Amphipathic antibacterial agents using cationic methacrylic polymers with natural rosin as pendant group
Ying
Chen
ab,
Perry A.
Wilbon
b,
Yung Pin
Chen
b,
Juhua
Zhou
c,
Mitzi
Nagarkatti
c,
Chunpeng
Wang
a,
Fuxiang
Chu
a,
Alan W.
Decho
b and
Chuanbing
Tang
*b
aInstitute of Chemical Industry of Forestry Products, Chinese Academy of Forestry, Nanjing, 210042, China
bDepartment of Chemistry and Biochemistry, Department of Environmental Health Sciences and USC Nanocenter, University of South Carolina, Columbia, South Carolina 29208, USA. E-mail: tang4@mailbox.sc.edu (C.T.)
cDepartment of Pathology, Microbiology and Immunology, University of South Carolina School of Medicine, Columbia, South Carolina 29209, USA
First published on 31st August 2012
Abstract
We prepared a class of novel cationic polymers as antimicrobial agents: quaternary ammonium-containing poly(N,N-dimethylaminoethyl methacrylate) with natural rosin as the pendant group (PDMAEMA-g-rosin). Different from most other amphipathic antimicrobial polymeric systems reported in the literature, our approach sandwiched the hydrophilic cationic group between the polymer backbone and bulky hydrophobic hydrophenanthrene side groups. A simple quaternization reaction was used to link the rosin ester chloride and PDMAEMA homopolymers. Both the Gram-positive bacterium Staphylococcus aureus (S. aureus) and Gram-negative bacterium Escherichia coli (E. coli) were tested against the PDMAEMA-g-rosin copolymers. PDMAEMA-g-rosin copolymers with the amphipathic structure exhibited effective antimicrobial activity against both E. coli and S. aureus. Both the degree of quaternization of rosin group and the molecular weight of PDMAEMA played roles in antimicrobial activities. Our results also indicated that conformation of hydrophobic group (particularly steric hindrance) played a role in dictating antibacterial efficacy. Scanning electron microscopy and confocal laser scanning microscopy were used to characterize morphological changes of bacteria after exposure with PDMAEMA-g-rosin copolymers. Possible mechanisms on a combination of ionic and hydrophobic interactions between bacterial cells and polymers are discussed.
Introduction
In recent years, considerable research has been focused on developing synthetic antibacterial polymers to prevent bacterial contamination of food, drinking water and medical implants and devices.1–5 When compared to other small molecule counterparts, the synthetic antibacterial polymers typically show better efficacy as well as reduced residual toxicity, increased selectivity, and prolonged lifetime. Recent reports indicate that synthetic polymers composed of conformational rigid polymer backbones coupled with regulated amphipathic structures show enhanced antibacterial activity and selectivity.6–11 Tew and colleagues synthesized amphipathic oxanorbornene-derived polymers by ring-opening metathesis polymerization that exhibited good antibacterial activity.8 However, it should be noted that the synthetic rigid polymers often need multi-step syntheses. Later, it was found that the amphipathicity and antibacterial activity could be also achieved from random copolymers of hydrophilic and hydrophobic monomers, which greatly simplified the synthesis of antibacterial materials.12 For example, DeGrado and colleagues synthesized amphipathic methacrylate based copolymers consisting of flexible polymer backbones and random amphipathic sequences possessing antibacterial activities that were comparable to those of natural peptides and exhibited relatively low toxicity.13It was reported that poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA) at pH 7 is partially-charged (hydrophilic) and partially-uncharged (hydrophobic), thus essentially an amphipathic macromolecule.14 Due to the amphipathicity, DMAEMA-based copolymers usually exhibit bactericidal activity and often are used as an antibacterial surface cation to inhibit the growth of bacteria, which can be easily attached to glass, filter paper, and plastics.15–18 Quaternization of PDMAEMA or its copolymers is one of the most widely used methods to improve antibacterial activity by increasing positive charge density and amphipathic structure of polymers. It was also noticed that most work with regard to PDMAEMA as an antibacterial agent is involved with an alkyl quaternized derivative or with the use of PDMAEMA as part of a copolymer coupled with other polymers.19,20
The antibacterial activity strongly depends on their overall molecular structure and the hydrophobicity of their substituent group. Generally, two methods are employed for immobilization of quaternary ammonium (QA) moieties on polymers. The first method involves the incorporation of QA into monomers followed by subsequent polymerization. This method has the advantage that the monomers can be copolymerized with various monomers having different compositions. The second method is to link the biocides directly onto preformed functional polymers. The advantage of this method is that the functional polymers can be modified with a variety of different biocides and the degree of modification can be well-controlled. The latter approach also allows more accurate structure-property evaluations by using a common base polymer structure and molecular weight to generate a family of derivatives having comparable structures.
