Revisiting the layered LiNi0.4Mn0.4Co0.2O2: a magnetic approach
Xiaofei
Bie
a,
Lina
Liu
a,
Helmut
Ehrenberg
bc,
Yingjin
Wei
a,
Kristian
Nikolowski
b,
Chunzhong
Wang
a,
Yutaka
Ueda
d,
Hong
Chen
e,
Gang
Chen
a and
Fei
Du
ad
aKey Laboratory of Physics and Technology for Advanced Batteries (Ministry of Education), College of Physics, Jilin University, Changchun 130012, P. R. China. E-mail: dufei@jlu.edu.cn
bInstitute for Applied Materials (IAM), Karlsruhe Institute of Technology (KIT), D-76344 Eggenstein-Leopoldshafen, Germany
cHelmholtz-Institute Ulm for Electrochemical Energy Storage (HIU), P.O. Box 3640, 76021 Karlsruhe, Germany
dInstitute for Solid State Physics, University of Tokyo, Kashiwa, Chiba 277-8581, Japan
eCollege of Physics, Beihua University, Jilin 132013, P. R. China
First published on 22nd August 2012
Abstract
Layered LiNi0.4Mn0.4Co0.2O2 has been synthesized by the co-precipitation method, and the structural, electrochemical and magnetic properties were comprehensively studied by Rietveld analysis, charge–discharge potential profiles, X-ray photoelectron spectroscopy, and dc and ac susceptibilities. The material shows initial discharge capacities of 166 and 206 mA h g−1 in potential windows of 2.5–4.4 V and 2.5–4.6 V, respectively, and a better capacity retention of 95% at 2.5–4.4 V after 50 cycles. The effective paramagnetic moment is calculated to be 3.02(3) μB/f.u. by fitting to the Curie–Weiss law, which is consistent with the averaged value, based on the specific contributions, as quantified by an analysis of the X-ray photoelectron spectroscopy data. The dc magnetization curves show irreversibility and spin freezing behavior at 77 K and 18 K, respectively. The evolution of irreversibility temperature under different applied fields indicates a spin-glass-like transition. The ac susceptibility data and the fitting using the frequency dependent spin-freezing temperatures also confirm this magnetic transition. In comparison with the previous results, the co-precipitation prepared sample shows a big difference in the magnetic parameters, coming from the different microscopic exchange interactions or the formation of a different scale of spin clusters, which is sensitive to the preparation procedure.
1. Introduction
As a promising candidate for cathode materials for lithium-ion batteries, LiNiyMnyCo1−2yO2 has been investigated widely for its low cost and environmentally benign properties.1–4 LiNiyMnyCo1−2yO2 has an α-NaFeO2 structure, with the oxygen atoms in a cubic close packed arrangement, where Ni2+ serves as a two-electron redox center, Co3+ increases the electronic conductivity and the non-Jahn–Teller Mn4+ helps to stabilize the rhombohedral framework during charge–discharge cycling.5,6 This system has been intensively studied by a number of experimental techniques, which usually focus on the long-range and short-range crystal structure, electronic structure, ionic and electronic transport.7–10 Although magnetic characterization cannot provide a direct method for improving the electrochemical performance, it can contribute to understanding the electronic interaction between ion clusters at the microscopic level, since the charge capacity and capacity retention of a cathode are closely related to the cation ordering and phase stability of the material.In an earlier report, Chernova et al.11 synthesized the LiNiyMnyCo1−2yO2 system by a high temperature solid state reaction. They found that the Weiss constant increased with decreasing level of Co-substitution y, indicating that the nonmagnetic Co3+ ions destroy the strong antiferromagnetic 90° superexchange interaction between Ni2+, O2− and Mn4+ ions, and a canonical spin glass transition was observed for the LiNiyMnyCo1−2yO2 system. However, another study on LiNi0.4Mn0.4Co0.2O2, synthesized by a citrate precursor method, showed cluster spin glass behavior with a higher irreversibility temperature and a more negative Weiss constant, in comparison with Chernova's results.12 From a magnetic point of view, the nearly 90° superexchange via O2− and the direct exchange between Ni2+, Mn4+ and Co3+ ions within the transition metal layers of LiNiyMnyCo1−2yO2 play a key role, because the transition metal layers are separated by nonmagnetic lithium layers.13 Within the transition metal layers, the geometrically frustrated triangle lattice would increase the quantum fluctuation of this system. On the other hand, the unavoidable disorder of Ni2+/Mn4+/Co3+ within the transition metal layers and the Li+/Ni2+ disorder in different layers would somewhat destroy the frustration effect. Note that the disordered arrangement is very sensitive to the preparation procedure, which is closely related to the formation of ion clusters. Hence, it is very interesting to study the competition between geometrical frustration and disorder and the influence of the preparation procedure on the magnetic and electrochemical properties, and highlight the link between the magnetism, electronic and atomic structure in the LiNiyMnyCo1–2yO2 system. In this work, a co-precipitation method was applied for the synthesis of the LiNi0.4Mn0.4Co0.2O2 compound, and the structural, electrochemical and magnetic properties are comprehensively studied and discussed in comparison with the previous reports.
