Cooperatively exfoliated fluorinated graphene with full-color emission†
Zhaofeng
Wang
a,
Jinqing
Wang
*a,
Zhangpeng
Li
ab,
Peiwei
Gong
ab,
Junfang
Ren
a,
Honggang
Wang
a,
Xiuxun
Han
*a and
Shengrong
Yang
a
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, P. R. China. E-mail: jqwang@licp.cas.cn; xxhan@licp.cas.cn; Fax: +86 931 8277088; Tel: +86 931 4968076
bGraduate University of Chinese Academy of Sciences, Beijing, 100080, P. R. China
First published on 25th September 2012
Abstract
We report a novel and effective method to prepare fluorinated graphene sheets (FGS) by the cooperative exfoliation of graphite fluoride using cetyl-trimethyl-ammonium bromide and dopamine. This facile, scalable preparation route results in wide (about 2 μm in width), long (at least 3 μm in length) and ultrathin (1–2 layers) FGS with uniform morphology. The chemical composition of FGS was characterized by X-ray photoelectron spectroscopy and energy dispersive X-ray spectroscopy. The obtained FGS exhibits full-color emission when excited by near ultraviolet (NUV) rays, suggesting its potential applications in luminescence devices, such as NUV-pumped FGS-based flexible light-emitting diodes.
Introduction
Fluorinated graphene, a derivative of graphene in which fluorine atoms are introduced in the form of C–F bonds, accompanied by the structural transformation of the C–C bonds from sp2 to sp3 configuration, has attracted more and more interest due to its unique electrical and optical properties.1–4 Theoretical and experimental studies confirm the covalent connection of fluorine to a carbon framework can alter the electronic structure of graphene sheets (GS), yielding a tunable bandgap ranged from 0 to ∼3 eV, which expands the applications of GS into optoelectronics and photonics.5–7 In particular, due to its ultrathin thickness and flexibility, fluorinated graphene sheets (FGS) have attracted much attention for the fabrication of transparent and flexible electronic devices.3,4,8FGS can also be considered as the structure units of graphite fluoride (FGR). Previous research into the photoluminescence properties of FGR suggest that it can emit visible light on irradiation by near-ultaviolet (NUV) rays.9,10 The excitation wavelength matches well with the NUV-chip (from 350 to 420 nm),11 indicating that FGR is a good candidate for NUV light-emitting diodes (LEDs). By comprehensive consideration of the relationship between FGR and FGS, and the electronic structure, thickness and flexibility of FGS, it is anticipated that FGS could inherit the photoluminescence properties of FGR and be applied as an efficient and ultrathin luminescence film in new generation flexible LEDs equipment.
To achieve this goal, the primary prerequisite is achieving low-cost and large-scale production of FGS. Present research suggests that liquid-phase exfoliation is one of the most promising methods for preparing graphene sheets (GS) and FGS, owing to its simple preparation process (usually converting raw materials into liquid reagent by ultrasonication).12–14 It has been recognized that the exfoliation effect of the liquid-phase method mainly depends on the choice of exfoliation agent.15 The reagents of sulfolane and 1-butyl-3-methylimidazolium bromide have been employed to exfoliate FGS; however, the former is highly toxic while the latter is difficult to rinse away.14,16 Therefore, a low-cost and environmentally friendly exfoliation route should be developed.
In this work, the cationic surfactant of cetyl-trimethyl-ammonium bromide (CTAB) was chosen as the exfoliation reagent to prepare FGS, because the implementing group of C16H33(CH3)3N+ (CTA+) could have strong electrostatic adsorption with the fluorine group in FGR, which possesses certain electronegativity. The intercalation of CTA+ cations can expand the interplanar space between adjacent FGR layers, which facilitates the exfoliation of FGR through subsequent ultrasonication. Moreover, CTAB also can reduce the surface tension and lower the surface energy, as well as eliminate the defect states through chemical interaction during the preparation process.17,18 In order to further improve the quality of FGS, a certain amount of dopamine (DA, which easily polymerizes into polydopamine) is introduced to utilize its excellent adhesive ability, which can assist sufficient dispersion of FGR.19 The morphology, chemical composition and exfoliation mechanism, as well as the photoluminescence properties, of the as-prepared sample were investigated and discussed in detail.