Rosin, also referred to as resin acids or rosin acids, is a natural product derived from pine trees, and its derivatives have been widely used as additives and modifiers for various applications such as tackifiers, surfactants, antifouling coatings, food additives, etc.17,21 These natural products have a characteristic bulky hydrophenanthrene moiety. Rosin acids have received much attention as a renewable feedstock in polymer synthesis.22–26 We recently prepared rosin-substituted polyesters with QA group located at the periphery of entire macromolecules.27 The QA group was exposed outside of both polymer backbone and rosin moiety. We demonstrated that these polymers showed excellent biocompatibility and hydrophobicity, as well as robust antibacterial activity. The high antibacterial activity may be due to their prominent hydrophobicity and the unique bulky hydrophenanthrene structure of rosin acids. However, the synthetic pathways involved at least six steps in order to obtain the antibacterial polymers.
Considering the hydrophilicity of PDMAEMA at pH 7 and the hydrophobicity of rosin moiety, herein we designed a novel structure of PDMAEMA copolymers with pendant group randomly decorated by the rosin moiety (PDMAEMA-g-rosin), in which the cationic QA group is sandwiched between polymer backbone and bulky rosin moiety. Such molecular geometry could transform the copolymers into novel amphipathic structures.12 This new strategy requires just a one-step quaternization reaction to obtain PDMAEMA-g-rosin copolymers. PDMAEMA with controlled molecular weight and a narrow molecular weight distribution was first prepared via reversible addition fragmentation transfer (RAFT) polymerization. Tertiary amines in the precursor polymers were then facilely quaternized with the chloride ester of a rosin acid to produce rosin-grafted copolymers (Scheme 1). The resulting copolymers were characterized and tested for antibacterial activities against the Gram-positive bacterium Staphylococcus aureus (S. aureus) and Gram-negative bacterium Escherichia coli (E. coli). We prepared a series of rosin-grafted PDMAEMA copolymers. The effects of molecular weight of copolymers and the degree of quaternization (DQ) on antibacterial activities were investigated. The morphology of bacteria was characterized using Scanning Electron Microscopy (SEM) and Confocal Laser Scanning Microscope (CLSM).
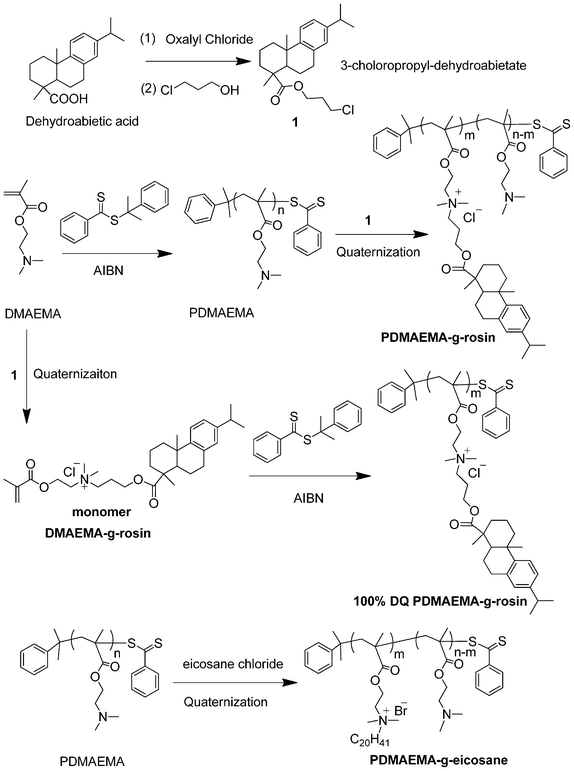 | ||
| Scheme 1 Synthesis of quaternary ammonium-containing PDMAEMA copolymers with rosin as pendant group (PDMAEMA-g-rosin). | ||
Experimental section
Materials
Dehydroabietic acid (DHAA, ∼90%) was obtained from Wuzhou Chemicals, China, and used as received. Oxalyl chloride (98%, Alfa Aesar), 3-chloro-1-propanol (98%, Alfa Aesar), N,N-dimethylaminoethyl methacrylate (DMAEMA, TCL), and Mueller-Hinton broth (Difco Laboratories) were used as received. Azobisisobutyronitrile (AIBN, Aldrich) was re-crystallized in methanol twice before use. All other solvents and agents were analytical grade, and used without further purification.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) were dissolved in a 10 mL Schlenk flask and the solution was degassed with three cycles of freeze-pump-thaw. The reaction mixture was heated at 60 °C for 5 h before being quenched by liquid nitrogen to stop the polymerization. The resulting PDMAEMA was obtained by dissolving in chloroform and precipitating in 20-fold cold hexane three times.
