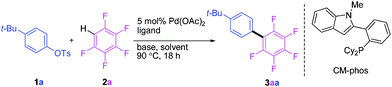Palladium-catalyzed direct arylation of polyfluoroarenes with aryl tosylates and mesylates†
Dong Sheng
Lee
ab,
Pui Ying
Choy
a,
Chau Ming
So
a,
Jun
Wang
a,
Chak Po
Lau
a and
Fuk Yee
Kwong
*a
aState Key Laboratory of Chirosciences and PolyU Shenzhen Research Institute (SZRI), Shenzhen; and Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong. E-mail: fuk-yee.kwong@polyu.edu.hk; Fax: (+) 852-2364-9932
bDepartment of Chemistry, National Chung-Hsing University, Taichung, 402, Taiwan R.O.C.
First published on 2nd August 2012
Abstract
This study reports the first general Pd-catalyzed direct arylation of polyfluoroarenes with aryl tosylates/mesylates. A wide range of polyfluoroarenes can be coupled with both aryl tosylates and mesylates under relatively mild reaction conditions (90 °C, in the presence of a weak base KOAc, without any additional acid additives). Moreover, a one-pot sequential C–H bond functionalization/C–N bond coupling has been successfully accomplished by employing one single Pd/CM-phos catalyst system.
Introduction
Polyfluorobiphenyl motifs are commonly found in numerous pharmaceutically attractive and materially valuable molecules.1 For instance, the perfluorinated polyphenylene sub-unit in photovoltaic polymer (P5FQ),2 perfluorosubstituted hydroxycoumarins (2H-1-benzopyran-2-one) in rodenticides,3 and trypanosomal cathepsin TbcatB inhibitors (9H-purine-2-carbonitrile)4 have aroused considerable focus in the polyfluorinated biaryl synthesis (Fig. 1).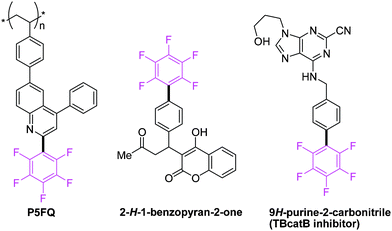 | ||
| Fig. 1 Examples of useful perfluoroarene containing molecules. | ||
Although the traditional cross-coupling repertoire has been successful for connecting two aromatic fragments, multi-step syntheses of organometallic nucleophiles are possible drawbacks. Indeed, in addition to the difficult preparation of highly electron-deficient nucleophiles (e.g. C6F5B(OH)2), the application of electron-poor nucleophilic partners in aromatic bond-forming reactions remain problematic.5 Recent notable findings showed a great achievement in cross-coupling by using direct arylation of a C–H bond from electron-deficient polyfluoroarenes.6 Aryl halides7 and aryl organometallic reagents8 such as arylboronic acids9 can both act as efficient coupling partners with polyfluoroarenes. These new protocols are attractive when compared to conventional coupling methods. However, the substrate scope is mainly limited to aryl halides associating with particular reactivity. Apart from aryl halides, it is worth establishing a method for phenolic electrophiles (pseudo-halides) in these reactions. In fact, aryl sulfonates would have a complementary advantage with respect to aryl halides. They potentially offer different or unique substitution patterns in the aromatic ring, in which the corresponding aryl halides may not be commonly available. Thus, the exploration of less expensive, yet more stable aryl arenesulfonates (when compared to aryl triflates) in coupling reactions is highly favourable. Precedence for palladium-catalyzed direct arylation of polyfluoroarenes using aryl sulfonates remains less explored. In 2006, the Fagnou group reported an example of palladium-catalyzed direct arylation of pentafluorobenzene using phenyl triflate.7b Very recently, the coupling of aryl triflates was further improved by Seayad and co-workers employing a Pd/MePhos catalyst system.10 Additionally, steric encumbered aryl triflates could be coupled with pentafluorobenzene using a Pd/RuPhos complex.10 Apart from triflates, the coupling of aryl tosylates with polyfluoroarenes was only developed recently. In 2011, Zhang reported the palladium-catalyzed direct coupling of polyfluoroarenes with activated heteroaryl tosylates.11 Yet, no examples of non-activated tosylates were shown.