2. Experimental
The precursor was synthesized by the co-precipitation method using transition-metal sulfate, sodium hydroxide and ammonia as starting materials. Stoichiometric amounts of NiSO4·6H2O, MnSO4·H2O and CoSO4·7H2O were dissolved with a molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and stirred continuously in deionized water. NaOH and NH3·H2O (Na
2 and stirred continuously in deionized water. NaOH and NH3·H2O (Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 = 1
NH3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were dissolved in deionized water to reach a 1M hydroxide solution. Then the precipitation was achieved by slowly dripping the alkaline solution into the sulfate solution until pH = 12. After vigorous stirring at 50 °C under a nitrogen atmosphere for 12 h, the homogenously precipitated hydroxide powder (Ni0.4Mn0.4Co0.2)(OH)2 was washed several times with 3 L deionized water by centrifuging and dried at 120 °C for 12 h. Synthesis of LiNi0.4Mn0.4Co0.2O2 was carried out using a solid state reaction. The obtained precursor (Ni0.4Mn0.4Co0.2)(OH)2 was mixed with a 5% excess of Li2CO3 by ball milling. The excess amount of Li salts was used to compensate for possible Li loss during calcination. After heating at 450 °C for 5h, the mixture was ground and pressed into pellets, then heated again at 900 °C for 12h to obtain the final products.
2) were dissolved in deionized water to reach a 1M hydroxide solution. Then the precipitation was achieved by slowly dripping the alkaline solution into the sulfate solution until pH = 12. After vigorous stirring at 50 °C under a nitrogen atmosphere for 12 h, the homogenously precipitated hydroxide powder (Ni0.4Mn0.4Co0.2)(OH)2 was washed several times with 3 L deionized water by centrifuging and dried at 120 °C for 12 h. Synthesis of LiNi0.4Mn0.4Co0.2O2 was carried out using a solid state reaction. The obtained precursor (Ni0.4Mn0.4Co0.2)(OH)2 was mixed with a 5% excess of Li2CO3 by ball milling. The excess amount of Li salts was used to compensate for possible Li loss during calcination. After heating at 450 °C for 5h, the mixture was ground and pressed into pellets, then heated again at 900 °C for 12h to obtain the final products.
The crystal structure of the material was studied by X-ray diffraction on a Bruker AXS diffractometer with Cu-Kα radiation. Diffraction data were recorded over the 2θ range 10°–80° with a step size of 0.01° and account time of 6s. The software package WINPLOTR was used for data analysis and Rietveld refinement of the structural model. X-ray photoelectron spectroscopy (XPS) was performed on an ESCALAB spectrometer (VG scientific) using a monochromatic Mg-Kα light source. All measured binding energies (BEs) were referenced to the C1s line at 284.6 eV. Magnetic characterization was performed using a superconducting quantum interference device magnetometer (Quantum Design MPMS-XL).
The electrochemical measurement was carried out using 2032 coin cells. A metallic lithium foil served as the anode electrode. The cathode electrode was composed of a mixture of LiNi0.4Mn0.4Co0.2O2 active material (80 wt%), super-P conductive additive (10 wt%), and polyvinylidenefluoride binder (PVDF, 10 wt%) dissolved in N-methylpyrrolidone (NMP). The slurry mixture was pasted on an aluminum foil, followed by drying at 120 °C for 24 h in a vacuum oven. Each electrode contained about 5 mg of active material. The electrolyte was 1 M lithium hexafluorophosphate (LiPF6) dissolved in ethylene carbonate (EC) and diethyl carbonate (DEC) (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC = 1
DEC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The battery cell was assembled in an argon-filled glove box with H2O and O2 concentrations below 1 ppm. The charge–discharge cycle was performed on a Land-2001A battery cycler.
1, v/v). The battery cell was assembled in an argon-filled glove box with H2O and O2 concentrations below 1 ppm. The charge–discharge cycle was performed on a Land-2001A battery cycler.