0.1) were dissolved in a 10 mL Schlenk flask and the solution was degassed with three cycles of freeze-pump-thaw. The reaction mixture was heated at 60 °C for 5 h before being quenched by liquid nitrogen to stop the polymerization. The resulting PDMAEMA was obtained by dissolving in chloroform and precipitating in 20-fold cold hexane three times.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were dissolved in 5 mL acetonitrile. The mixture was refluxed at 80 °C for 72 h. After the completion of the reaction, the solvent was evaporated. The product was then dissolved in chloroform and precipitated in hexane twice. The obtained solid product was dried in a vacuum oven at 50 °C for 12 h to remove residual solvents. The mole percentage of quaternization for the polymers was calculated by integrating the ratio of methylene protons (–C(O)–O–CH2–) to aromatic protons from rosin group in the 1H NMR spectra (CDCl3).
5) were dissolved in 5 mL acetonitrile. The mixture was refluxed at 80 °C for 72 h. After the completion of the reaction, the solvent was evaporated. The product was then dissolved in chloroform and precipitated in hexane twice. The obtained solid product was dried in a vacuum oven at 50 °C for 12 h to remove residual solvents. The mole percentage of quaternization for the polymers was calculated by integrating the ratio of methylene protons (–C(O)–O–CH2–) to aromatic protons from rosin group in the 1H NMR spectra (CDCl3).
Results and discussion
(1) Synthesis and characterization of PDMAEMA-g-rosin copolymers
As shown in Scheme 1, we designed a straightforward and effective synthetic pathway to prepare amphipathic PDMAEMA-g-rosin copolymers. Hydrophobic rosin moiety was directly linked onto PDMAEMA polymers by a simple quaternization reaction. We chose dehydroabietic acid (DHAA) as the starting rosin, while the carboxylic acid group allowed derivatization.28,29 Dehydroabietic acid was treated with oxalyl chloride to give the corresponding acyl chloride, and followed by an esterification reaction with 3-chloropropan-1-ol, yielding 3-choloropropyl-dehydroabietate (Compound 1). The structure of 1 was confirmed by 1H NMR (Fig. 1A). The precursor polymer PDMAEMA was synthesized via RAFT polymerization of DMAEMA using CDB as a chain transfer agent and AIBN as an initiator. Three PDMAEMA polymers with molecular weights of 7500 g mol−1, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 41
000 g mol−1 and 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 were synthesized by tuning the molar ratio of [DMAEMA]
000 g mol−1 were synthesized by tuning the molar ratio of [DMAEMA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CDB]
[CDB]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN] to 100
[AIBN] to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 200
0.1, 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 500
0.1 and 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, respectively. Finally, a quaternization reaction between the precursor PDMAEMA and 1 was carried out. The degree of quaternization (DQ) of PDMAEMA could be tuned by adjusting the molar ratio of tertiary amine of PDMAEMA to 1. Using this facile approach, it was feasible to tune various molecular parameters such as molecular weight, DQ and therefore overall amphipathicity, as shown in Table 1. Due to the steric hindrance, a 100% quaternization cannot be achieved even if the quaternization molar ratio of [tertiary amine]
0.1, respectively. Finally, a quaternization reaction between the precursor PDMAEMA and 1 was carried out. The degree of quaternization (DQ) of PDMAEMA could be tuned by adjusting the molar ratio of tertiary amine of PDMAEMA to 1. Using this facile approach, it was feasible to tune various molecular parameters such as molecular weight, DQ and therefore overall amphipathicity, as shown in Table 1. Due to the steric hindrance, a 100% quaternization cannot be achieved even if the quaternization molar ratio of [tertiary amine]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used as high as 1
1 was used as high as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. To prepare PDMAEMA-g-rosin with complete quaternization of the tertiary amine group, a quaternary ammonium-containing monomer DMAEMA-g-rosin was prepared between DMAEMA and 1. The monomer DMAEMA-g-rosin was subsequently polymerized by RAFT (Scheme 1).
5. To prepare PDMAEMA-g-rosin with complete quaternization of the tertiary amine group, a quaternary ammonium-containing monomer DMAEMA-g-rosin was prepared between DMAEMA and 1. The monomer DMAEMA-g-rosin was subsequently polymerized by RAFT (Scheme 1).