Aryl mesylates are more atom economical than the corresponding aryl tosylates due to their significantly lower molecular weight. However, aryl mesylates are relatively more inactive and it is challenging to apply them in C–H bond functionalization under palladium catalysis. In 2012, Seayad showed the first palladium-catalyzed coupling of aryl mesylates with polyfluoroarenes.10 Only activated aryl mesylates were successfully applied in this transformation under the conditions of high Pd loading (10 mol%) and at high temperature (120 °C). To the best of our knowledge, a general procedure for non-activated (hetero)aryl mesylates and aryl tosylates has been sporadically reported to date. Herein, we report our efforts in developing a general and efficient catalyst system for handling aryl tosylates/mesylates in the direct arylation of perfluoroarenes.
Results and discussion
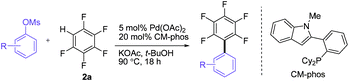
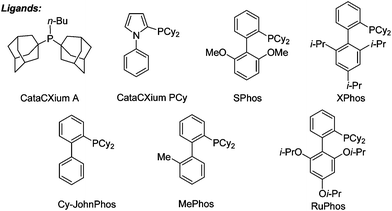
We initially embarked on the coupling of aryl tosylates with polyfluoroarenes by using non-activated 4-tert-butylphenyl tosylate and pentafluorobenzene as the benchmark substrates (Table 1). A series of reaction parameter screenings were then deployed. Commercially available and well-recognized phosphine ligands12 such as CataCXium®A, CataCXium®PCy, SPhos, Xphos, Cy-JohnPhos, MePhos and RuPhos were initially screened (entries 1–7). Moderate substrate conversions and fair product yields were afforded by using biaryl-type monodentate phosphines (entries 3–7). A catalyst system comprising Pd(OAc)2 and CM-phos13 was found superior for this tosylate coupling (entry 8). Of the commonly used organic solvents examined, the alcoholic solvent t-BuOH was found to be the best solvent of choice whereas DMF and dioxane gave moderate and poor conversions, respectively (entries 8, 11, 13). t-BuOH/DMF solvent mixture also afforded a good yield for this direct arylation (entries 12). The addition of pivalic acid (to the K2CO3 system) greatly improved the conversion (entries 8 vs. 10). Surprisingly, weak bases such as KOAc or Na2CO3 were equally efficient and gave excellent yields even without the employment of additional PivOH (entries 14–15).|
|
||||
|---|---|---|---|---|
| Entry | Ligand | Solvent | Base | Yield (%)b |
a Reaction conditions: ArOTs 1a (0.3 mmol), C6F5H (0.6 mmol), Pd(OAc)2 (5.0 mol%), (Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand = 1 ligand = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), base (0.45 mmol) and t-BuOH (1.0 mL) under N2 at 90 °C for 18 h.
b GC yields were reported.
c 2.0 mol% of Pd(OAc)2 was used.
d 10 mol% of PivOH was added. 4), base (0.45 mmol) and t-BuOH (1.0 mL) under N2 at 90 °C for 18 h.
b GC yields were reported.
c 2.0 mol% of Pd(OAc)2 was used.
d 10 mol% of PivOH was added.