Experimental details
Raw materials
FGR with a fluorine content of about 20% was purchased from Shanghai CarFluor Chemicals Co., Ltd. CTAB (99%) was purchased from Sinopharm Chemical Reagent Co., Ltd. Dopamine hydrochloride (99%) was obtained from Acros Organics and used as received. Tris-HCl (pH = 8.5) was prepared as a solvent and ultrapure water (>18 MΩ cm) was used for rinsing.Preparation processes
The preparation was carried out at room temperature in air. In a typical procedure, 0.1 g of FGR was weighted and added to 50 mL Tris-HCl solution (pH = 8.5) with constant stirring. FGR was difficult to disperse until 0.4 g of DA was added. The dispersion was stirred for about 1 h. Then, 0.2 g of CTAB was weighed and introduced to the solution. After being stirred for another 3 h, the obtained black dispersion was treated by ultrasonication for 10 min. Last, the product was filtered using microporous membrane and thoroughly washed with ultrapure water, followed by freeze drying. FGS was thus obtained.For comparison, samples exfoliated only using DA or CTAB were also prepared. The process is the same as that for FGS, except in the addition of raw materials.
Characterizations
The crystal phase of the obtained materials were determined by powder X-ray diffraction (XRD, Rigaku D/MAX-2400 X-ray diffractometer with Ni-filtered Cu-Kα radiation). The morphology, thickness and microstructure of different sheet-like samples were observed by atomic force microscopy (AFM, VEECO Nanoscope IIIa), transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM, FEI Tecnai F30, operated at 300 kV).The chemical composition was investigated by energy dispersive X-ray spectroscopy (EDX) and X-ray photoelectron spectroscopy (XPS, PHI-5702, Physical Electronics) using a monochromated Al-Kα irradiation. Thermal gravimetric analysis (TGA) was carried out by DSC/DTA-TG (NETZSCH Ltd., Germany) from 40 to 800 °C at a heating rate of 10 °C min−1 in air. The photoluminescence excitation and emission spectra were recorded on a FLS-920T fluorescence spectrophotometer equipped with a 450 W Xe light source and double excitation monochromators.
Results and discussion
TEM was employed to measure the quality of the as-prepared FGS. As shown in Fig. 1, it is obvious that cooperative exfoliation of FGR by PDA and CTAB yields large-dimensional (about 2 μm in width and 3 μm in length) and transparent FGS, and the preparation process is simple, facile and easily scalable. In this approach, FGR is totally exfoliated into FGS and the concentration of the final dispersion can reach as high as 2 mg mL−1. The higher contrast edges (dark regions) in Fig. 1a can be attributed to scrolling of the sheet, which is common for exfoliated FGS.16 Moreover, a transparent nanosheet bending nearly 180° in Fig. 1b proves the flexibility of the as-prepared FGS.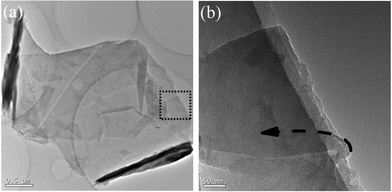 | ||
| Fig. 1 Morphology observations of the cooperatively exfoliated FGS revealed by TEM: (a) TEM micrograph of typically scrolled FGS nanosheet; (b) TEM image with higher magnification of the dashed line region in (a). | ||
Fig. 2a provides a TEM micrograph of two similar FGS nanosheets that have separated from each other, clearly displaying how few-layered sheets exfoliate from FGR to create FGS. EDX analysis (Fig. 2b) obtained from the region in Fig. 2a reveals that the nanosheets are mainly composed of carbon and fluorine elements, and the absence of nitrogen and bromine indicates that residual PDA and CTAB have been completely removed. To accurately determine the thickness and layer number of sheets, AFM and HRTEM observations were performed. As shown in Fig. 2c, the morphology is similar to that observed in TEM images and the thickness of sheet is measured as about 1.735 nm, corresponding to 1–2 layers given that the thickness of monolayer fluorinated graphene is between 0.783 nm and 0.87 nm.14 The HRTEM micrograph on the edge of FGS (as shown in Fig. S1a, ESI†) confirms the AFM result and indicates that the interlayer space is about 0.667 nm. Hence, an ultrathin FGS has been obtained successfully.