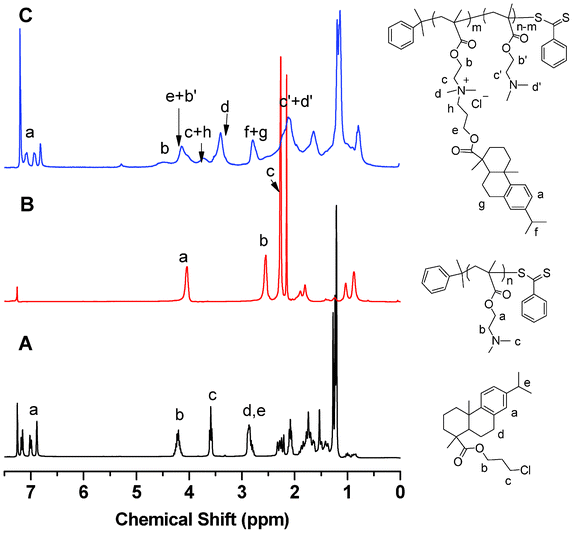 | ||
| Fig. 1 1H NMR spectra: (A) 3-choloropropyl-dehydroabietate (1); (B) PDMAEMA before quaternization; and (C) PDMAEMA after quaternization. | ||
| Sample | M n (g mol−1) (PDMAEMA) | PDI (PDMAEMA) | DQ (%) | M n (g mol−1) (PDMAEMA-g-rosin) | MIC (μg mL−1) | Solubility Limit (μg mL−1) | |
|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | ||||||
| Monomer | 157 | — | — | 534 | 32 | 64 | >256 |
| a Calculated by 1H NMR end group analysis. | |||||||
| Polymer 1 | 7500 | 1.25 | 5 | 8300 | 128 | 128 | >256 |
| Polymer 2 | 7500 | 1.25 | 15 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
48 | 96 | >256 |
| Polymer 3 | 7500 | 1.25 | 40 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
64 | 128 | 256 |
| Polymer 4 | 7500 | 1.25 | 68 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
128 | >256 | 256 |
| Polymer 5 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.28 | 17 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
64 | 128 | >256 |
| Polymer 6 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.23 | 19 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
128 | 128 | >256 |
| Polymer 7 | 5490a | — | 100 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600a 600a |
>256 | >256 | 128 |
1H NMR analysis of PDMAEMA, before and after quaternization, confirmed that the pendant rosin group was successfully attached. As shown in Fig. 1B, the chemical shifts at 2.6 ppm and 2.3 ppm were attributed to the protons (–CH2–N–) and methyl protons (–N(CH3)2) on the tertiary amine of PDMAEMA, respectively. After quaternization of PDMAEMA with compound 1, new peaks were observed at 3.7 and 3.4 ppm, corresponding to methylene protons (–CH2–N+–CH2–) and methyl protons (–N+(CH3)2–) of quaternized DMAEMA units. Methylene protons next to the backbone ester group shifted from 4.1 ppm (the protons from original DMAEMA unit) to 4.5 ppm (Fig. 1C). In addition, the peaks at 6.8 ∼7.2 ppm corresponded to aromatic protons from the dehydroabietate group on the pendant side chain of PDMAEMA, similar to those in compound 1 (Fig. 1A). The DQ was calculated based on the integration areas of methylene protons next to the backbone ester group and aromatic protons, as described by the following equation:
| DQ(%) = 2*Ipeak at 6.8∼7.2 ppm/[3*(Ipeak at 4.54 ppm + Ipeak at 4.10 ppm) − 2*Ipeak at 6.8∼7.2 ppm]*100% |
(2) Antibacterial assessment
Preliminary studies of antibacterial activities of PDMAEMA-g-rosin against E. coli and S. aureus were carried out by the disk diffusion method. Fig. 2 shows the size (diameter, mm) of inhibition zones on agar plates after incubation of bacteria with different amounts of PDMAEMA-g-rosin (Polymer 2) placed on the central disks. Obviously, PDMAEMA-g-rosin exhibited good antibacterial activity against both E. coli and S. aureus, although slightly better against E. coli. To further confirm that the antibacterial activities were derived from the rosin moiety, which likely enhanced the penetration of the polymers into cell membranes and subsequently killed the bacteria, two control experiments were carried out, in which neat PDMAEMA and PDMAEMA quaternized with an eicosyl group (PDMAEMA-g-eicosane) were used. The eicosyl group has the same number of carbons as the rosin moiety, although conformationally it is a linear alkyl group. PDMAEMA-g-eicosane was synthesized via a similar synthetic procedure to the preparation of PDMAEMA-g-rosin, except eicosane chloride was used as the quaternization agent (Scheme 1). As shown in Fig. 2, even when the amount of neat PDMAEMA homopolymers placed on the central disk was as high as 150 μg in agar plates incubated with either E. coli or S. aureus, no inhibition zones were observed, indicating negligible antibacterial activities of PDMAEMA against both bacterial strains. On the other hand, PDMAEMA-g-eicosane also showed no activity against E. coli and only exhibited very weak activity against S. aureus. This result is consistent with previous work on some PDMAEMA derivatives,19,30 suggesting that antibacterial activity of PDMAEMA-g-rosin was due to not only the hydrophobicity of rosin, but also its unique fused-ring structure, which could result in different amphipathicity, as discussed later in this paper.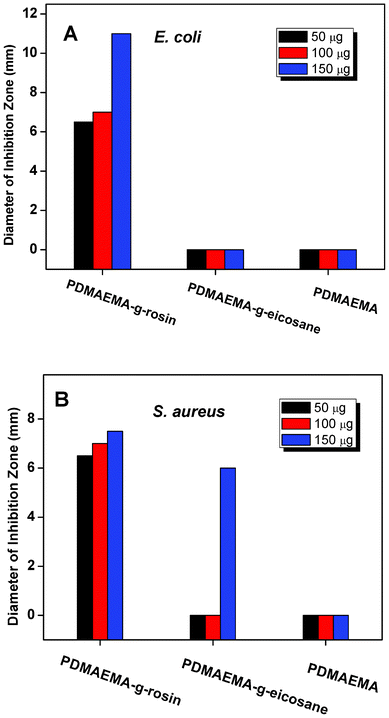 | ||