|
||||
| 1 | CataCXium® A | t-BuOH | K2CO3 | 0 |
| 2 | CataCXium® PCy | t-BuOH | K2CO3 | 0 |
| 3 | SPhos | t-BuOH | K2CO3 | 47 |
| 4 | XPhos | t-BuOH | K2CO3 | 37 |
| 5 | Cy-JohnPhos | t-BuOH | K2CO3 | 4 |
| 6 | MePhos | t-BuOH | K2CO3 | 12 |
| 7 | RuPhos | t-BuOH | K2CO3 | 43 |
| 8 | CM-phos | t-BuOH | K2CO3 | 76 |
| 9c | CM-phos | t-BuOH | K2CO3 | 43 |
| 10d | CM-phos | t-BuOH | K2CO3 | 87 |
| 11 | CM-phos | DMF | K2CO3 | 51 |
| 12 | CM-phos |
t-BuOH/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
K2CO3 | 77 |
| 13 | CM-phos | Dioxane | K2CO3 | 32 |
| 14 | CM-phos | t-BuOH | Na2CO3 | 90 |
| 15 | CM-phos | t-BuOH | KOAc | 90 |
| 16d | CM-phos | t-BuOH | KOAc | 92 |
| 17 | CM-phos | t-BuOH | NaOAc | 73 |
|
||||
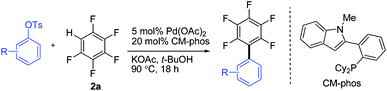
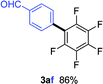
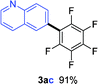
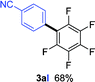
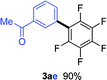
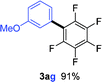
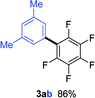
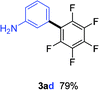
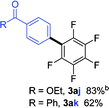
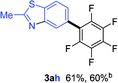
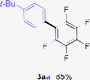
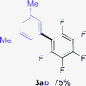
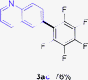
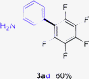
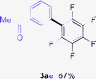
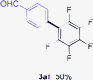
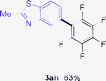
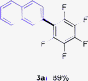
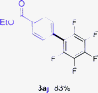
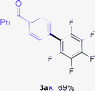
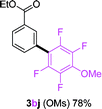
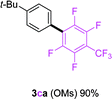
Having the preliminary optimized reaction conditions in hand, we examined a range of aryl tosylates in this reaction (Table 2). To the best of our knowledge, there has been no successful example of non-activated aryl tosylates reported to date in direct arylation of perfluoroarenes. Electronically neutral aryl tosylates were effective and gave the corresponding products in good to excellent yields. Functional groups such as methoxy, keto, cyano, ester and aldehyde were compatible under these reaction conditions. Heterocyclic benzothiazoyl and quinolinyl tosylates gave the corresponding coupling products smoothly.
The Pd/CM-phos catalytic system was also found to be effective in promoting the coupling of aryl mesylates (Table 3). An array of aryl mesylates were tested in this coupling reaction. Heteroaryl mesylates were found to be applicable in this system.
|
|
|||
|---|---|---|---|
a Reaction conditions: ArOMs (0.3 mmol), C6F5H (0.6 mmol), Pd(OAc)2 (5.0 mol%), (Pd:CM-phos = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), KOAc (0.45 mmol) and t-BuOH (1.0 mL) under N2 at 90 °C for 18 h (reaction time for each substrate was not optimized). Isolated yields are reported. 4), KOAc (0.45 mmol) and t-BuOH (1.0 mL) under N2 at 90 °C for 18 h (reaction time for each substrate was not optimized). Isolated yields are reported.
|
|||
|
|
|
|
|
|
|
|

|
|
|
|
|
|
||
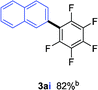
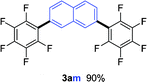
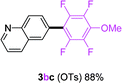
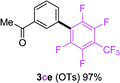
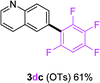
To further expand the substrate scope, we next investigated the feasibility of using other polyfluoroarenes as the coupling partners (Table 4). Moderate to excellent yields resulted.
a Reaction conditions: ArOTs/OMs (0.3 mmol), polyfluoroarene (0.9 mmol), Pd(OAc)2 (5.0 mol%), (Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CM-phos = 1 CM-phos = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), KOAc (0.45 mmol) and t-BuOH (1.0 mL) under N2 at 90 °C for 18 h (reaction time for each substrate was not optimized). Isolated yields are reported.