3. Results and discussions
In order to check the R-3m structure of the obtained LiNi0.4Mn0.4Co0.2O2 material and to determine the degree of Li+/Ni2+ cation disorder, a Rietveld refinement is performed, based on the powder X-ray diffraction data, as shown in Fig. 1. A structural model based on the α-NaFeO2 structure is used, with the detailed refined parameters listed in Table 1. During the refinement, the occupancy of the 3a sites by Mn and Co is fixed at 0.4 and 0.2 for each ion, and the total amount of Li and Ni is also fixed. In agreement with other structural studies of the LiNiyMnyCo1−2yO2 system,14,15 a partial Li+/Ni2+ mixing between different layers is also considered and allowed to vary. Such refinement results in a model in which 3.6% of Ni ions in the transition metal layers are exchanged with Li ions, being best described by the formula [Li0.964Ni0.036]3b[Li0.036Ni0.364Mn0.4Co0.2]3aO2. The degree of Li+/Ni2+ cation disorder depends on many parameters during synthesis (time, temperature and atmosphere for annealing, particle morphology, residual organic components etc.),16 so that even very similar synthesis conditions can result in slightly different cation distributions. In general, the introduction of Co ions reduces the degree of Li+/Ni2+ mixing, for example from 8% in LiNi0.5Mn0.5O2 to nearly 4%.11 In comparison with previous reports on LiNi0.4Mn0.4Co0.2O2 material,17 our refined values exhibit good agreement among cell parameters, but a lower degree of Li+/Ni2+ cation disorder (3.64%).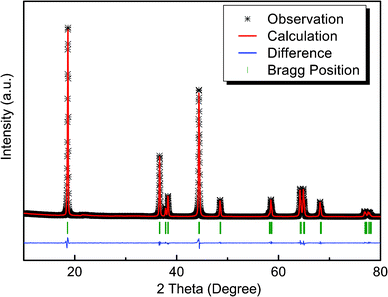 | ||
| Fig. 1 Results from the Rietveld refinement of the LiNi0.4Mn0.4Co0.2O2 structure model, based on power X-ray diffraction using FULLPROF. | ||
| Crystal system | Hexagonal | ||||
|---|---|---|---|---|---|
| Space group | R-3m | ||||
| Cell parameter | a = b = 2.874(8) Å, c = 14.278(5) Å | ||||
| Atom | Site | x | y | z | Occupancy |
| Li(1) | 3b | 0.000 | 0.000 | 0.500 | 0.964 |
| Ni(1) | 3b | 0.000 | 0.000 | 0.500 | 0.036 |
| Li(2) | 3a | 0.000 | 0.000 | 0.000 | 0.036 |
| Ni(2) | 3a | 0.000 | 0.000 | 0.000 | 0.364 |
| Mn | 3a | 0.000 | 0.000 | 0.000 | 0.400 |
| Co | 3a | 0.000 | 0.000 | 0.000 | 0.200 |
| O | 6c | 0.000 | 0.000 | 0.241 | 1 |
| R wp (%) = 7.67 | R p (%) = 6.82 | R f (%) = 1.32 | |||
Fig. 2 displays the charge–discharge potential profiles and the cycling performance of LiNi0.4Mn0.4Co0.2O2 in different potential windows. The material exhibits an initial discharge capacity of 166 and 206 mA h g−1 in the potential windows of 2.5–4.4 V and 2.5–4.6 V, respectively. The capacity retention is relatively poor when the material is cycled between 2.5 V and 4.6 V. This may be due to the formation of a solid electrolyte interface film on the particle surface or the dissolving of Co3+ into the electrolyte.18,19 In spite of this possible disadvantage, the LiNi0.4Mn0.4Co0.2O2 material prepared by the described co-precipitation method demonstrates a high reversible capacity and capacity retention. Such an improvement in the observed electrochemical performance is probably related to the low level of Li+/Ni2+ cation disorder or a superior morphology of the material. Further electrochemical measurements are in progress to reveal the underlying mechanisms.
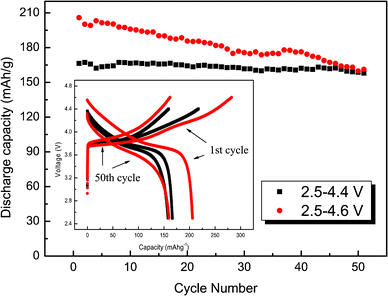 | ||
| Fig. 2 Cycling performance of LiNi0.4Mn0.4Co0.2O2 in the voltage windows of 2.5–4.4 V and 2.5–4.6 V. | ||
The valence states of the transition metals in LiNi0.4Mn0.4Co0.2O2 should be determined before a reliable magnetic property analysis is possible, since magnetic exchange interactions are very sensitive to the specific electron configuration of the involved ions. Fig. 3 shows the XPS of the Ni2p, Mn2p and Co2p of the LiNi0.4Mn0.4Co0.2O2 material. The fitting method is based on ref. 20, which uses the linear background and a constant full width at half maximum. The Ni2p3/2 and Ni2p1/2 spectra are located at 854.2 and 871.6 eV, respectively. After fitting to the Ni2p3/2 peak, a mixed valence state of Ni is proposed, with two binding energies located at 853.6 eV and 855.0 eV, which correspond to Ni2+ and Ni3+, respectively.21,22 The ratio of Ni2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni3+ is nearly 80
Ni3+ is nearly 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, based on the corresponding integrated areas. A similar interpretation describes the Mn2p3/2 doublet, which can also be separated into two peaks, located at 641.3 eV and 642.2 eV, corresponding to the Mn3+ and Mn4+ cations, respectively.23 The Co2p3/2 and Co2p1/2 binding energies, which are localized at 779.4 eV and 794.4 eV, are consistent with those of the Co3+ ions.24 In a typical LiNi0.4Mn0.4Co0.2O2 material, the oxidation states of nickel, cobalt and manganese are expected to be +2, +3 and +4, respectively. But the present material shows a mixed valence state for the Ni and Mn ions. This mixed valence state will not only affect the electrochemical performance strongly, but also plays an important role in the magnetic behavior, because the magnetic ordering in the LiNiyMnyCo1−2yO2 system is dependent on the magnetic exchange interaction via nearly 90° Ni2+/3+–O2−–Mn3+/4+ superexchange paths and 180° Ni2+/3+–Mn3+/4+ direct exchange.