| Fig. 2 The size (diameter, mm) of inhibition zones after incubation of bacteria with different concentrations of PDMAEMA-g-rosin (Polymer 2), PDMAEMA-g-eicosane and neat PDMAEMA placed on the central disk of agar plates for (A) E. coli; and (B) S. aureus. | ||
The molecular weight of polymers and the degree of quaternization (DQ) are two important factors that dictate the efficacy of many cationic antibacterial macromolecular systems. As shown in Table 1, a series of PDMAEMA-g-rosin copolymers (Polymer 1–7) with different molecular weight and DQ were prepared. The minimum inhibitory concentration (MIC), the lowest polymer concentration to completely inhibit bacterial growth, of these polymers was determined using the broth dilution method. It can be seen from Table 1 that PDMAEMA-g-rosin copolymers showed slightly higher antibacterial activities against E. coli than S. aureus, in agreement with agar diffusion tests. Fig. 3A shows the DQ dependence of the MICs of PDMAEMA-g-rosin with the same molecular weight of parent PDMAEMA (Mn = 7500 g mol−1) against E. coli and S. aureus. It can be seen that PDMAEMA-g-rosin showed the similar dependence on DQ against both E. coli and S. aureus. The increased antibacterial activity was first observed when DQ increased from 7 to 15%, owing to the increase in positive charge density and also hydrophobicity of the polymers chain. Although Polymer 2 (DQ = 15%) was found to still have a higher inhibition concentration than the monomer itself, taking into account that the polymer contains only about 15% active substance (rosin moiety), one can affirm that polymeric conjugates of rosin compounds have improved antimicrobial activity than the small molecular compound. However, further increase of DQ (i.e., 40% and 68%) decreased the antibacterial activity. One possible explanation is that the increase of rosin moiety fraction made polymers more hydrophobic, which then may have resulted in partial aggregation within the culture medium and decreased antibacterial action, similar to the observed aggregation of antimicrobial peptides that weaken their antimicrobial potency.31 In fact, when the concentration of polymer solution >256 μg mL−1, polymer solutions with DQ levels at 40% and 68% (Polymers 3 and 4) became turbid once culture medium was added, as confirmed by the solubility test (Table 1). As a result of the decrease of solubility, the antibacterial activity of polymers may be limited because there was less interaction between polymers and bacteria.
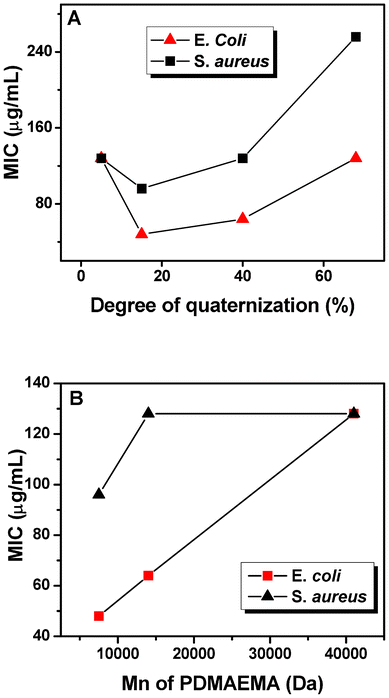 | ||
| Fig. 3 The MIC of PDMAEMA-g-rosin against E. coli and S. aureus with (A) different degree of quaternization (Polymers 2, 3 and 4) and (B) molecular weight of PDMAEMA (Polymers 2, 5 and 6). | ||
With similar DQs (15%, 17% and 19% for Polymers 2, 5 and 6, respectively), the effect of molecular weight of polymers on antibacterial activity is shown in Fig. 3B. Low molecular weight polymers showed higher antibacterial activities than their high molecular weight counterparts. Similar results of molecular dependence on the antibacterial activity of some quaternary ammonium-containing polymers have also been reported.8 As previously reported,32 the complexation of one polyion with an oppositely charged polyion is essentially irreversible, while complexes of a polyion with a less charged species are reversible. Therefore, considering that the negatively charged outer leaf layer forms a polyion–polyion complex with the positively charged polymers, the dissociation of such complexes becomes increasingly more difficult with increasing molecular weight (i.e., more charges on the polymers).