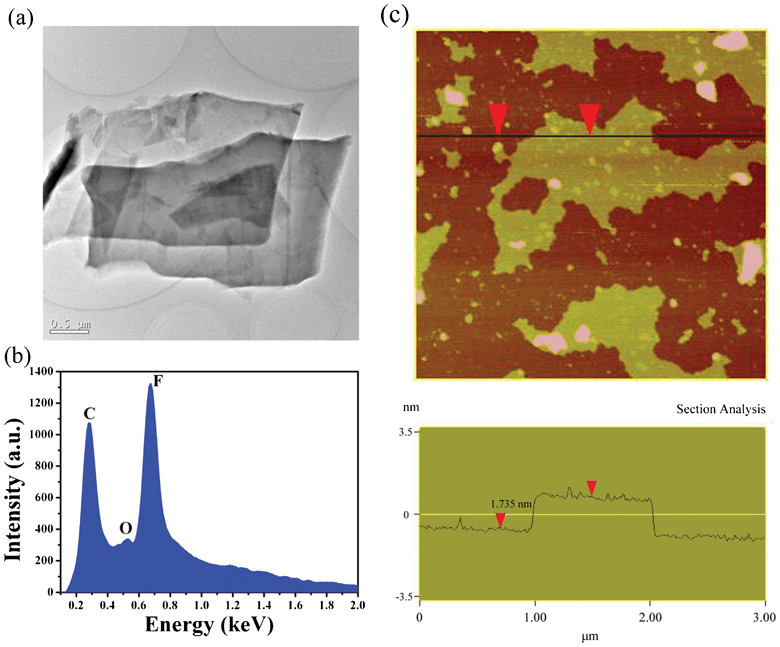 | ||
| Fig. 2 (a) TEM micrograph of two FGS sheets that just exfoliated from each other; (b) EDX spectrum of the sheets in (a) for exploring the chemical composition; (c) AFM image and the corresponding cross section analysis of the as-prepared FGS sample. | ||
The chemical functionalities of the FGS were further studied using XPS characterization and the results are shown in Fig. 3. It is observed from the survey XPS spectra in Fig. 3a that no elements other than C, O and F are detected on the surfaces of FGR and FGS. O atoms may come from O2, H2O or CO2 adsorbed onto the surfaces of FGR and FGS.21 The comparable fluorine content in FGS and FGR suggests that no obvious defluorination occurs during the exfoliation process. Two main peaks centered at 284.8 and 289.8 eV in C1s core level XPS spectrum (see Fig. 3b) are attributed to the binding energy of C1s in the form of C–C and C–F bonds,2,20 respectively, confirming that the fluorine in FGS connects with the carbon atoms via covalent bonds. The relative atomic ratio of carbon to fluorine (RC/F) can be estimated from the peak areas of C1s and F1s as well as their atomic sensitivity factors, which are often used to describe the element content.22,23 By calculation, the RC/F values are determined to be 3.95 for FGR and 4.08 for FGS. The slightly lower fluorine content of FGS could be attributed to the removal of the unstable fluorine groups which are physically absorbed or entrapped on the framework surfaces.20
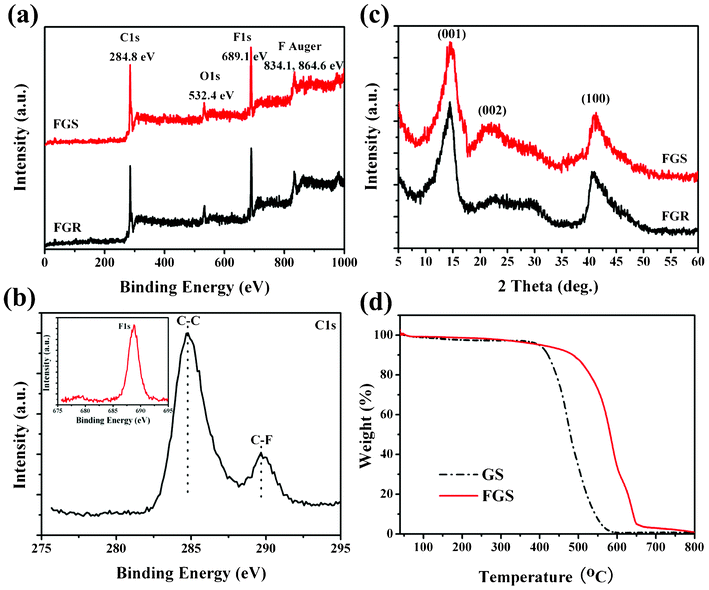 | ||
| Fig. 3 (a) Survey XPS spectra of FGR and FGS samples. (b) C1s spectrum of the prepared FGS to investigate the type of chemical bonds between carbon and fluorine elements; the inset in (b) shows the F1s core line of the as-synthesized FGS. (c) Typical XRD patterns of FGR and exfoliated FGS. (d) TGA curves of GS and FGS from 40 to 800 °C at a heating rate of 10 °C min−1 in air. | ||
The crystal structures of FGR and exfoliated FGS were measured by XRD, as shown in Fig. 3c. It can be observed that both FGR and FGS display a peak centered at about 2θ = 14.46° with a d-spacing of 0.612 nm, which can be assigned as the (001) reflection in a hexagonal system, particularly in compounds possessing a very high fluorine level.24 The peaks at around 41.28° can be assigned to the (100) reflections, which are associated with the C–C in-plane length in the reticular system.25 The (002) reflection in these samples is very broad, suggesting that the samples are poorly ordered along the stacking direction and composed of free graphene-like sheets.26 The appearance of (002) confirms the existence of sp2 C in FGR and FGS. Comparing with FGR, the (002) peak of FGS exhibits a pronounced increase, proving the successful exfoliation of FGR. Furthermore, TGA investigation was carried out to examine the thermal stability of the obtained FGS. Fig. 3d shows the TGA curves of GS and FGS from 40 to 800 °C at a heating rate of 10 °C min−1 in air. The onset temperature of the weight loss of GS is 412 °C, while the decomposition onset of FGS occurs at 510 °C, which proves that FGS possesses a better thermal stability.
