to the equation as follows:30
| A0J = A01 (I0J/I01) (υ01/υ0J) | (5) |
A 01 is Einstein's coefficient of spontaneous emission between the 5D0 and 7F1 levels and could be considered to be equal to 50 s−1.31υ01 and υ0J (υ0J = 1/λJ) are the energy gaps of the 5D0→7F1 and 5D0→7FJ transitions, respectively. Moreover, the I01 and I0J are the integrated intensities of the 5D0→7F1 and 5D0→7FJ transitions, respectively.
Finally, the quantum efficiencies of the three kinds of europium hybrids were determined in this order: Eu(ZnO-MAA-PHEMA-SBA-15)(BTA)3 > Eu(ZnO-MAA-PHEMA-SBA-15)(DBM)3 > Eu(ZnO-MAA-PHEMA-SBA-15)(TTA)3, but the difference was not apparent and similar to the fact of their lifetimes. For the three kinds of hybrid materials, the only difference was the species of the assistant β-diketonates ligands, but it was not the same as the order of pure europium complexes of β-diketonates. This was explained from the excitation spectra. The energy absorption and excitation was mainly from the ZnO-MAA-PHEMA-SBA-15 composite mesoporous host, not only from the β-diketonates. It could be concluded that in the hybrid systems, the ZnO polymer mesoporous silane composite host was the main energy donor for Eu3+.
Furthermore, the number of coordination molecules of Eu3+ hybrid material systems was selectively estimated to elucidate the negative influence of vibration caused by the water molecules and to further study the coordination environment surrounding the lanthanide ions. On the basis of the previous research of Supkowski and Horrocks,32 possible number of coordinated water molecules (nw) was determined by the follow equation:
| nw = 1.11 × (τ−1 − Ar − 0.31) | (6) |
It was seen from the results that the coordination number of water molecules in the Eu hybrids was estimated to be about 1.5. The possible 1–2 hydroxyl groups vibration of the coordinated water molecules resulted in nonradiative transition and a decrease in the luminescent efficiency.
Conclusions
In summary, ZnO and ternary lanthanide (Eu3+, Tb3+, Sm3+, Dy3+) complexes are assembled through chemical bonds with the polymer (PHEMA) functionalized SBA-15 mesoporous silica. All these multi-component hybrid materials are characterized in detail, ranging from structure to property. These hybrids can show the characteristic luminescence of lanthanide ions mainly through the energy transfer from the ZnO-polymer-SBA-15 composite host, whose photoluminescent behaviors are discussed deeply. The results may provide a representative strategy to assemble multi-component (ZnO nanocomposite, lanthanide complexes, polymer, mesoporous silica) photofunctional hybrid materials with covalent bonds.Acknowledgements
This work is supported by the National Natural Science Foundation of China (20971100, 91122003) and Program for New Century Excellent Talents in University (NCET-08-0398).References
- (a) L. D. Carlos, R. A. S. Ferreira, V. D. Bermudez and S. J. L. Ribeiro, Adv. Mater., 2009, 21, 509 CrossRef CAS; (b) K. Binnemans, Chem. Rev., 2009, 109, 4283 CrossRef CAS; (c) Y. Li, Y. Bian, M. Yan, P. S. Thapaliya, D. Johns, X. Yan, D. Galipeau and J. Jiang, J. Mater. Chem., 2011, 21, 11131 RSC.
- (a) F. Y. Liu, L. D. Carlos, R. A. S. Ferreira, J. Rocha, M. C. Gaudino, M. Robitzer and F. Quignard, Biomolecules, 2008, 9, 1945 CAS; (b) S. S. Nobre, C. D. S. Brites, R. A. S. Ferreira, V. D. Bermudez, C. Carcel, J. I. E. Moreau, J. Rocha, M. W. C. Man and L. D. Carlos, J. Mater. Chem., 2008, 18, 4172 RSC; (c) H. S. He, M. Dubey, A. G. Sykes and P. S. May, Dalton Trans., 2010, 39, 6466 RSC; (d) J. L. Liu, B. Yan and L. Guo, Eur. J. Inorg. Chem., 2010, 2290 CrossRef.
- (a) H. R. Li, N. N. Lin, Y. G. Wang, Y. Feng, Q. Y. Gan, H. J. Zhang, Q. L. Dong and Y. Chen, Eur. J. Inorg. Chem., 2009, 519 CrossRef CAS; (b) Y. Feng, H. R. Li, Q. Y. Gan, Y. G. Wang, B. Y. Liu and H. J. Zhang, J. Mater. Chem., 2010, 20, 972 RSC; (c) L. Guo and B. Yan, Eur. J. Inorg. Chem., 2010, 1267 CrossRef CAS; (d) J. L. Liu and B. Yan, Dalton Trans., 2011, 40, 1961 RSC.