Morphological changes of E. coli and S. aureus cells after incubation with PDMAEMA-g-rosin (Polymer 2) were characterized by FE-SEM. Normally, intact E. coli cells have a rod-like shape, whereas the shape of S. aureus cells is spherical (i.e., cocci). As shown in Fig. 4A and 4C, in control experiments, both types of bacteria maintained their integrity of smooth cell surfaces, and intact bacterial outer envelopes were clearly observed. After 1 h incubation with PDMAEMA-g-rosin copolymers, the cells were easily observable, but had different structural damage depending on the type of bacterium (Fig. 4B and 4D). The surfaces of E. coli cells appeared to be much rougher with the presence of many holes, suggesting that the cell outer membrane was significantly damaged by the copolymers. The S. aureus cells, in contrast, also had observable damage to their structures and completely lost their cellular integrity. In addition, both E. coli and S. aureus cells significantly shrank after incubation, which implied that these cells were lysed and had released their contents as a result of the formation of holes induced by antibacterial copolymers. The drastic morphological change could be explained by a combination of ionic and hydrophobic interactions between the bacterial cellular membrane and polymers.33,34 Our SEM results confirmed that PDMAEMA-g-rosin copolymers have effective bactericidal properties.
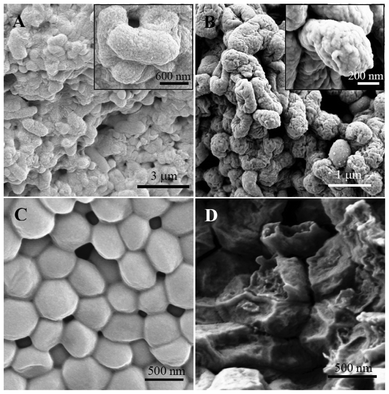 | ||
| Fig. 4 FE-SEM images of E. coli (A, B) and S. aureus (C, D) before (left) and after (right) incubation with PDMAEM-g-rosin copolymers. | ||
LIVE/DEAD bacterial viability assays were carried out to examine the interactions between bacterial cells and polymers. Using this assay, bacterial cells that appear green are live cells with intact membranes, whereas bacterial cells that stain red are dead cells that have damaged membranes. It was observed that bacterial cells contained within aggregates typically included both live cells and dead cells. The coexistence of live and dead cells in the aggregates was shown by the representative CLSM images. Fig. 5A and 5D shows the images of the control samples where no polymers were added to E. coli and S. aureus suspensions, respectively. No aggregates were observed and all cells were live (green). After treatment with Polymer 2 for 1 h, it was observed (Fig. 5B and 5E) that both live cells (green) and dead cells (red) were found in the aggregates. Fig. 5C and 5F show the dead cells after only 2 h incubation, demonstrating that a majority of dead cells were in aggregates, where the cells were in direct contact with polymers. The red-staining of cells also indicated damaged membranes into which the propidium iodide dye penetrated. The CLSM images are consistent with the FE-SEM images, and indicative of the degradation of cellular integrity observed in some aggregate cells.
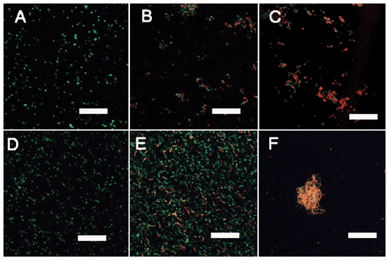 | ||
| Fig. 5 CLSM images of E. coli (A–C) and S. aureus (D–F) cells without- and with- PDMAEMA-g-rosin copolymers stained with the LIVE/DEAD bacterial viability kit: (A) control sample of E.coli; (B) E. coli cells treated with PDMAEMA-g-rosin after 1 h; (C) E. coli cells treated with PDMAEMA-g-rosin after 2 h; (D) control sample of S. aureus; (E) S. aureus cells treated with PDMAEMA-g-rosin after 1 h; and (F) S. aureus cells treated with PDMAEMA-g-rosin after 2 h. Scale bars: 20 μm. | ||
In this study, we observed that the charge location has a significant effect on antibacterial activity. A PDMAEMA-g-rosin polymer with 100% DQ was prepared by direct polymerization of monomer DMAEMA-g-rosin (Scheme 1). However, this polymer exhibited very low antibacterial activities, as shown in Table 1 (Polymer 7). In one of our studies reported earlier, we prepared a rosin-grafted polymer, in which the QA group was located at the periphery of the entire polymer (Scheme 2A).27 Differences in the antibacterial activities of these two kinds of polymers most likely resulted from differences in charge location on the polymers. For the earlier work, the cationic groups were located on the termini of the pendant moiety, acting like small needles (Scheme 2A). Thus this cationic polymer could be easily absorbed onto the bacterial cell surface through electrostatic interactions and subsequently diffuse through the cell wall and kill bacteria. However, in the present case (Scheme 2B), the positive charges were sandwiched between the PDMAEMA backbone and rosin moiety. There could exist a significant steric hindrance effect from its pendent rosin moiety to impede the interaction of the polycation with the bacterial cell wall. In order to expose the positive charges, a partial quaternization was needed. This was exactly what we observed: a balanced quaternization exhibited higher antibacterial activities (e.g., Polymer 2). Our results imply that the position of the positive charge plays an important role in the antibacterial activities of cationic polymers. Such steric hindrance effects have not been reported in the literature.