It is notable that the raw material utilized in this work is partially fluorinated graphite in which sp2 C and sp3 C coexist. The distribution of sp2 C and sp3 C in FGR can be investigated from the Gaussian fitting of its C1s XPS spectrum (Fig. S2, ESI†). The C1s spectrum can be fitted to five peaks locating at about 284.7 eV (sp2 C), 285.6 eV (sp3 C), 289.2 eV (sp2 C covalently linked to an F atom), 290.4 eV (sp3 C covalently linked to an F atom; C–F) and 292.1 (sp3 C covalently linked to two F atoms; C–F2), respectively.2,20 The ratio of the area percentage of sp2 C and sp3 C is calculated to be about 2.81. Due to the coexistence of sp2 C and sp3 C in the structure, fracture of the carbon framework can occur and structural defects are inevitable in FGR. Therefore, structural defects also exist in the exfoliated FGS, and this can be demonstrated by the magnified HRTEM image of the FGS sample (Fig. S1b, ESI†).
To understand the mechanism of cooperative exfoliation, a further insight into the structures and functions of raw materials is instructive. It is well known that FGR is an intercalation compound in which fluorine alternates the “top” and “down” positions of carbon plane.27 The interlayer space of FGR revealed by HRTEM is about 0.667 nm, which is smaller than that previously reported, due to the partial fluorination.28 Compared with the interlayer space of 0.335 nm for graphite,29 the value for FGR indicates that it can be more easily intercalated by heterogeneous species. However, FGR is a hydrophobic material, which hinders its exfoliation in aqueous solution (Fig. S3a, ESI†). Recent research in our group has demonstrated that DA can attach to the surfaces of almost all materials as an effective adhesive, and can auto-polymerize into polydopamine (PDA), which contains large quantity of hydrophilic groups that can facilitate the dispersion of FGR.19,30,31 The combination process of PDA with surfaces involves both physical and chemical interactions. In this case, PDA can adsorb on the surfaces of FGR by physical action since no obvious defluorination occurred (revealed by the comparison of fluorination degrees of FGR and FGS in Fig. 3a). As shown in Fig. S3, ESI,† with the addition of PDA, the hydrophobic FGR is efficiently dispersed in Tris-HCl solution, which is beneficial for further exfoliation. To determine the function of individual components in peeling FGR sheets, products utilizing only PDA or CTAB were prepared and their TEM micrographs are shown in Fig. S4, ESI†. The results suggest that CTAB demonstrates a good effect on exfoliation, while the product is still composed of large particles when only PDA is utilized. The function of CTAB in the process is explained in the Introduction as being due to the electrostatic interaction. However, the effect of using CTAB alone is inferior to that of the combination, which results from its greater dispersibility as shown in Fig. S3, ESI.† As a result, the generation mechanism of FGS in this work can be considered as the cooperative exfoliation by PDA and CTAB, as schematically illustrated in Fig. 4. As far as we know, exfoliating processes which have previously been implemented successfully utilize either matching the surface energy between solvent molecular and FGR layers or strong dipole interactions.13,16,32,33 The utilization of electrostatic interaction by CTA+ and fluorine groups to peel FGR is thus reported for the first time.