20, based on the corresponding integrated areas. A similar interpretation describes the Mn2p3/2 doublet, which can also be separated into two peaks, located at 641.3 eV and 642.2 eV, corresponding to the Mn3+ and Mn4+ cations, respectively.23 The Co2p3/2 and Co2p1/2 binding energies, which are localized at 779.4 eV and 794.4 eV, are consistent with those of the Co3+ ions.24 In a typical LiNi0.4Mn0.4Co0.2O2 material, the oxidation states of nickel, cobalt and manganese are expected to be +2, +3 and +4, respectively. But the present material shows a mixed valence state for the Ni and Mn ions. This mixed valence state will not only affect the electrochemical performance strongly, but also plays an important role in the magnetic behavior, because the magnetic ordering in the LiNiyMnyCo1−2yO2 system is dependent on the magnetic exchange interaction via nearly 90° Ni2+/3+–O2−–Mn3+/4+ superexchange paths and 180° Ni2+/3+–Mn3+/4+ direct exchange.
 | ||
| Fig. 3 X-Ray photoelectron spectra for LiNi0.4Mn0.4Co0.2O2. | ||
The magnetic properties of LiNi0.4Mn0.4Co0.2O2 are firstly studied by dc magnetization measurements, as shown in Fig. 4, including a de Almeida–Thouless (AT) line fitting and the inverse magnetization curve. Magnetization data in zero-field cooled (ZFC) and field-cooled (FC) modes in three different applied fields are compared in Fig. 4(a). Apparently the ZFC magnetization displays a sharp peak at the freezing temperature (Tf = ~18 K). Unlike ferromagnetism or canonical spin glass, the FC magnetization does not exhibit a “Brillouin-like” behavior or temperature-independent platform-like behavior.25 At low temperature, a pronounced cusp is also observed in the FC mode and will be quenched by a stronger applied field of 1000 Oe. In addition the bifurcation between the ZFC and FC curves is still observed at the irreversibility temperature (Tirr = ~77 K) under 100 Oe. The irreversibility temperature shifts to a lower temperature with increasing magnetic field and can also be fitted by the de Almeida–Thouless (AT) line, which predicts one critical line for a spin glass in the mean-field theory.26 The AT equation is expressed as follows
| HAT(Tirr)/ΔJ ∝ (1 − Tirr/TF)3/2 | (1) |
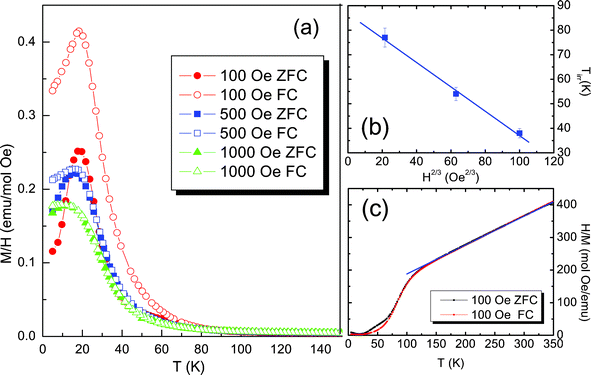 | ||
| Fig. 4 (a) ZFC and FC susceptibility as a function of temperature between 5 and 150 K in an applied magnetic field of 100, 500 and 1000 Oe; (b) fitting to the de Almeida–Thouless (AT) line with the irreversibility temperatures; (c) the inverse magnetization data with the Curie–Weiss fitting. | ||
At higher temperatures the magnetization shows a paramagnetic dependence on temperature, as shown in Fig. 4(c). By fitting the high-temperature linear behavior to the Curie–Weiss law,27 the Weiss constant is calculated to be −98 K, suggesting a strong antiferromagnetic interaction. The Curie constant is fitted to be 1.15 emu K mol−1 Oe, with which the effective moment μeff = 3.02(3) μB/f.u. is obtained by evaluating the relation 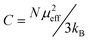 , where N is the number density of magnetic ions per mole and kB is Boltzmann's constant.28 Theoretically the effective moment μeff = 3.02(5) μB/f.u. can be obtained by
, where N is the number density of magnetic ions per mole and kB is Boltzmann's constant.28 Theoretically the effective moment μeff = 3.02(5) μB/f.u. can be obtained by 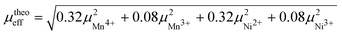 , where the Mn4+/Mn3+ and Ni3+/Ni2+ ratios are taken from the XPS analysis. The consistency between the experimental and the theoretical moment not only confirms that the surface-sensitive XPS results are representative for the bulk material, but also suggests that nearly all the electrons are localized at the magnetic ions and are not itinerant.