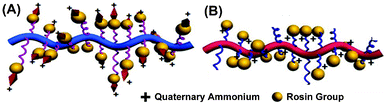 | ||
| Scheme 2 A comparison between two amphipathic structures having cationic charges at different locations with respect to the rosin moiety and polymer backbone: (A) an early-reported polymer with cationic charges located at the periphery;30 (B) a polymer used in the current work with cationic charges embedded inside. | ||
Conclusions
In conclusion, we have developed a simple and efficient method to synthesize amphipathic quaternary ammonium-containing PDMAEMA copolymers with the rosin moiety as pendant group via a quaternization reaction to directly link the hydrophobic rosin moiety onto PDMAEMA polymers. The PDMAEMA-g-rosin copolymers showed effective antibacterial activities against E. coli and S. aureus. Antibacterial activities were found to be dependent on both the degree of quaternization of rosin group, the molecular weight of copolymers as well as the conformation of hydrophobic group. Results of FE-SEM and CLSM imaging indicated that the PDMAEMA-g-rosin copolymers can effectively kill the bacteria by damaging bacterial cellular membranes. Together, these results suggest that PDMAEMA-g-rosin copolymers are potentially useful as antibacterial agents in a wide variety of biomedical and general use applications.Acknowledgements
This work was supported by China International Science and Technology Cooperation (2011DFA32440), Forestry Public Sector Research Fund of State Forestry Administration of China (CAFYBB2010003-3), the University of South Carolina (start-up funds), and USDA NIFA (Award 2011-51160-31205) and National Science Foundation (DMR-1206072).References
- E. R. Kenawy, S. D. Worley and R. Broughton, Biomacromolecules, 2007, 8, 1359–1384 CrossRef CAS.
- K. A. Brogden, Nat. Rev. Microbiol., 2005, 3, 238–250 CrossRef CAS.
- K. Page, M. Wilson and I. P. Parkin, J. Mater. Chem., 2009, 19, 3819–3831 RSC.
- P. Li, Y. F. Poon, W. Li, H. Y. Zhu, S. H. Yeap, Y. Cao, X. Qi, C. Zhou, M. Lamrani, R. W. Beuerman, E. T. Kang, Y. Mu, C. M. Li, M. W. Chang, S. S. J. Leong and M. B. Chan-park, Nat. Mater., 2011, 10, 149–156 CrossRef CAS.
- F. Nederberg, Y. Zhang, J. P. K. Tan, K. Xu, H. Wang, C. Yang, S. Gao, X. D. Guo, K. Fukushima, L. Li, J. L. Hedrick and Y. Y. Yang, Nat. Chem., 2011, 3, 409–414 CrossRef CAS.
- M. F. Ilker, K. Nusslein, G. N. Tew and E. B. Coughlin, J. Am. Chem. Soc., 2004, 126, 15870–15785 CrossRef CAS.
- Z. M. Al-Badri, A. Som, S. Lyon, C. F. Nelson, K. Nusslein and G. N. Tew, Biomacromolecules, 2008, 9, 2805–2810 CrossRef CAS.
- K. Lienkamp, A. E. Madkour, A. Musante, C. F. Nelson, K. Nusslein and G. N. Tew, J . Am. Chem. Soc., 2008, 130, 9836–9843 CrossRef CAS.
- G. J. Gabriel, A. E. Madkour, J. M. Dabkowski, C. F. Nelson, K. Nusslein and G. N. Tew, Biomacromolecules, 2008, 9, 2980–2983 CrossRef CAS.
- C. M. Goodman, S. Choi, S. Shandler and W. F. DeGrado, Nat. Chem. Biol., 2007, 3, 252–262 CrossRef CAS.