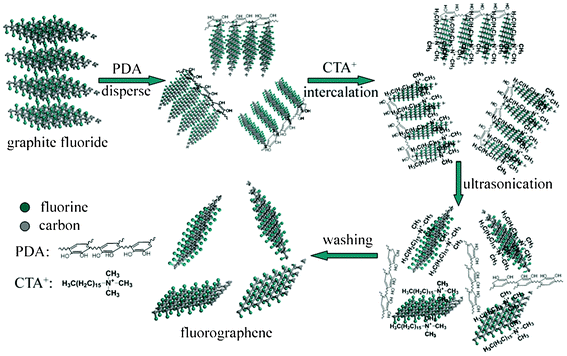 | ||
| Fig. 4 Illustration of the cooperative exfoliation process by PDA and CTAB to prepare FGS. | ||
With the purpose of exploring the optical applications of FGS, luminescence properties were measured as shown in Fig. 5. In the emission spectrum, full-color emission from violet light to red light (3.1–1.65 eV) is observed when excited by a 365 nm excitation line (3.40 eV). The emission curve is very smooth, which is directly related to the quality of FGS prepared by the cooperative exfoliation process. The color coordinate is calculated to be (0.343, 0.392), located in the greenish white light region (Fig. S5, ESI†). The excitation spectrum monitored at the green light (2.34 eV) exhibits a broad band from 2.76 to 4.41 eV, suggesting that continuous optical excited-state energy levels are generated when GS is fluorinated. These results indicate that FGS inherits the photoluminescence of FGR.
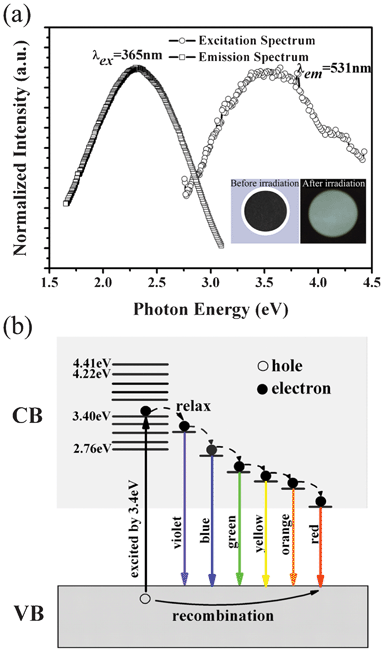 | ||
| Fig. 5 Optical properties of the cooperatively exfoliated FGS: (a) Left curve is the emission spectrum of FGS under 365 nm (3.40 eV) excitation; the right one is the excitation spectrum monitored by 531 nm (2.34 eV); the inset figures on the lower right exhibit its optical photographs before and after the 365 nm irradiation using ultraviolet lamp. (b) Luminescence mechanism for the full-color emission (from violet light to red light) when excited by 3.4 eV. | ||
It is worth noting that the photoluminescence property of FGS is dependent on its fluorination degree and the variation of fluorine content in FGS could cause a shift in the emission peak. For example, the FGS in this work exhibits visible light with a maximum peak at about 531 nm, while the fully fluorinated graphene (fluorographene) reported by K. J. Jeon et al. is a wide bandgap semiconductor with ultraviolet luminescence.34 This is because the electronic structure of FGS could be altered by changing the fluorine content, that is, FGS with a higher fluorination degree possesses a wider bandgap.