, where the Mn4+/Mn3+ and Ni3+/Ni2+ ratios are taken from the XPS analysis. The consistency between the experimental and the theoretical moment not only confirms that the surface-sensitive XPS results are representative for the bulk material, but also suggests that nearly all the electrons are localized at the magnetic ions and are not itinerant.
The field dependence of the magnetization at four temperatures is also measured and shown in Fig. 5. It is obvious that the lower the temperature, the more the M(H) curve bends. However, magnetic saturation is not reached, even at 5 T. The magnetization becomes a nonlinear function of the field and displays ferromagnetic behavior with a small hysteresis in the low field region, whose coercive field and remanence at 5 K are listed in Table 2. Both the absence of magnetization saturation at high fields and the pronounced hysteresis loop in the low-field region are characteristics of spin-glass-like behavior.13,29–32
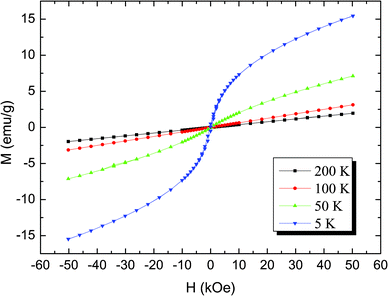 | ||
| Fig. 5 The magnetic field variation of magnetization at different fixed temperatures for LiNi0.4Mn0.4Co0.2O2. | ||
In order to confirm the spin-glass-like transition, the temperature dependence of the ac susceptibilities over the frequency range 10 to 1000 Hz is recorded as shown in Fig. 6(a). It can be seen that the real part of the ac susceptibility (χ′) exhibits a peak at ~20 K, corresponding to Tf in the dc curves. And with the increase in driving frequency the peak position shifts towards a higher temperature and the height of the ac peak decreases, which are characteristics of a spin glass or superparamagentic transition. In order to distinguish the spin glass from superparamagnetism, the criterion parameter δ is considered33,34
| δ = ΔTf/TfΔlog ω | (2) |
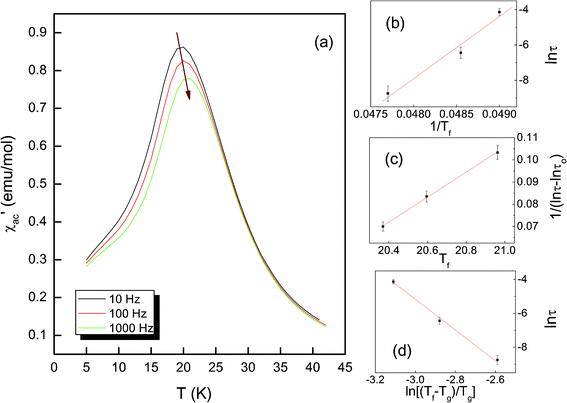 | ||
| Fig. 6 (a) Temperature dependence of the real part of ac susceptibility in the frequency range 10 to 1000 Hz; frequency dependence of the dynamic spin freezing temperature Tf in LiNi0.4Co0.2Mn0.4O2, analyzed using (b) the Néel–Arrhenius law, (c) the Vogel–Fulcher law, and (d) the power law. | ||
So far, three kinds of models can be employed to quantitatively analyze the dynamic behavior near Tf: the Néel–Arrhenius law,35–39 the Vogel–Fulcher law34,40,41,43 and the power law38,39,42. For a system of non-interacting superparamagnetic particles, the relaxation time follows the Néel–Arrhenius law
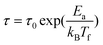 | (3) |
The Vogel–Fulcher law is a modification of the Néel–Arrhenius law and takes the interactions among spin clusters into account, as expressed by
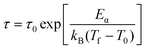 | (4) |
The power law, which supposes the existence of an equilibrium phase transition with a divergence of relaxation time near the transition temperature, has been used to explain the relaxation behavior in a spin glass system,
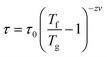 | (5) |
The above experimental analysis confirms the spin-glass-like behavior in the LiNi0.4Mn0.4Co0.2O2 material prepared by co-precipitation. From the point of view of spin glass formation, geometrical frustration and random distribution of transition metal ions are regarded as the two main reasons. By calculating the frustration parameter 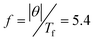 , which is far below the limit where frustration plays an important role, we can rule out the influence of geometrical frustration.44 On the other hand, in the local structure of LiNi0.5Mn0.5O2, a superstructurally ordered “flower” structure has been suggested to be the ground state by first-principle calculations, where Li+ ions occupy the center surrounded by six Mn4+ ions, and the outer leaves are made up of twelve Ni2+ ions. In such a magnetic model, magnetic ordering with a short-range net magnetic moment formation happens at 95 K. When nonmagnetic Co3+ ions are introduced into the TM layers, they will act as a dilution of the magnetic network, destroy the superstructure ordering in [LiMn6] “flowers”, and form ion clusters isolated by nonmagnetic Co3+ ions. Within the clusters there might be short-range ferromagnetic ordering coming from ferromagnetic 90° Mn4+–O–Mn4+ and direct 180° Ni2+–Mn4+ interactions,12 as manifested by the small magnetic hysteresis in the low field range at 5 K. However, we cannot fully exclude the strong 180° Ni2+–O2−–Ni2+ antiferromagnetic interaction between different layers, although Co3+ doping can decrease the degree of Li+/Ni2+ disorder to 3.64%. The negative Weiss constant and no magnetic saturation, even at 5 T, strongly suggest dominant antiferromagnetic interactions among clusters. So the competition between ferromagnetic and antiferromagnetic interactions of clusters will induce a spin-glass-like transition, rather than the geometrical frustration effect.