- G. N. Tew, R. W. Scott, M. L. Klein and W. F. DeGrado, Accts. Chem. Res., 2010, 43, 30–39 CrossRef CAS.
- B. P. Mowery, S. E. Lee, D. A. Kissounko, R. F. Epand, R. M. Epand, B. Weisblum, S. S. Stahl and S. H. Gellman, J. Am. Chem. Soc., 2007, 129, 15474–15476 CrossRef CAS.
- K. Kuroda, G. A. Caputo and W. F. Degrado, Chem. Eur. J., 2009, 15, 1123–1133 CrossRef CAS.
- G. Liu, D. Wu, C. Ma, G. Zhang, H. Wang and S. Yang, ChemPhysChem, 2007, 8, 2254–2259 CrossRef CAS.
- A. Kusumo, L. Bombalski, Q. Lin, K. Matyjaszewski, J. W. Schneider and R. D. Tilton, Langmuir, 2007, 23, 4448–4454 CrossRef CAS.
- S. B. Lee, R. R. Koepsel, S. W. Morley, K. Matyjaszewski, Y. Sun and A. J. Russell, Biomacromolecules, 2004, 5, 877–882 CrossRef CAS.
- D. Roy, J. S. Knapp, J. T. Guthrie and S. Perrier, Biomacromolecules, 2008, 9, 91–99 CrossRef CAS.
- J. Huang, H. Murata, R. R. Koepsel, A. J. Russell and K. Matyjaszewski, Biomacromolecules, 2007, 8, 1396–1399 CrossRef CAS.
- L. B. Rawlinson, S. M. Ryan, G. Mantovani, J. A. Syrett, D. M. Haddleton and D. J. Brayden, Biomacromolecules, 2010, 11, 443–453 CrossRef CAS.
- H. Dong, J. Huang, R. R. Koepsel, P. Ye, A. J. Russell and K. Matyjaszewski, Biomacromolecules, 2011, 12, 1305–1311 CrossRef CAS.
- J. J. W. Coppen and G. A. Hone, Gum naval stores: Turpentine and rosin from pin resin, Natural Resources Institute, Food and Agriculture Organization of the United Nations, Rome, 1995 Search PubMed.
- J. Wang, K. Yao, A. L. Korich, S. Li, S. Ma, H. J. Ploehn, P. M. Iovine, C. Wang, F. Chu and C. Tang, J. Polym. Sci. Part A: Polym. Chem., 2011, 49, 3728–3738 CrossRef CAS.
- J. Wang, M. Lin, C. Wang and F. Chu, J. Appl. Polym. Sci., 2009, 113, 3757–3765 CrossRef CAS.
- P. A. Wilbon, Y. Zheng, K. Yao and C. Tang, Macromolecules, 2010, 43, 8747–8754 CrossRef CAS.
- K. Yao, J. Wang, W. Zhang, J. S. Lee, C. Wang, F. Chu, X. He and C. Tang, Biomacromolecules, 2011, 12, 2171–2177 CrossRef CAS.
- Y. Zheng, K. Yao, J. Lee, D. Chandler, J. Wang, C. Wang, F. Chu and C. Tang, Macromolecules, 2010, 43, 5922–5924 CrossRef CAS.
- J. Wang, Y. Chen, K. Yao, P. A. Wilbon, W. Zhang, L. Ren, J. Zhou, M. Nagarkatti, C. Wang, F. Chu, X. He, A. W. Decho and C. Tang, Chem. Comm., 2012, 48, 916–918 RSC.
- J. S. Lee and S. Hong, Eur. Polym. J., 2002, 38, 387–392 CrossRef CAS.
- H. S. Do, J. H. Park and H. J. J. Kim, Appl. Polym. Sci., 2009, 111, 1172–1176 CrossRef CAS.
- E. Yancheva, D. Paneva, V. Maximova, L. Mespouille, P. Dubois, N. Manolova and I. Rashkov, Biomacromolecules, 2007, 8, 976–984 CrossRef CAS.
- K. Matsuzaki, Biochimica et Biophysica Acta (BBA) – Biomembranes, 2009, 1788, 1687–1692 CrossRef CAS.
- H. Dautzenberg, W. Jaeger, J. Kotz, B. Phlipp, C. Seidel and D. Stscherbina, Polyelecrolytes: formation, Characterization and Application; Hanser Gardner: Munich, Germany, 1994 Search PubMed.
- T. Ikeda, H. Hirayama, K. Suzuki, H. Yamaguchi and S. Tazuke, Macromol. Chem. Phys., 1986, 187, 333–340 CrossRef CAS.
- T. Ikeda, H. Hirayama, H. Yamaguchi, S. Tazuke and M. Watanabe, Antimicrob. Agents Chemother., 1986, 30, 132–136 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