b 1.2 mmol of polyfluoroarene was used.
c 0.6 mmol of polyfluoroarene was used.
d 24 h was used. 4), KOAc (0.45 mmol) and t-BuOH (1.0 mL) under N2 at 90 °C for 18 h (reaction time for each substrate was not optimized). Isolated yields are reported.
b 1.2 mmol of polyfluoroarene was used.
c 0.6 mmol of polyfluoroarene was used.
d 24 h was used.
|
||
|---|---|---|
|
|
|
|
|

|
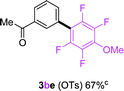
|
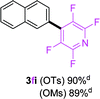
|
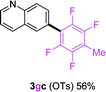
|
To further show the potential application of this coupling process in synthesizing related cathepsin TbcatB inhibitors (consisting of –C6F5, N-Ar and –CN moieties),4 a tandem reaction was attempted. To our delight, the one-pot synthesis of an N-aryl aminobenzonitrile (65% yield in two steps) was successful from direct coupling of aryl tosylate with pentafluorobenzene and subsequent N-arylation of the amino moiety (Scheme 1).
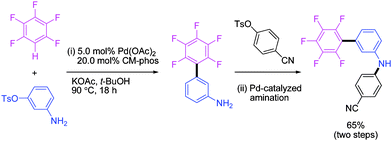 | ||
| Scheme 1 One pot sequential reaction (see ESI† for detailed procedures). | ||
In order to investigate the dependence of the C–H bond cleavage, a kinetic isotope effect (KIE) experiment was carried out. A KIE of 1.2 was observed from the competitive experiment of deuteropentafluorobenzene and pentafluorobenzene. This result indicated that the C–H bond cleavage is likely to be not the kinetically rate-determining step in this catalysis.
Conclusions
In summary, we reported the first general palladium-catalyzed direct arylation of polyfluoroarenes with aryl tosylates and mesylates. This protocol offers a convenient access to polyfluorobiaryl scaffolds from phenolic derivatives. Particularly noteworthy is that the reaction conditions are relatively mild (weak base, KOAc; at 90 °C, without acid additives). Moreover, the cascade direct arylation/C–N bond-coupling sequence could be carried out by using one Pd/CM-phos catalyst system. We believe this direct arylation protocol using sulfonate coupling partners is versatile for diversified functional materials and pharmaceuticals.Acknowledgements
We thank the Research Grants Council of Hong Kong (CERG: PolyU5012/09P) and State Key Laboratory of Chirosciences (4-BBX3) for financial support.References
- For selected reviews: (a) H. Amii and K. Uneyama, Chem. Rev., 2009, 109, 2119 CrossRef CAS; (b) E. A. Meyer, R. K. Castellano and F. Diederich, Angew. Chem., Int. Ed., 2003, 42, 1210 CrossRef CAS; (c) F. Babudri, G. M. Farinola, F. Naso and R. Ragni, Chem. Commun., 2007, 1003 RSC; (d) T. Okamoto, K. Nakahara, A. Saeki, S. Seki, J. H. Oh, H. B. Akkerman, Z. Bao and Y. Matsuo, Chem. Mater., 2011, 23, 1646 CrossRef CAS.
- A. A. Stefopoulos, S. N. Kourkouli, S. Economopoulos, F. Ravani, A. Andreopoulou, K. Papagelis, A. Siokou and J. K. Kallitsis, Macromolecules, 2010, 43, 4827 CrossRef CAS.
- A. J. Whittle, J. J. Swanborough, D. R. Parry and R. L. Sunley, Brit. UK Pat. Appl., 2003, 38pp Search PubMed.
- J. P. Mallari, A. A. Shelat, A. Kosinski, C. R. Caffrey, M. Connelly, F. Zhu, J. H. McKerrow and R. K. Guy, J. Med. Chem., 2009, 52, 6489 CrossRef CAS.