, which is far below the limit where frustration plays an important role, we can rule out the influence of geometrical frustration.44 On the other hand, in the local structure of LiNi0.5Mn0.5O2, a superstructurally ordered “flower” structure has been suggested to be the ground state by first-principle calculations, where Li+ ions occupy the center surrounded by six Mn4+ ions, and the outer leaves are made up of twelve Ni2+ ions. In such a magnetic model, magnetic ordering with a short-range net magnetic moment formation happens at 95 K. When nonmagnetic Co3+ ions are introduced into the TM layers, they will act as a dilution of the magnetic network, destroy the superstructure ordering in [LiMn6] “flowers”, and form ion clusters isolated by nonmagnetic Co3+ ions. Within the clusters there might be short-range ferromagnetic ordering coming from ferromagnetic 90° Mn4+–O–Mn4+ and direct 180° Ni2+–Mn4+ interactions,12 as manifested by the small magnetic hysteresis in the low field range at 5 K. However, we cannot fully exclude the strong 180° Ni2+–O2−–Ni2+ antiferromagnetic interaction between different layers, although Co3+ doping can decrease the degree of Li+/Ni2+ disorder to 3.64%. The negative Weiss constant and no magnetic saturation, even at 5 T, strongly suggest dominant antiferromagnetic interactions among clusters. So the competition between ferromagnetic and antiferromagnetic interactions of clusters will induce a spin-glass-like transition, rather than the geometrical frustration effect.
Note that the specific chemical preparation method, including the sintering temperature, annealing time and so on, plays an important role in the electronic structure, ordered/disordered arrangement of transition metal ions and stoichiometric ratios in the transition metal oxides. For example, the A-site ordered RBaMn2O6 systems show a clearly separated charge-ordering and Néel transition temperature, instead of the spin glass transition observed in the disordered R0.5Ba0.5MnO3.45,46 As listed in Table 2, we summarize the magnetic parameters of three samples with different preparation conditions. It can be seen that our previous study of the LiNi0.4Mn0.4Co0.2O2 material prepared by the citrate precursor method shows the largest Curie constant and effective moment, which may come from the divergence of oxidation of bivalence in Ni and tetravalence in Mn, while having the largest coercive field and remanence, highest irreversibility, freezing temperature and negative Weiss constant imply a large size of the spin clusters with the strongest antiferromagnetic interactions, due to the high degree of Li+/Ni2+ disorder. In this paper we employ the co-precipitation method for synthesis, similar to the method used in ref. 12. But the magnetic parameters also show considerable differences. We think that although the preparation method, sintering temperature and time are nearly the same, the degree of disorder arrangement of the Ni2+/Mn4+/Co3+ ions is not exactly the same, in spite of the similar chemical preparation methods, and results in different sizes of ion clusters. The disorder in the LiNiyMnyCo1−2yO2 compounds would not only induce different magnetic behavior, but would undoubtedly influence the electrochemical performances in two possible ways. The first one is the disordered Li+/Ni2+ in the different layers, since rhombohedral LiNiyMnyCo1−2yO2 is characteristic of the layered structure, which provides two-dimensional channel transport of the Li ions during the discharge/charge cycles. The disorder means that some of the Li+ ions are introduced into the transition metal layers, which cannot be released in the discharge cycle. Hence, the electrochemical capacity will decrease correspondingly. The other cause lies in the disorder of the transition metal layers. It has been reported that in the LiNi0.4Mn0.4Co0.2O2 system, Ni2+ serves as a two-electron redox center, Co3+ increases the electronic conductivity and the non-Jahn–Teller Mn4+ helps to stabilize the rhombohedral layered framework. If large ion clusters were formed in the transition metal layers, the material would show the electronic phase separation state to some extent, and all the clusters would exhibit a separated electrochemical performance as LiNiO2, LiMnO2 or LiCoO2. The effect of the three co-doped ions would be fully restrained during the electrochemical cycling. So reaching a perfect ordering of Ni2+/Mn4+/Co3+ ions in the transition metal layers might still be a serious challenge for future work.