In our case, the origin of the luminescence could be ascribed to excitonic recombination.34 However, the reason that FGS emits so broad a band instead of a relatively narrow one is unclear. We believe this phenomenon could be related to the microstructure of FGS. From the magnified HRTEM image of the FGS surface (Fig. S1b, ESI†), it is found that the pore sizes of the carbon framework are non-uniform, indicating that there are various C forms with different C–C bond lengths coexisting, and the distribution of these C atoms with different forms is random. Therefore, although the RC/F of our FGS is estimated to be about 4.08, it can be inferred that fluorine atoms would not be arranged consistently on the carbon plane following this proportion.35 In some parts the fluorination degrees would be higher (CF, C2F, etc.), while it would be lower (C6F, C8F, etc.) in other locations. Thus, the charge density and electronic structure of the π orbits of carbon in these positions would be modified to different extents, generating various unexpected bandwidths, which could be responsible for the broad emission curve covering the whole visible light region (the possible luminescence mechanism of FGS excited by 3.4 eV is illustrated in Fig. 5b).
The luminescence of the cooperatively exfoliated FGS covers the whole visible light spectrum, which suggests broad applications for it in many fields, such as illumination and display devices. In particular, due to its flexibility and broad excitation band in NUV region, FGS sheets could be applied in flexible NUV LEDs as an ultrathin white light emitting luminescence coating.
Conclusion
In summary, a facile and feasible route has been successfully exploited for preparing single- or few-layered FGS with high yield. This approach is carried out at room temperature in air and is suitable for large-scale production. The observation of visible light emission of the prepared FGS is exciting, as it suggests a practical significance in fabricating flexible NUV-LEDs. This research may arouse a great deal of interest in the photoluminescence of FGS-based materials from researchers, and also expand its applications.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant Nos. 20823008 and 51075384), “Top Hundred Talents Program” of Chinese Academy of Sciences and the “Funds for Distinguished Young Scientists of Gansu”.References
- S. B. Bon, L. Valentini, R. Verdejo, J. L. Garcia Fierro, L. Peponi, M. A. Lopez-Manchado and J. M. Kenny, Chem. Mater., 2009, 21, 3433 CrossRef.
- J. T. Robinson, J. S. Burgess, C. E. Junkermeier, S. C. Badescu, T. L. Reinecke, F. K. Perkins, M. K. Zalalutdniov, J. W. Baldwin, J. C. Culbertson, P. E. Sheehan and E. S. Snow, Nano Lett., 2010, 10, 3001 CrossRef CAS.
- F. Withers, T. H. Bointon, M. Dubois, S. Russo and M. F. Craciun, Nano Lett., 2011, 11, 3912 CrossRef CAS.
- R. R. Nair, W. Ren, R. Jalil, I. Riaz, V. G. Kravets, L. Britnell, P. Blake, F. Schedin, A. S. Mayorov, S. Yuan, M. I. Katsnelson, H. M. Cheng, W. Strupinski, L. G. Bulusheva, A. V. Okotrub, I. V. Grigorieva, A. N. Grigorenko, K. S. Novoselov and A. K. Geim, Small, 2010, 6, 2877 CrossRef CAS.
- D. K. Samarakoon, Z. Chen, C. Nicolas and X. Q. Wang, Small, 2011, 7, 965 CrossRef CAS.
- H. Chang, J. Cheng, X. Liu, J. Gao, M. Li, J. Li, X. Tao, F. Ding and Z. Zheng, Chem.–Eur. J., 2011, 17, 8896 CrossRef CAS.
- H. Şahin, M. Topsakal and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 115432 CrossRef.
- F. Withers, S. Russo, M. Dubois and M. F. Craciun, Nanoscale Res. Lett., 2011, 6, 526 CrossRef CAS.
- T. Nakajima, Fluorine-carbon and fluoride-carbon materials: chemistry, physics, and applications, The Chemical Rubber Company Press, 1995 Search PubMed.
- B. Wang, J. R. Sparks, H. R. Gutierrez, F. Okino, Q. Hao, Y. Tang, V. H. Crespi, J. O. Sofo and J. Zhu, Appl. Phys. Lett., 2010, 97, 141915 CrossRef.
- Y.-S. Tang, S.-F. Hu, W.-C. Ke, C. C. Lin, N. C. Bagkar and R.-S. Liu, Appl. Phys. Lett., 2008, 93, 131114 CrossRef.