- (a) B. Yan, Q. M. Wang and D. J. Ma, Inorg. Chem., 2009, 48, 36 CrossRef CAS; (b) H. F. Lu, B. Yan and J. L. Liu, Inorg. Chem., 2009, 48, 3966 CrossRef CAS; (c) L. Guo, B. Yan and J. L. Liu, Dalton Trans., 2011, 40, 4933 RSC; (d) L. Guo, L. S. Fu, R. A. S. Ferreira, L. D. Carlos, Q. P. Li and B. Yan, J. Mater. Chem., 2011, 21, 15600 RSC.
- (a) R. Shunmugam and G. N. Tew, Macromol. Rapid Commun., 2008, 29, 1355 CrossRef CAS; (b) Z. Q. Huang, H. H. Zhang, J. X. Guo, J. L. Wang and Y. Q. Chen, Polym. Eng. Sci., 2009, 49, 1273 CrossRef CAS; (c) X. G. Huang, Q. Wang, X. H. Yan, J. Xu, W. S. Liu, Q. Wang and Y. Tang, J. Phys. Chem. C, 2011, 115, 2332 CrossRef CAS.
- (a) J. Feng, S. Y. Song, Y. Xing, H. J. Zhang, Z. F. Li, L. N. Sun, X. M. Guo and W. Q. Fan, J. Solid State Chem., 2009, 182, 435 CrossRef CAS; (b) J. Feng, S. Y. Song, W. Q. Fan, L. N. Sun, X. M. Guo, C. Y. Peng, J. B. Y. N. Yu and H. J. Zhang, Microporous Mesoporous Mater., 2009, 117, 278 CrossRef CAS; (c) H. R. Li, W. J. Cheng, Y. Wang, B. Y. Liu, W. J. Zhang and H. J. Zhang, Chem.–Eur. J., 2010, 16, 2125 CrossRef CAS; (d) Y. Wang, H. R. Li, Y. Feng, H. J. Zhang, G. Calzaferri and T. Z. Ren, Angew. Chem., Int. Ed., 2010, 49, 1434 CrossRef CAS.
- (a) B. Yan, B. Zhou and Y. Li, Microporous Mesoporous Mater., 2009, 120, 317 CrossRef CAS; (b) Y. Li and B. Yan, Microporous Mesoporous Mater., 2010, 128, 62 CrossRef CAS; (c) B. Yan and Y. Li, Dalton Trans., 2010, 39, 1480 RSC; (d) Y. J. Li, B. Yan and Y. Li, Microporous Mesoporous Mater., 2010, 131, 82 CrossRef CAS; (e) Y. Y. Li, B. Yan, L. Guo and Y. J. Li, Microporous Mesoporous Mater., 2012, 48, 73 Search PubMed.
- (a) Y. Li, B. Yan and Y. J. Li, Microporous Mesoporous Mater., 2010, 132, 87 CrossRef CAS; (b) Y. J. Li, B. Yan and L. Wang, Dalton Trans., 2011, 40, 6722 RSC; (c) Y. J. Li, L. Wang and B. Yan, J. Mater. Chem., 2011, 21, 1130 RSC; (d) Y. J. Li and B. Yan, J. Mater. Chem., 2011, 21, 8129 RSC.
- (a) P. P. Lima, R. A. S. Ferreira, S. A. Júnior, O. L. Malta and L. D. Carlos, J. Photochem. Photobiol., A, 2009, 201, 204 CrossRef; (b) M. E. Mesquita, S. S. Nobre, M. Fernandes, R. A. S. Ferreira, S. C. G. Santos, M. O. Rodrigues, L. D. Carlos and V. D. Bermudezc, J. Photochem. Photobiol., A, 2009, 205, 156 CrossRef CAS; (c) D. M. Wang, J. H. Zhang, Q. Lin, L. S. Fu, H. J. Zhang and B. Yang, J. Mater. Chem., 2003, 13, 2279 RSC; (d) X. F. Qiao and B. Yan, New J. Chem., 2011, 35, 568 RSC; (e) X. F. Qiao and B. Yan, Inorg. Chem., 2009, 48, 4714 CrossRef CAS.