4. Conclusion
LiNi0.4Mn0.4Co0.2O2 was prepared by the co-precipitation method. Powder X-ray diffraction confirmed the rhombohedral layered structure of the material. The sample exhibited an initial discharge capacity of 166 and 206 mA h g−1 in different potential windows of 2.5–4.4 V and 2.5–4.6 V, respectively. X-ray photoelectron spectroscopy showed some divergence of +2 in Ni ions and +4 in Mn ions, which corresponds well to the high-temperature Curie–Weiss law fitting. A spin-glass-like transition was suggested at low temperature, based on the analysis of dc temperature and field dependent magnetization and ac frequency dependent susceptibility. In comparison with previous results, we considered that the disordered arrangement of Ni2+/Mn4+/Co3+ ions in the transition metal layer and of Li+/Ni2+ in the different layers, induced by slight differences in the synthetic procedure, was one of the most important factors that influenced the magnetic and electrochemical behavior of the material.Acknowledgements
This work was supported by the Special Funds for Major State Basic Research Project of China (2009CB220104), Jilin Province Project of Research and Development, China (20075007), the National Natural Science Foundation of China (Grand No.11004073) and Research Fund for the Doctoral Program of Higher Education of China (new teacher) 20090061120020. Parts of this work was also sponsored by the PhD. Candidates Interdiscipline Research Project of Jilin University (2011J015) and the Development Program of Science and Technology of Jilin Province, China (No. 201205035)References
- Wang Li, Jiangang Li, Xiangming He, Weihua Pu, Chunrong Wan and Changyin Jiang, J. Solid State Electrochem., 2009, 13, 1157 CrossRef.
- Kenji Shizuka, Shouji Takano and Kenji Okahara, J. Power Sources, 2007, 174, 1074 CrossRef CAS.
- Zhonghua Lu, D. D. MacNeil and J. R. Dahn, Electrochem. Solid-State Lett., 2001, 4, A200 CrossRef CAS.
- Seung-Taek Myung, Atsushi Ogata, Ki-Soo Lee, Shinichi Komaba, Yang-Kook Sun and Hitoshi Yashiro, J. Electrochem. Soc., 2008, 155, A374 CrossRef CAS.
- X. Li, Y. J. Wei, H. Ehrenberg, F. Du, C. Z. Wang and G. Chen, Solid State Ionics, 2008, 178, 1969 CrossRef CAS.
- Aniruddha Deb, Uwe Bergmann, Stephen P. Cramer and Elton J. Cairns, J. Appl. Phys., 2005, 97, 113523 CrossRef.
- K. M. Shaju, G. V. Subba Rao and B. V. R. Chowdari, Electrochim. Acta, 2002, 48, 145 CrossRef CAS.
- J. Saavedra-Arias José, Naba K. Karan, Dillip K. Pradhan, Arun Kumar, Santander Nieto, Reji Thomas and Ram S. Katiyar, J. Power Sources, 2008, 183, 761 CrossRef.
- Makoto Gozu, Konrad Świerczek and Janina Molenda, J. Power Sources, 2009, 194, 38 CrossRef CAS.
- K. M. Shaju, G. V. Subba Rao and B. V. R. Chowdari, J. Electrochem. Soc., 2004, 151, A1324 CrossRef CAS.
- A. Chernova Natasha, Miaomiao Ma, Jie Xiao, M. Stanley Whittingham, Julien Breger and Clare P. Grey, Chem. Mater., 2007, 19, 4682 CrossRef.
- Fei Du, Xiaofei Bie, Yan Chen, Yingjin Wei, Lina Liu, Chunzhong Wang, Guangtian Zou and Gang Chen, J. Appl. Phys., 2009, 106, 053904 CrossRef.
- A. Chernova Natasha, Gene M. Nolis, Fredrick O. Omenya, Hui Zhou, Zheng Li and M. Stanley Whittingham, J. Mater. Chem., 2011, 21, 9865 RSC.
- W. Li, J. N. Reimers and J. R. Dahn, Phys. Rev. B: Condens. Matter, 1992, 46, 3236 CrossRef CAS.
- J. B. Goodenough, D. G. Wickham and W. J. Croft, J. Phys. Chem. Solids, 1958, 5, 107 CrossRef CAS.