- T. Korenaga, T. Kosaki, R. Fukumura, T. Ema and T. Sakai, Org. Lett., 2005, 7, 4915 CrossRef CAS.
- For recent selected references: (a) Y. Wei and W. Su, J. Am. Chem. Soc., 2010, 132, 16377 CrossRef CAS; (b) H. Li, J. Liu, C.-L. Sun, B.-J. Li and Z.-J. Shi, Org. Lett., 2011, 13, 276 CrossRef CAS; (c) Y. Wei, H. Zhao, J. Kan and M. Hong, J. Am. Chem. Soc., 2010, 132, 2522 CrossRef CAS; (d) C.-Y. He, S. Fan and X. Zhang, J. Am. Chem. Soc., 2010, 132, 12850 CrossRef CAS; (e) R. G. Kalkhambkar and K. K. Laali, Tetrahedron Lett., 2011, 52, 5525 CrossRef CAS; (f) C. –Y. He, Q. –Q. Min and X. Zhang, Organometallics, 2012, 31, 1335 CrossRef CAS; (g) F. Chen, Z. Feng, C. –Y. He, H. –Y. Wang, Y.–L Guo and X. Zhang, Org. Lett., 2012, 14, 1176 CrossRef CAS.
- For recent selected references with aryl halides as coupling partners: (a) M. Lafrance, C. N. Rowley, T. K. Woo and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 8754 CrossRef CAS; (b) M. Lafrance, D. Shore and K. Fagnou, Org. Lett., 2006, 8, 5097 CrossRef CAS; (c) H.-Q. Do and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 1128 CrossRef CAS; (d) H.-Q. Do, R. M. K. Khan and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 15185 CrossRef CAS; (e) H.-Q. Do and O. Daugulis, Chem. Commun., 2009, 6433 RSC; (f) O. Rene and K. Fagnou, Org. Lett., 2010, 12, 2116 CrossRef CAS; (g) F. Chen, Q.-Q. Min and X. Zhang, J. Org. Chem., 2012, 77, 2992 CrossRef CAS.
- H. Zhao, Y. Wei, J. Xu, J. Kan, W. Su and M. Hong, J. Org. Chem., 2011, 76, 882 CrossRef CAS.
- Y. Wei, J. Kan, M. Wang, W. Su and M. Hong, Org. Lett., 2009, 11, 3346 CrossRef CAS.
- J. W. W. Chang, E. Y. Chia, C. L. L. Chai and J. Seayad, Org. Biomol. Chem., 2012, 10, 2289 CAS.
- S. Fan, J. Yang and X. Zhang, Org. Lett., 2011, 13, 4374 CrossRef CAS.
- From Strem Chemicals catalogue 2010–2012.
- For our previous developments on CM-phos and related indolylphosphines see: (a) C. M. So and F. Y. Kwong, Chem. Soc. Rev., 2011, 40, 4963 RSC; (b) C. M. So, C. P. Lau and F. Y. Kwong, Angew. Chem. Int. Ed., 2008, 47, 8059 CrossRef CAS; (c) C. M. So, C. P. Lau, A. S. C. Chan and F. Y. Kwong, J. Org. Chem., 2008, 73, 7731 CrossRef CAS; (d) C. M. So, H. W. Lee, C. P. Lau and F. Y. Kwong, Org. Lett., 2009, 11, 317 CrossRef CAS; (e) W. K. Chow, C. M. So, C. P. Lau and F. Y. Kwong, J. Org. Chem., 2010, 75, 5109 CrossRef CAS; (f) P. Y. Yeung, C. M. So, C. P. Lau and F. Y. Kwong, Angew. Chem. Int. Ed., 2010, 49, 8918 CrossRef CAS; (g) C. M. So, C. P. Lau and F. Y. Kwong, Chem. Eur. J., 2011, 17, 761 CrossRef CAS; for review of our previous ligand development, see: (h) F. Y. Kwong and A. S. C. Chan, Synlett, 2008, 1440 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra21667a |
| This journal is © The Royal Society of Chemistry 2012 |