- (a) X. F. Qiao, H. Y. Zhang and B. Yan, Dalton Trans., 2010, 39, 8882 RSC; (b) L. Guo, B. Yan, K. Sheng and X. L. Wang, Dalton Trans., 2011, 40, 632 RSC; (c) X. F. Qiao and B. Yan, J. Phys. Chem. B, 2009, 113, 11865 CrossRef CAS; (d) K. Sheng, B. Yan, H. F. Lu and L. Guo, Eur. J. Inorg. Chem., 2010, 3498 CrossRef CAS; (e) B. Yan, L. M. Zhao, X. L. Wang and Y. Zhao, RSC Adv., 2011, 1, 1064–1071 RSC.
- (a) J. P. Yang, F. Zhang, Y. R. Chen, S. Qian, P. Hu, W. Li, Y. H. Deng, Y. Fang, L. Han, M. Luqman and D. Y. Zhao, Chem. Commun., 2011, 47, 11618 RSC; (b) S. Sarkar, A. Makhal, T. Bora, S. Baruah, J. Dutta and K. Samir, Phys. Chem. Chem. Phys., 2011, 13, 12488 RSC; (c) S. Bhandari, M. Deepa, S. N. Sharma, A. G. Joshi, A. K. Srivastava and R. Kant, J. Phys. Chem. C, 2010, 114, 14606 CAS; (d) R. Kas, E. Sevinc, U. Topal and H. Y. Acar, J. Phys. Chem. C, 2010, 114, 7758 CrossRef CAS.
- (a) H. Xu, L. Cheng, C. Wang, X. X. Ma, Y. G. Li and Z. Liu, Biomaterials, 2011, 32, 9364 CrossRef CAS; (b) M. Darbandi, G. Urban and M. Krueger, J. Colloid Interface Sci., 2012, 365, 41 CrossRef CAS; (c) X. D. He, Y. Liu, H. T. Li, H. Huang, J. L. Liu, Z. H. Kang and S. T. Lee, J. Colloid Interface Sci., 2011, 356, 107 CrossRef CAS; (d) K. Kim, J. Y. Woo, S. Jeong and S. H. Han, Adv. Mater., 2011, 23, 911 CrossRef CAS.
- (a) B. H. Kwon, H. S. Jang, H. S. Yoo, S. W. Kim, D. S. Kang, S. Maeng, D. S. Jang, H. Kimand and D. Y. Jeon, J. Mater. Chem., 2011, 21, 12812 RSC; (b) Y. J. Li and B. Yan, Photochem. Photobiol., 2010, 86, 1008 CrossRef CAS; (c) B. Yan, Y. Zhao and Y. J. Li, Photochem. Photobiol., 2011, 87, 757 CrossRef CAS; (d) B. Yan, Y. Zhao and Q. P. Li, J. Photochem. Photobiol., A, 2011, 222, 351 CrossRef CAS; (e) Y. Zhao and B. Yan, Photochem. Photobiol., 2012, 88, 21 CrossRef CAS.
- (a) W. Q. Fan, J. Feng, S. Y. Song, Y. Q. Lei, G. L. Zheng and H. J. Zhang, Chem.–Eur. J., 2010, 16, 1903 CrossRef CAS; (b) W. Q. Fan, J. Feng, S. Y. Song, Y. Q. Lei, L. Zhou, G. L. Zheng, S. Dang, S. Wang and H. J. Zhang, Nanoscale, 2010, 2, 2096 RSC; (c) W. Q. Fan, J. Feng, S. Y. Song, Y. Q. Lei, Y. Xing, R. P. Deng, S. Dang and H. J. Zhang, Eur. J. Inorg. Chem., 2008, 5513 CrossRef CAS; (d) Y. Zhao and B. Yan, Dalton Trans., 2012, 41, 5334 RSC.
- (a) D. P. Liu, G. D. Li, Y. Su and J. S. Chen, Angew. Chem., Int. Ed., 2006, 45, 7370 CrossRef CAS; (b) H. M. Xiong, Z. D. Wang, D. P. Liu, J. S. Chen, Y. G. Wang and Y. Y. Xia, Adv. Funct. Mater., 2005, 15, 1751 CrossRef CAS; (c) H. M. Xiong, Z. D. Wang and Y. Y. Xia, Adv. Mater., 2006, 18, 748 CrossRef CAS; (d) H. M. Xiong, X. Zhao and J. S. Chen, J. Phys. Chem. B, 2001, 105, 10169 CrossRef CAS.