- T. Gross, Th. Buhrmester, K. G. Bramnik,, N. N. Bramnik, K. Nikolowski, C. Baehtz, H. Ehrenberg and H. Fuess, Solid State Ionics, 2005, 176, 1193 CrossRef CAS.
- Miaomiao Ma, Natasha A. Chernova, Brian H. Toby, Peter Y. Zavalij and M. Stanley Whittingham, J. Power Sources, 2007, 165, 517 CrossRef CAS.
- Ling Liu, Kening Sun, Naiqing Zhang and Tongyong Yang, J. Solid State Electrochem., 2009, 13, 1381 CrossRef CAS.
- B. –R. Lee, H. –J. Noh, S. –T. Myung, K. Amine and Y. –K. Sun, J. Electrochem. Soc., 2011, 158, A180 CrossRef CAS.
- J. –G. Kim, D. L. Pugmire, D. Battaglia and M. A. Langell, Appl. Surf. Sci., 2000, 165, 70 CrossRef CAS.
- J. Katana Ngala, Natasha A. Chernova, Miaomiao Ma, Marc Mamak, Peter Y. Zavalij and M. Stanley Whittingham, J. Mater. Chem., 2004, 14, 214 RSC.
- K. M. Shaju, G. V. Subba Rao and B. V. R. Chowdari, Electrochim. Acta, 2003, 48, 1505 CrossRef CAS.
- N. V. Kosova, E. T. Devyatkina and V. V. Kaichev, J. Power Sources, 2007, 174, 965 CrossRef CAS.
- Shumei Dou and Wenlou Wang, J. Solid State Electrochem., 2011, 15, 399 CrossRef CAS.
- Z. Śniadecki, B. Idzikowski, J.-M. Greneche, P. Kerschl, U. K. Röβler and L. Schultz, J. Phys.: Condens. Matter, 2008, 20, 425212 CrossRef.
- M. Gruyters, Phys. Rev. Lett., 2005, 95, 077204 CrossRef CAS PubMed.
- Zu-Fei Huang, Fei Du, Chun-Zhong Wang, Deng-Pan Wang and Gang Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 054411 CrossRef.
- J. Sugiyama, H. Nozaki, J. H. Brewer, E. J. Ansaldo, G. D. Morris and C. Delmas, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 144424 CrossRef.
- Fang Wang, Jian Zhang, Yuan-fu Chen, Guang-jun Wang, Ji-rong Sun, Shao-ying Zhang and Bao-gen Shen, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 094424 CrossRef.
- Ya-Qiong Liang, Nai-li Di and Zhao-hua Cheng, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 134416 CrossRef.
- Fei Du, Zu-Fei Huang, Chun-Zhong Wang, Xing Meng, Gang Chen, Yan Chen and Shou-Hua Feng, J. Appl. Phys., 2007, 102, 113906 CrossRef.
- Yan-kun Tang, Xiao Ma, Zhi-qi Kou, Young Sun, Nai-li Di, Zhao-hua Cheng and Qing-an Li, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 132403 CrossRef.
- Yong Tian Wang, Hai Yang Bai, Ming Xiang Pan, De Qian Zhao and Wei Hua Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 064422 CrossRef.
- S. D. Tiwari and K. P. Rejeev, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 104433 CrossRef.
- L. Néel, Ann. Geophys., 1949, 5, 99 Search PubMed.
- W. F. Brown Jr, Phys. Rev., 1963, 130, 1677 CrossRef.
- I. S. Jacobs and C. P. Bean, Magnetism, Academic, New York, 1963 Search PubMed.
- J. Souletie and J. L. Tholence, Phys. Rev. B, 1985, 32, 516 CrossRef CAS.
- B. Martínez, A. Labarta, R. Rodríguez-Solá and X. Obradors, Phys. Rev. B: Condens. Matter, 1994, 50, 15779 CrossRef.
- R. N. Bhowmik and R. Ranganathan, J. Magn. Magn. Mater., 2001, 237, 27 CrossRef CAS.
- D. X. Li, S. Nimori, Y. Shiokawa, Y. Haga, E. Yamamoto and Y. Onuki, Phys. Rev. B: Condens. Matter, 2003, 68, 172405 CrossRef.
- T. O. Andrew, Phys. Rev. B, 1985, 32, 7384 CrossRef.
- Z. Śniadecki, J. –M. Grenèche and B. Idzikowski, J. Appl. Phys., 2011, 109, 123921 CrossRef.
- E. G. John, J. Mater. Chem., 2001, 11, 37 RSC.
- T. Nakajima, H. Yoshizawa and Y. Ueda, J. Phys. Soc. Jpn., 2004, 73, 2283 CrossRef CAS.
- T. Nakajima and Y. Ueda, J. Appl. Phys., 2005, 98, 461 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