- M. Lotya, Y. Hernandez, P. J. King, R. J. Smith, V. Nicolosi, L. S. Karlsson, F. M. Blighe, S. De, Z. Wang, I. McGovern, G. S. Duesberg and J. N. Coleman, J. Am. Chem. Soc., 2009, 131, 3611 CrossRef CAS.
- A. B. Bourlinos, V. Georgakilas, R. Zboril, T. A. Steriotis and A. K. Stubos, Small, 2009, 5, 1841 CrossRef CAS.
- R. Zbořil, F. Karlický, A. B. Bourlinos, T. A. Steriotis, A. K. Stubos, V. Georgakilas, K. Šafářová, D. Jančík, C. Trapalis and M. Otyepka, Small, 2010, 6, 2885 CrossRef.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. McGovern, B. Holland, M. Byrne, Y. K. Gun'Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563 CrossRef CAS.
- H. Chang, J. Cheng, X. Liu, J. Gao, M. Li, J. Li, X. Tao, F. Ding and Z. Zheng, Chem.–Eur. J., 2011, 17, 8896 CrossRef CAS.
- D. K. Smith and B. A. Korgel, Langmuir, 2008, 24, 644 CrossRef CAS.
- T. Chakraborty, I. Chakraborty, S. P. Moulik and S. Ghosh, J. Phys. Chem. B, 2007, 111, 2736 CrossRef CAS.
- Y. Mi, Z. Wang, X. Liu, S. Yang, H. Wang, J. Ou, Z. Li and J. Wang, J. Mater. Chem., 2012, 22, 8036 RSC.
- J. M. Lee, S. J. Kim, J. W. Kim, P. H. Kang, Y. C. Nho and Y. S. Lee, J. Ind. Eng. Chem., 2009, 15, 66 CrossRef CAS.
- W. Lu, S. Liu, X. Qin, L. Wang, J. Tian, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, J. Mater. Chem., 2012, 22, 8775 RSC.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760–764 CrossRef CAS.
- D. Deng, X. Pan, L. Yu, Y. Cui, Y. Jiang, J. Qi, W.-X. Li, Q. Fu, X. Ma, Q. Xue, G. Sun and X. Bao, Chem. Mater., 2011, 23, 1188 CrossRef CAS.
- K. Guérin, J. P. Pinheiro, M. Dubois, Z. Fawal, F. Masin, R. Yazami and A. Hamwi, Chem. Mater., 2004, 16, 1786 CrossRef.
- S. Mouras, A. Hamm, D. Djurado and J. C. Cousseins, Rev. Chim. Miner., 1987, 24, 572 Search PubMed.
- C. Nethravathi and M. Rajamathi, Carbon, 2008, 46, 1994 CrossRef CAS.
- S. H. Cheng, K. Zou, F. Okino, H. R. Gutierrez, A. Gupta, N. Shen, P. Eklund, J. Sofo and J. Zhu, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 205435 CrossRef.
- Y. Kita, N. Watanabe and Y. Fujii, J. Am. Chem. Soc., 1979, 101, 3832 CrossRef CAS.
- A. V. Murugan, T. Muraliganth and A. Manthiram, Chem. Mater., 2009, 21, 5004 CrossRef CAS.
- M. Qu, B. Zhang, S. Song, L. Chen, J. Zhang and X. Cao, Adv. Funct. Mater., 2007, 17, 593 CrossRef CAS.
- J. Ou, J. Wang, S. Liu, J. Zhou and S. Yang, J. Phys. Chem. C, 2009, 113, 20429 CAS.
- B. J. Landi, H. J. Ruf, J. J. Worman and R. P. Raffaelle, J. Phys. Chem. B, 2004, 108, 17089 CrossRef CAS.
- W. Qian, R. Hao, Y. Hou, Y. Tian, C. Shen, H. Gao and X. Liang, Nano Res., 2009, 2, 706 CrossRef CAS.
- K. J. Jeon, Z. Lee, E. Pollak, L. Moreschini, A. Bostwick, C. M. Park, R. Mendelsberg, V. Radmilovic, R. Kostecki and T. J. Richardson, ACS Nano, 2011, 5, 1042 CrossRef CAS.
- M. Chen, H. Zhou, C. Qiu, H. Yang, F. Yu and L. Sun, Nanotechnology, 2012, 23, 115706 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra21871b |
| This journal is © The Royal Society of Chemistry 2012 |