- (a) D. W. Bahnemann, C. Kromann and M. R. Hoffmann, J. Phys. Chem., 1987, 91, 3789 CrossRef CAS; (b) L. Spanhel and M. A. Anderson, J. Am. Chem. Soc., 1991, 113, 2826 CrossRef CAS; (c) E. A. Meulenkamp, J. Phys. Chem. B, 1998, 102, 5566 CrossRef CAS; (d) H. Althues, R. Palkovits, A. Rumplecker, P. Simon, W. Sigle, M. Bredol, U. Kynast and S. Kaskel, Chem. Mater., 2006, 18, 1068 CrossRef CAS.
- U. Schubert, Macromol. Symp., 2008, 267, 1 CrossRef CAS.
- Y. F. Shao and B. Yan, Dalton Trans., 2012, 41, 7423 RSC.
- (a) G. B. Deacon and R. J. Phillips, Coord. Chem. Rev., 1980, 33, 227 CrossRef CAS; (b) S. Sakohara, M. Ishida and M. A. Anderson, J. Phys. Chem. B, 1998, 102, 10169 CrossRef CAS.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic Coordination Compound (translated by D. R. Huang), Chemical Industry Press, Beijing, 1986 Search PubMed.
- (a) M. H. Lim and A. Stein, Chem. Mater., 1999, 11, 3285 CrossRef CAS; (b) W. H. Zhang, X. B. Lu, J. H. Xiu, Z. L. Hua, L. X. Zhang, M. Robertson, J. L. Shi, D. S. Yan and J. D. Holmes, Adv. Funct. Mater., 2004, 14, 544 CrossRef CAS.
- Q. Y. Hu, J. E. Hampsey, N. Jiang, C. J. Li and Y. F. Lu, Chem. Mater., 2005, 17, 1561 CrossRef CAS.
- M. D. Regulacio, M. H. Pablico, J. A. Vasquez, P. N. Myers, S. Gentry, M. Prushan, S. W. Tam-Changand and S. L. Stoll, Inorg. Chem., 2008, 47, 1512 CrossRef CAS.
- (a) G. A. Crosby, J. Chem. Phys., 1961, 34, 743 CrossRef CAS; (b) S. Sato and M. Wada, Bull. Chem. Soc. Jpn., 1970, 43, 1955 CrossRef CAS.
- H. T. Xu, Y. X. Wang, L. Zhao and J. Univ, Sci. Technol. China, 2010, 40, 58 CAS.
- K. S. Guan and Y. S. Yin, Mater. Chem. Phys., 2005, 92, 10 CrossRef CAS.
- (a) F. Eckes, V. Bulach, A. Guenet, C. A. Strassert, L. De Cola and M. W. Hosseini, Chem. Commun., 2010, 46, 619 RSC; (b) A. Beeby, R. S. Dickins, S. FitzGerald, L. J. Govenlock, C. L. Maupin, D. Parker, J. P. Riehl, G. Siligardi and J. A. G. Williams, Chem. Commun., 2000, 1183 RSC; (c) H. S. He, M. Dubey, A. G. Sykes and P. S. May, Dalton Trans., 2010, 39, 6466 RSC.
- (a) S. I. Klink, H. Keizer and F. C. J. M. van Veggel, Angew. Chem., Int. Ed., 2000, 39, 4319 CrossRef CAS; (b) I. Oueslati, R. A. S. Ferreira, L. D. Carlos, C. Baleizao, M. N. Berberan-Santos, B. de Castro, J. Vicens and U. Pischel, Inorg. Chem., 2006, 45, 2652 CrossRef CAS.
- P. C. R. Soares-Santos, H. I. S. Nogueira, V. Felix, M. G. B. Drew, R. A. S. Ferreira, L. D. Carlos and T. Trindade, Chem. Mater., 2003, 15, 100 CrossRef CAS.
- (a) R. A. S. Ferreira, L. D. Carlos, R. R. Goncalves, S. J. L. Ribeiro and V. D. Bermudez, Chem. Mater., 2001, 13, 2991 CrossRef; (b) L. D. Carlos, Y. Messaddeq, H. F. Brito, R. A. Sá Ferreira, V. D. Bermudez and S. J. L. Ribeiro, Adv. Mater., 2002, 12, 594 CrossRef.
- M. H. V. Werts, R. T. F. Jukes and J. W. Verhoeven, Phys. Chem. Chem. Phys., 2002, 4, 1542 RSC.
- R. M. Supkowski and W. D. Horrocks, Inorg. Chim. Acta, 2002, 340, 44 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21605a/ |
| This journal is © The Royal Society of Chemistry 2012 |
